Benzylpyrazinium Salts as Photo-Initiators in the Polymerization of Epoxide Monomers
Abstract
:1. Introduction
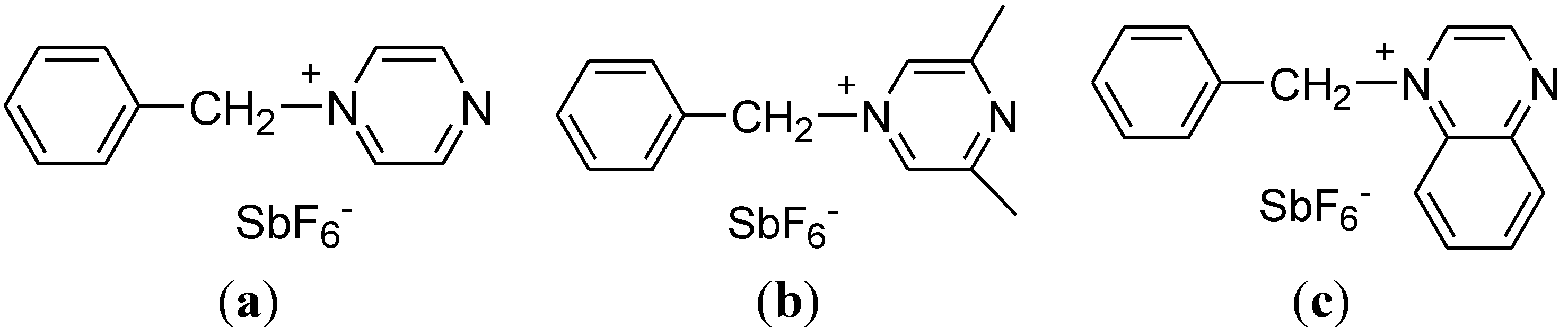
2. Experimental Section
2.1. Materials
2.2. Measurements
2.3. Synthesis of BPH and BDH
2.4. Synthesis of BQH
2.5. Differential Photo-Calorimeter (DPC)
2.6. Polymerization
3. Results and Discussion
3.1. Preparation and Characterization of BPH, BDH and BQH

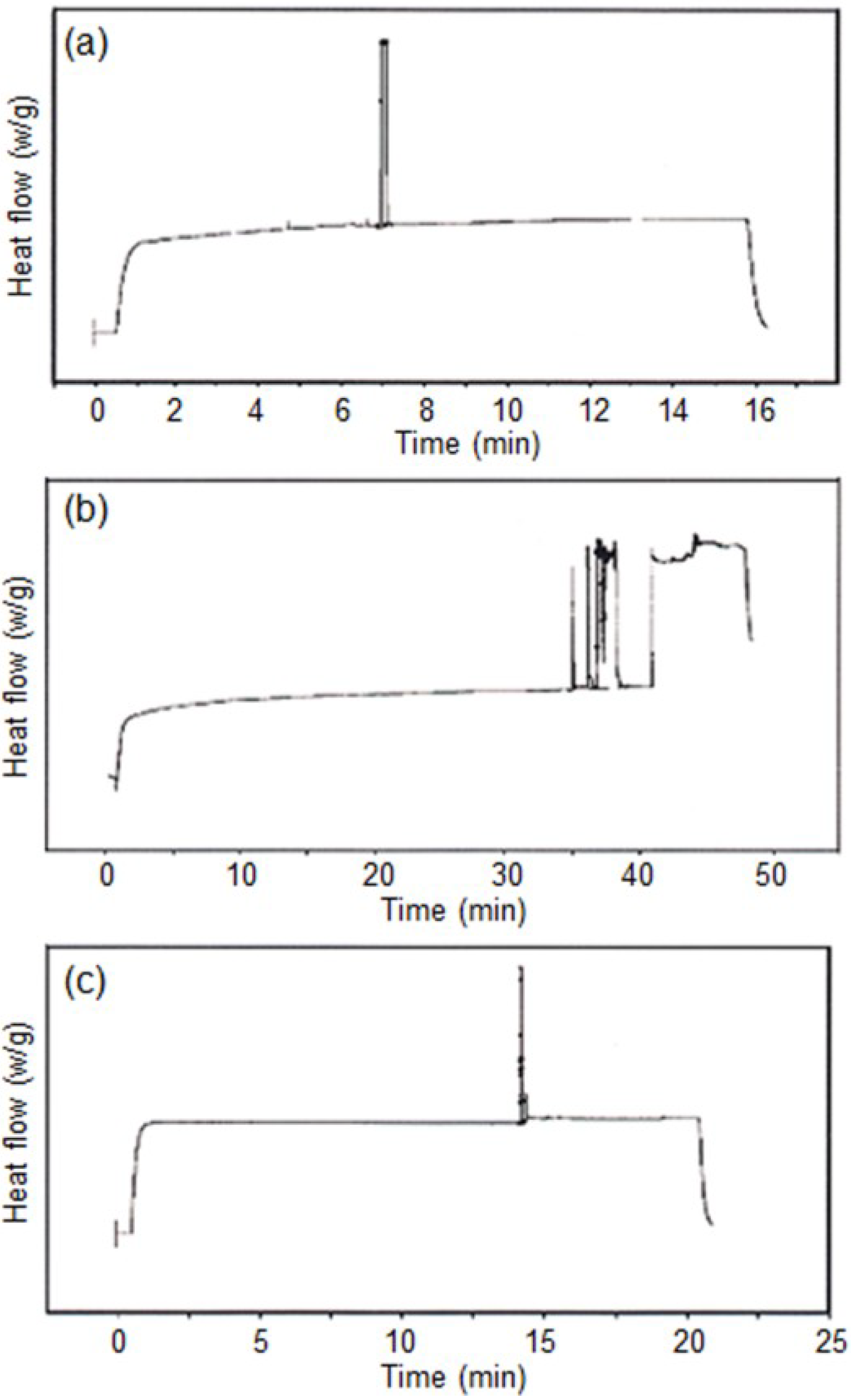
3.2. Photo-Polymerization
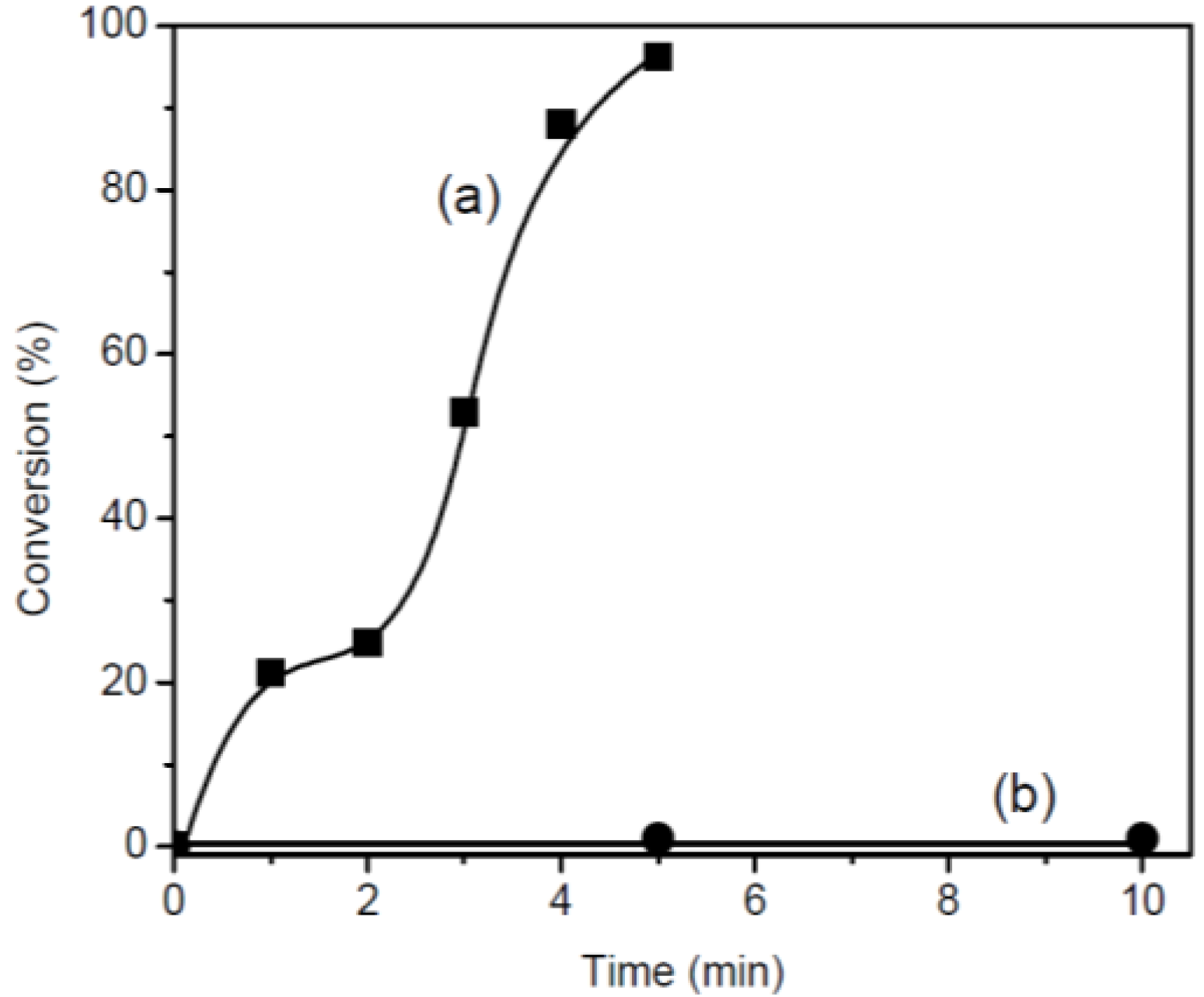
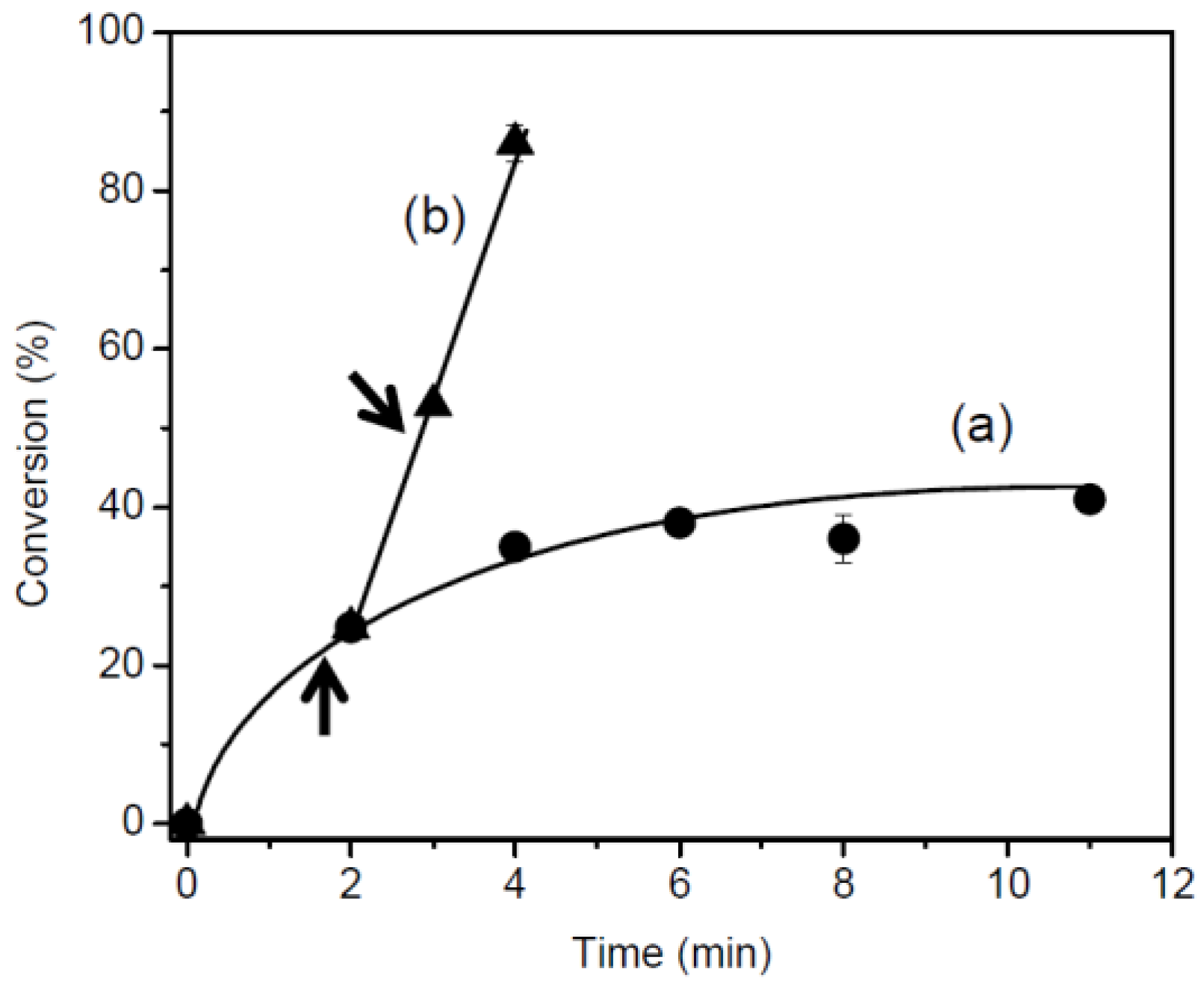
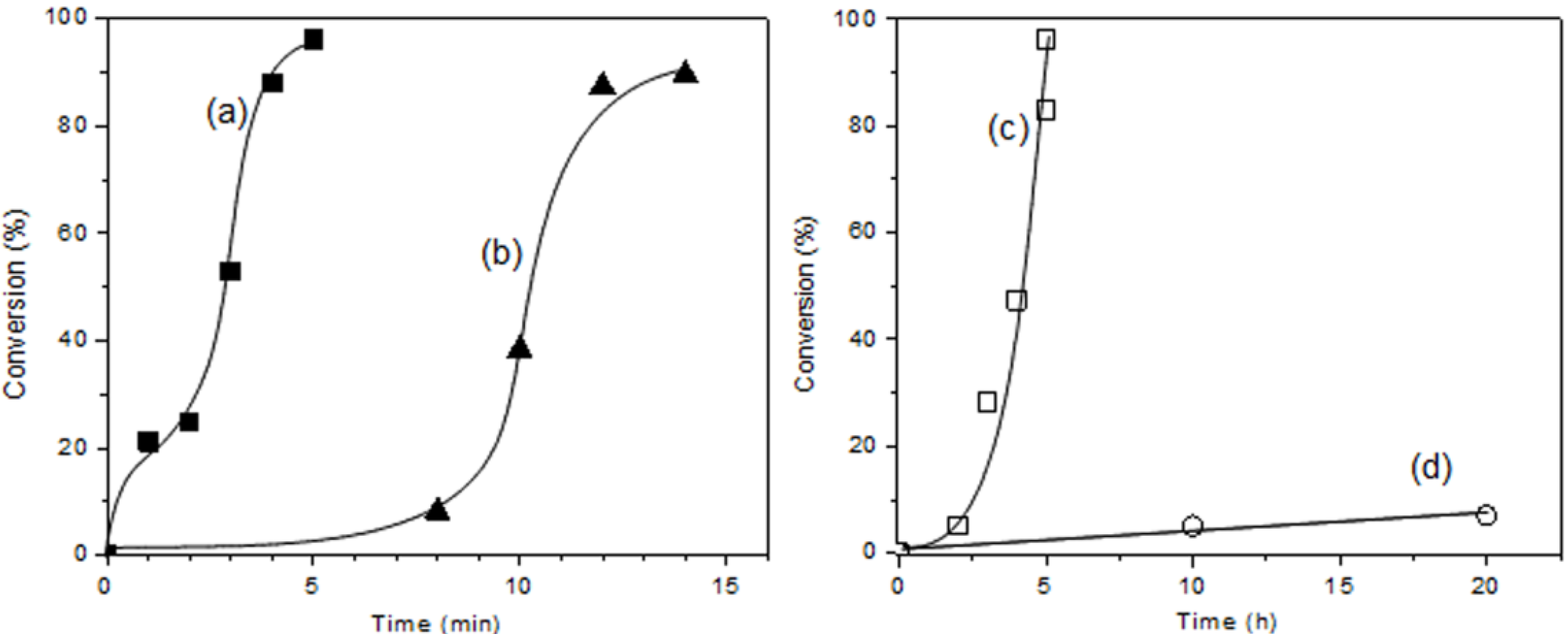
| Monomer | Time (min) | Conversion (%) | Mn | Mw/Mn |
|---|---|---|---|---|
| CHO | 2 | 25 | 4180 | 2.86 |
| 3 | 53 | 5920 | 2.61 | |
| 4 | 88 | 7460 | 2.49 | |
| 5 | 96 | 7000 | 2.76 | |
| STO | 8 | trace | - | - |
| 10 | 38 | - | - | |
| 12 | 87 | 900 | 1.28 | |
| 14 | 90 | 730 | 1.26 | |
| GME | 2 | trace | - | - |
| 3 | 28 | 840 | 1.38 | |
| 4 | 47 | 760 | 1.27 | |
| 5 | 83 | 810 | 1.40 |

