Combustion Synthesis and Photoluminescence Properties of Red-Emitting CaAlSiN3:Eu2+ Phosphor for White-LEDs
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimum Synthesis Condition and Combustion Phenomena
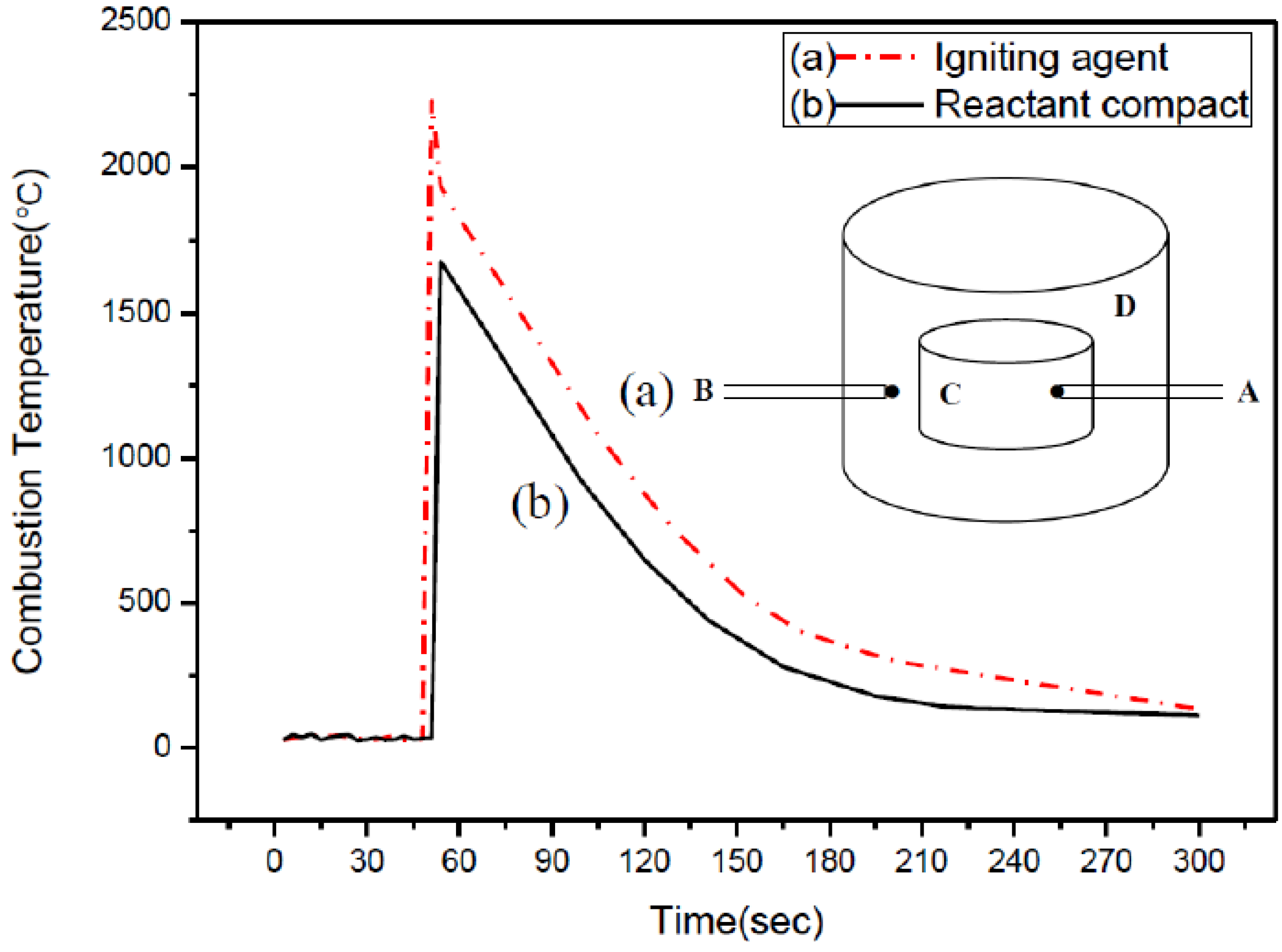

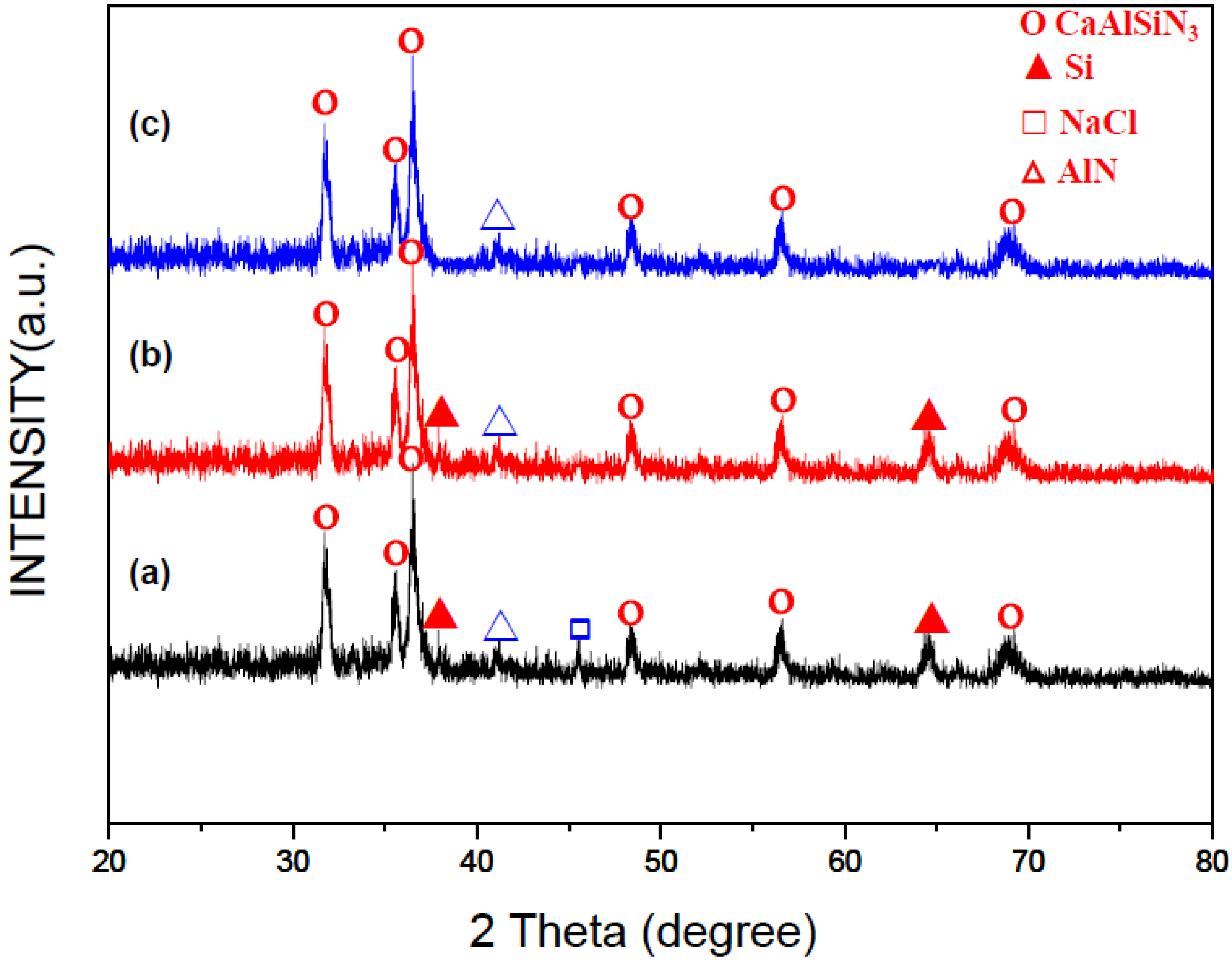
