Preparation, Surface and Pore Structure of High Surface Area Activated Carbon Fibers from Bamboo by Steam Activation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological Characteristics of BACFs
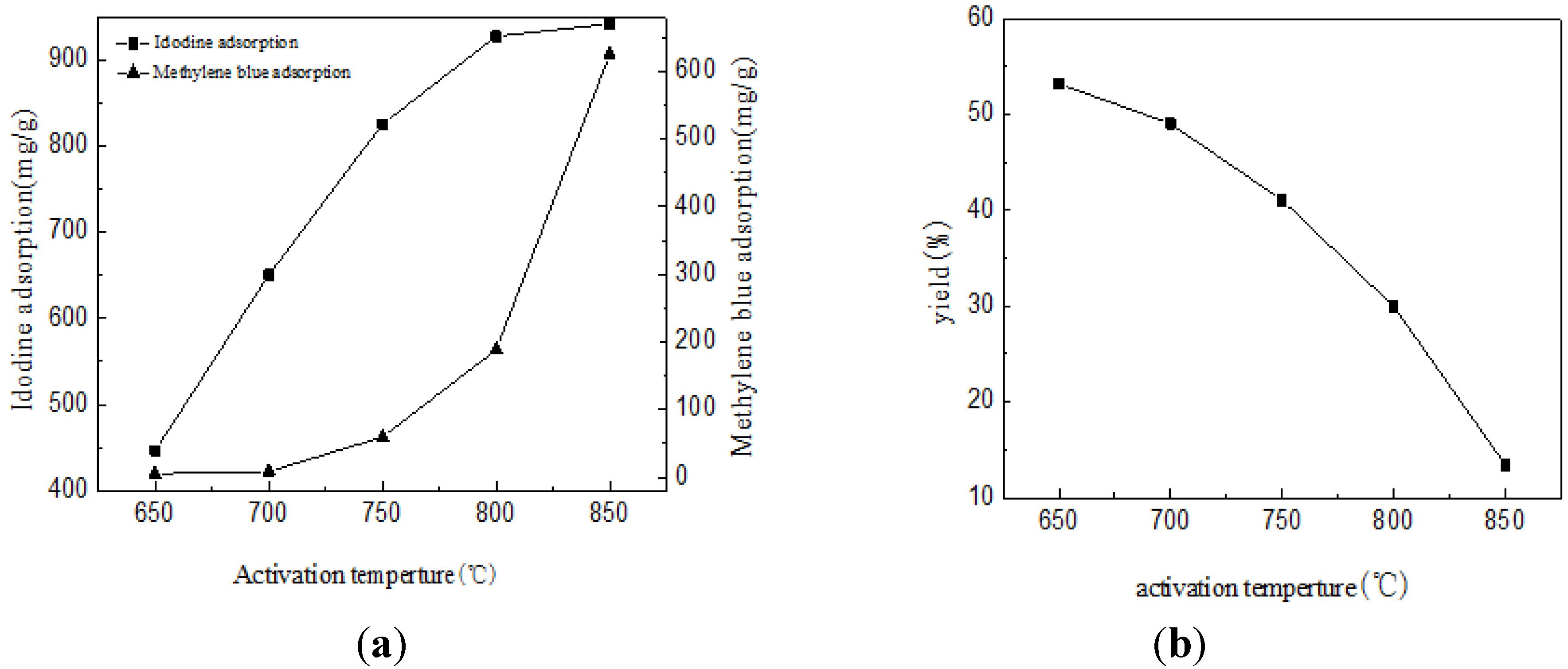
2.2. The Iodine, MB Adsorption and Yield Rate of BACFs

2.3. XRD Analysis of BACFs

| Temperature (°C) | d002/nm | Lc/nm | La/nm | Lc/d002 | g/% |
|---|---|---|---|---|---|
| 650 | 0.4072 | 0.88 | 1.83 | 2.17 | −7.25 |
| 700 | 0.4053 | 1.98 | 1.98 | 2.37 | −7.14 |
| 750 | 0.4053 | 2.06 | 2.06 | 2.46 | −7.14 |
| 800 | 0.3930 | 2.11 | 2.11 | 2.60 | −5.69 |
| 850 | 0.3879 | 2.39 | 2.39 | 2.98 | −5.10 |
2.4. XPS Analysis of BACFs
| Temperature (°C) | C (wt%) | O (wt%) | P (wt%) | C/O |
|---|---|---|---|---|
| 650 | 80.72 | 18.91 | 0.37 | 4.26 |
| 700 | 82.85 | 16.71 | 0.40 | 4.95 |
| 750 | 83.08 | 16.83 | 0.08 | 4.94 |
| 800 | 84.40 | 15.30 | 0.30 | 5.51 |
| 850 | 88.55 | 11.19 | 0.26 | 7.91 |

| Temperature (°C) | Graphite (CP1) | C–OH (CP2) | C=O (CP3) | C–OOH (CP4) | CO32−, CO, CO2 (CP5) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| BE (eV) | M (%) | BE (eV) | M (%) | BE (eV) | M (%) | BE (eV) | M (%) | BE (eV) | M (%) | |
| 650 | 284.6 | 68.5 | 286.0 | 14.7 | 287.2 | 7.0 | 288.7 | 6.2 | 290.1 | 3.7 |
| 700 | 284.6 | 70.9 | 286.0 | 9.6 | 286.8 | 6.8 | 288.5 | 8.7 | 290.2 | 4.5 |
| 750 | 284.6 | 69.4 | 285.8 | 10.8 | 286.7 | 6.5 | 288.3 | 7.2 | 289.9 | 6.1 |
| 800 | 284.6 | 66.0 | 286.0 | 15.5 | 287.5 | 7.5 | 288.9 | 3.2 | 289.7 | 7.8 |
| 850 | 284.6 | 65.3 | 286.0 | 12.4 | 287.2 | 10.5 | 289.0 | 6.4 | 290.8 | 5.4 |
2.5. N2 Adsorption of BACFs


| Temperature (°C) | SBET (m2/g) | Smic (m2/g) | Vtot (cm3/g) | Vmic (cm3/g) | Vmes (cm3/g) | Vmi/Vt (%) |
|---|---|---|---|---|---|---|
| 650 | 483 | 386 | 0.256 | 0.181 | 0.075 | 70.7 |
| 700 | 561 | 449 | 0.293 | 0.212 | 0.081 | 72.2 |
| 750 | 724 | 601 | 0.411 | 0.291 | 0.131 | 70.9 |
| 800 | 1125 | 856 | 0.566 | 0.387 | 0.165 | 68.4 |
| 850 | 2024 | 1275 | 0.999 | 0.569 | 0.416 | 57.0 |
3. Experimental
3.1. Samples
3.2. Measurements



4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, R.; Liu, G.Q.; Xu, X.H.; Li, M.; Zhang, J.C.; Hao, X.M. Surface texture, chemistry and adsorption properties of acid blue 9 of hemp (Cannabis sativa L.) bast-based activated carbon fibers prepared by phosphoric acid activation. Biomass Bioenergy 2011, 35, 437–445. [Google Scholar] [CrossRef]
- Suzuki, M. Activated carbon fiber: Fundamentals and applications. Carbon 1994, 32, 577–586. [Google Scholar] [CrossRef]