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Endo, T.; Sanda, F. Design of latent catalysts and their application to polymer synthesis. Macrom. Symp. 1996, 107, 237–242. [Google Scholar] [CrossRef]
- Yağci, Y.; Reetz, I. Externally stimulated initiator systems for cationic polymerization. Prog. Polym. Sci. 1998, 23, 1485–1538. [Google Scholar] [CrossRef]
- Temin, S.C. Recent advances in cross-linking. J. Macromol. Sci. Part. C Polym. Rev. 2007, 22, 131–167. [Google Scholar] [CrossRef]
- Zhou, X.; Essawy, H.A.; Pizzi, A.; Li, X.; Pasch, H.; Pretorius, N.; Du, G. Poly(amidoamine)s dendrimers of different generations as components of melamine urea formaldehyde (MUF) adhesives used for particleboards production: What are the positive implications? J. Polym. Res. 2013, 20. [Google Scholar] [CrossRef]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Anne-Laure, B.; Christos, M.; Deniz, T.; Stephane, C. Polyether synthesis: From activated or metal-free anionic ring-opening polymerization of epoxides to functionalization. Prog. Polym. Sci. 2013, 38, 845–873. [Google Scholar]
- Nechifor, M. Synthesis and properties of novel aromatic polyamides with non-conjugated bichromophoric units in the main chains. J. Polym. Res. 2011, 18, 2477–2485. [Google Scholar] [CrossRef]
- Hou, G.; Gao, J.; Tian, C. Hybrid free radical-cationic thermal polymerization of methylacryloylpropyl-POSS/epoxy resins nanocomposites. J. Polym. Res. 2013, 20. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Lalevée, J. Cationic Photoinitiating Systems. In Photoinitiators for Polymer Synthesis: Scope, Reactivity and Efficiency; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; Chapter 12; pp. 289–341. [Google Scholar]
- Crivello, J.V.; Lee, J.L. Redox initiated cationic polymerization: Silane-N-aryl heteroaromatic onium salt redox couples. J. Polym. Sci. Part. A Polym. Chem. 2010, 48, 4484–4495. [Google Scholar] [CrossRef]
- Maeda, K.; Nakano, K.; Iwasa, S.; Hasegawa, E. Photo-acid generator having aromatic ketone structure for ArF chemically amplified resist. Microelectron. Eng. 2002, 61–62, 771–776. [Google Scholar] [CrossRef]