- Pastor, A.C.; Rodriguez-Reinoso, F.; Marsh, H.; Martinez, M.A. Preparation of activated carbon cloths from viscous rayon. Part I. Carbonization procedures. Carbon 1999, 37, 1275–1283. [Google Scholar] [CrossRef]
- Park, S.J.; Jang, Y.S.; Shim, J.W.; Ryu, S.K. Studies on pore structures and surface functional groups of pitch-based activated carbon fibers. J. Colloid. Interface. Sci. 2003, 260, 259–264. [Google Scholar] [CrossRef]
- Eduardo, M.C.C.; Antonio, M.G.M.; Angeles, D.D.; Angel, L.O. Textural and morphological study of activated carbon fibers prepared from kenaf. Microporous Mesoporous Mater. 2008, 111, 523–529. [Google Scholar] [CrossRef]
- Li, K.Q.; Li, Y.; Hu, H.L. Adsorption characteristics of lead on cotton-stalk-derived activated carbon fibre by steam activation. Desalin. Water Treat. 2011, 30, 1–9. [Google Scholar]
- Phan, N.H.; Rio, S.; Faur, C.; Coq, L.L.; Cloirec, P.L.; Nguyen, T.H. Production of fibrous activated carbons from natural (jute, coconut) fibers for water treatment applications. Carbon 2006, 44, 2569–2577. [Google Scholar] [CrossRef]
- Uraki, Y.; Nakatani, A.; Kubo, S.; Sano, Y. Preparation of activated carbon fibers with large specific surface area from softwood acetic acid lignin. J. Wood. Sci. 2001, 47, 465–469. [Google Scholar] [CrossRef]
- Asakura, R.; Morita, M.; Maruyama, K.; Hatori, H.; Yamada, Y. Preparation of fibrous activated carbons from wood fiber. J. Mater. Sci. 2004, 39, 201–206. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Li, J.; Sun, F.S.; Ng, D.H.L.; Kwong, F.L.; Liu, S.Q. Preparation and characterization of activated carbon fiber from paper. Chin. J. Chem. Phys. 2011, 24, 503–504. [Google Scholar]
- Asada, T.; Ohkubo, T.; Kawata, K.; Oikawa, K. Ammonia adsorption on bamboo charcoal with acid treatment. J. Health. Sci. 2006, 52, 585–589. [Google Scholar] [CrossRef]
- Wang, S.Y.; Tsai, M.H.; Lo, S.F.; Tsai, M.J. Effects of manufacturing conditions on the adsorption capacity of heavy metal ions by Makino bamboo charcoal. Bioresour. Technol. 2008, 99, 7027–7033. [Google Scholar] [CrossRef]
- Ip, A.W.M.; Barford, J.P.; McKay, G. Production and comparison of high surface area bamboo derived active carbons. Bioresour. Technol. 2008, 98, 8909–8916. [Google Scholar]
- Lianggui, W. Removal of Disperse Red dye by bamboo-based activated carbon: Optimisation, kinetics and equilibrium. Environ. Sci. Pollut. Res. 2013, 20, 4635–464. [Google Scholar] [CrossRef]
- Dagang, L.; Tuhua, Z.; Kaifu, L.; Qinglin, W. Starch composites reinforced by bamboo cellulosic crystals. Bioresour. Technol. 2010, 101, 2529–2536. [Google Scholar] [CrossRef]