- Hino, T.; Endo, T. Cationic ring-opening polymerization of an epoxide by tropylium salts as thermal- and photolatent initiators. J. Polym. Sci. Part. A Polym. Chem. 2004, 42, 2166–2170. [Google Scholar] [CrossRef]
- Takahashi, E.; Sanda, F.; Endo, T. Novel pyridinium salts as cationic thermal and photoinitiators and their photosensitization properties. J. Polym. Sci. Part. A Polym. Chem. 2002, 40, 1037–1046. [Google Scholar] [CrossRef]
- Everett, J.P.; Schmidt, D.L.; Rose, G.D.; Argritis, P.; Aidinis, C.J.; Hatzakis, M. Synthesis of some onium salts and their comparison as cationic photoinitiators in an epoxy resist. Polymer 1997, 38, 1719–1723. [Google Scholar] [CrossRef]
- Denizligil, S.; Yagci, Y.; McArdle, C. Photochemically and thermally induced radical promoted cationic polymerization using an allylic sulfonium salt. Polymer 1995, 36, 3093–3098. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, K.W.; Endo, T.; Lee, S.B. Benzylpyrazinium salts as thermally latent initiators in the polymerization of glycidyl phenyl ether: Substituent effect on the initiator activity and mechanistic aspects. Macromolecules 2004, 37, 5830–5834. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.I.; Park, C.S.; Lee, S.B.; Kim, J.H.; Kim, M.S. Preparation of intercross-linked poly(l-lactide) and epoxy resin using N-benzyl pyrazine hexafluoroantimonate. J. Polym. Res. 2013, 20. [Google Scholar] [CrossRef]
- Golaz, B.; Michaud, V.; Leterrier, Y.; Månson, J.-A.E. UV intensity, temperature and dark-curing effects in cationic photo-polymerization of a cycloaliphatic epoxy resin. Polymer 2012, 53, 2038–2048. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, M.S.; Lee, S.B. Benzylpyrazinium Salts as Photo-Initiators in the Polymerization of Epoxide Monomers. Materials 2014, 7, 5581-5590. https://doi.org/10.3390/ma7085581
Kim MS, Lee SB. Benzylpyrazinium Salts as Photo-Initiators in the Polymerization of Epoxide Monomers. Materials. 2014; 7(8):5581-5590. https://doi.org/10.3390/ma7085581
Chicago/Turabian StyleKim, Moon Suk, and Sang Bong Lee. 2014. "Benzylpyrazinium Salts as Photo-Initiators in the Polymerization of Epoxide Monomers" Materials 7, no. 8: 5581-5590. https://doi.org/10.3390/ma7085581