- Masahiko, K.; Toshiyuki, A.; Mikio, K.; Bunichiro, T. Analysis on residue formation during wood liquefaction with polyhydric alcohol. J. Wood. Sci. 2004, 50, 407–414. [Google Scholar] [CrossRef]
- Joanne, Y.; Meijuan, C.; Szeto, Y.S.; Siucheong, Y. Comparative study of liquefaction process and liquefied products from bamboo using different organic solvents. Bioresour. Technol. 2009, 100, 6674–6678. [Google Scholar] [CrossRef]
- Liyi, Y.; Jingmiao, Z.; Jie, Z.; Song, T. Liquefaction of bamboo shoot shell for the production of polyols. Bioresour. Technol. 2014, 153, 147–153. [Google Scholar] [CrossRef]
- Xiaojun, M.; Guangjie, Z. Preparation of carbon fibers from liquefied wood. Wood Sci. Technol. 2010, 44, 3–11. [Google Scholar] [CrossRef]
- Xiaojun, M.; Guangjie, Z. Variations in the microstructure of carbon fibers prepared from Liquefied wood during carbonization. J. Appl. Polym. Sci. 2011, 121, 3525–3530. [Google Scholar] [CrossRef]
- Pelekani, C.; Snoeyink, V.L. Competitive adsorption between atrazine and methylene blue on activated carbon: The importance of pore size distribution. Carbon 2000, 38, 1423–1436. [Google Scholar] [CrossRef]
- Lei, S.; Miyamoto, J.; Kanoh, H.; Nakahigashi, Y.; Kaneko, K. Enhancement of the methylene blue adsorption rate for ultramicroporous carbon fiber by addition of mesopores. Carbon 2006, 44, 1884–1890. [Google Scholar] [CrossRef]
- Cuesta, A.; Dhamelincout, P.; Laureyns, J.; Martínez-Alonso, A.; Tascon, J.M.D. Comparative performance of X-ray diffraction and Raman microprobe techniques for the study of carbon materials. J. Mater. Chem. 1998, 8, 2875–2879. [Google Scholar]
- Kercher, A.K.; Nagle, D.C. Microstructural evolution during charcoal carbonization by X-ray diffraction analysis. Carbon 2003, 41, 15–27. [Google Scholar] [CrossRef]
- Chong, Y.B.; Ohara, H. Modification of carbon fiber surfaces by direct fluorination. J. Fluor. Chem. 1992, 57, 169–175. [Google Scholar]
- Paiva, M.C.R.; Bernardo, C.A.; Nardin, M. Mechanical, surface and interfacial characterisation of pitch and PAN-carbon fibres. Carbon 2000, 38, 1323–1327. [Google Scholar] [CrossRef]
- El-Merraoui, M.; Tamai, H.; Yasuda, H.; Kanata, T.; Mondori, J.; Nadai, K.; Kaneko, K. Pore structures of activated carbon fibers from organometallics/pitch composites by nitrogen adsorption. Carbon 1998, 36, 1769–1776. [Google Scholar] [CrossRef]
- Kang, E.T.; Neoh, K.G.; Tan, K.L.; Liaw, D.J. Surface Graft Polymerization and Grafting of Polymers for Adhesion Improvement. In Handbook of Adhesion Improvement Techniques in Advanced Materials; Marcel Dekker Inc.: New York, NY, USA, 1999; pp. 289–321. [Google Scholar]
- Kang, E.T.; Neoh, K.G.; Liaw, D.J. Surface Modification of Polymers via Molecular Design, Advanced Functional Molecules and Polymers; Gordon and Breach Publisher: Amsterdam, The Netherlands, 2001; pp. 1–33. [Google Scholar]
- Yuchun, C.; ChienCheng, L.; HungChih, L. Characterization of microstructure and surface properties of heat-treated PAN-and rayon-based activated carbon fibers. Porous. Mater. 2007, 14, 227–237. [Google Scholar] [CrossRef]
- Qingsong, L.; Tong, Z.; Nan, L.; Peng, W.; Gulizhaer, A. Modification of bamboo-based activated carbon using microwave radiation and its effects on the adsorption of methylene blue. Appl. Surf. Sci. 2010, 256, 3309–3315. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and PorousSolids, Principles, Methodology and Applicatiions; Academic Press: London, UK, 1999. [Google Scholar]
- Shujuan, Z.; HanQing, Y.; HuiMin, F. PVA-based activated carbon fibers with lotus root-like axially porous structure. Carbon 2006, 44, 2059–2068. [Google Scholar] [CrossRef]
- Warhurst, A.M.; Fowler, G.D.; McConnachie, G.L.; Pollard, S.J.T. Pore structure and adsorption characteristics of steam pyrolysis carbons from moringa oleifera. Carbon 1997, 35, 1039–1045. [Google Scholar]
- Mizuta, K.; Matsumoto, T.; Hatate, Y.; Nishihara, K.; Nakanishi, T. Removal of nitrate-nitrogen from drinking water using bamboo powder charcoal. Bioresour. Technol. 2004, 95, 255–257. [Google Scholar] [CrossRef]
- Toshihide, H.; Yoshiyuki, K.; Tomoki, S.; Jun’ichi, H.; Masahiro, K. Characteristics and humidity control capacity of activated carbon from bamboo. Bioresour. Technol. 2010, 101, 3964–3969. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ma, X.; Yang, H.; Yu, L.; Chen, Y.; Li, Y. Preparation, Surface and Pore Structure of High Surface Area Activated Carbon Fibers from Bamboo by Steam Activation. Materials 2014, 7, 4431-4441. https://doi.org/10.3390/ma7064431
Ma X, Yang H, Yu L, Chen Y, Li Y. Preparation, Surface and Pore Structure of High Surface Area Activated Carbon Fibers from Bamboo by Steam Activation. Materials. 2014; 7(6):4431-4441. https://doi.org/10.3390/ma7064431
Chicago/Turabian StyleMa, Xiaojun, Hongmei Yang, Lili Yu, Yin Chen, and Ying Li. 2014. "Preparation, Surface and Pore Structure of High Surface Area Activated Carbon Fibers from Bamboo by Steam Activation" Materials 7, no. 6: 4431-4441. https://doi.org/10.3390/ma7064431