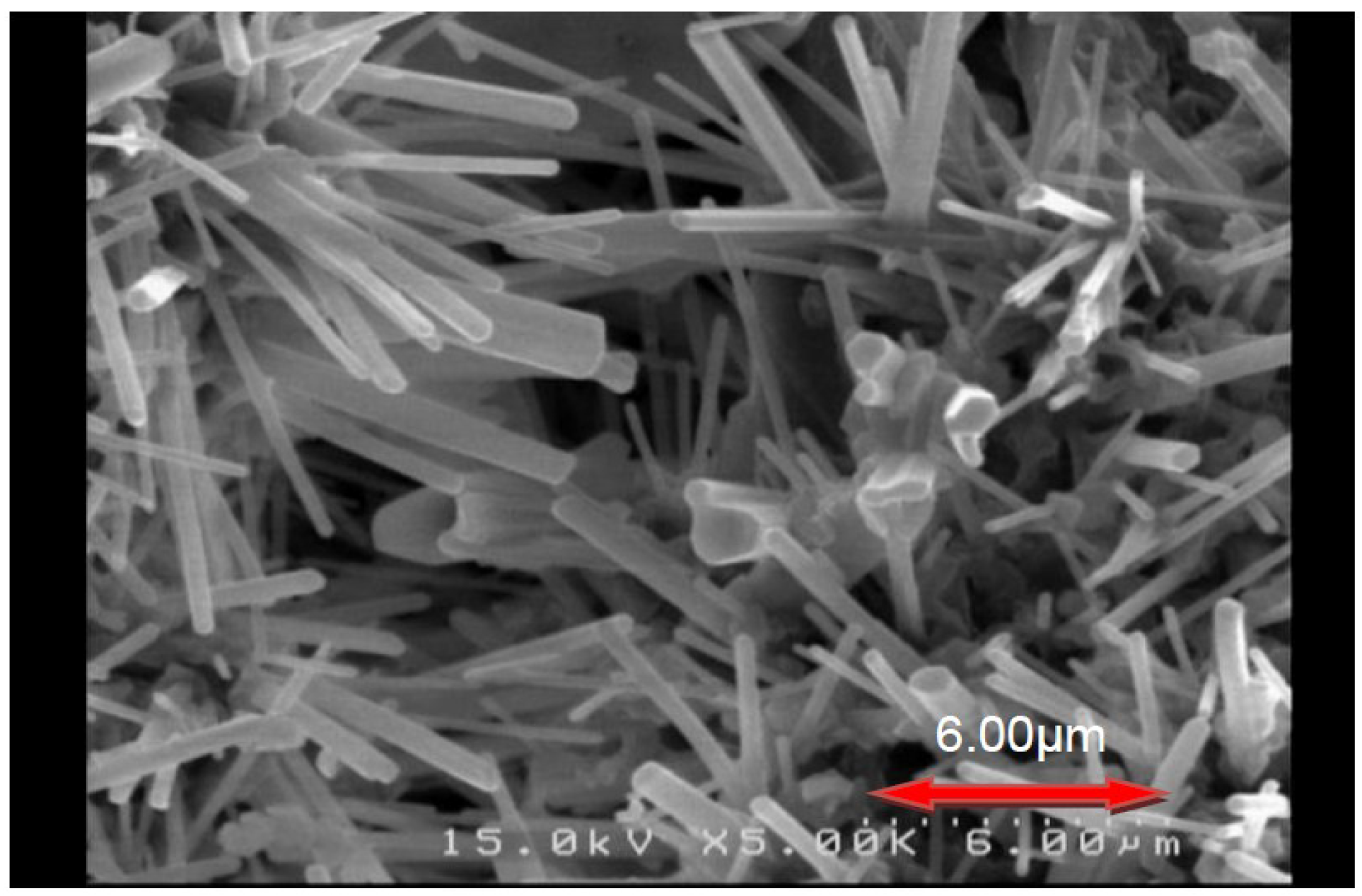
2.2. Effects of Process Parameters on Product Yield
2.2.1. Effects of NH4Cl and NaN3
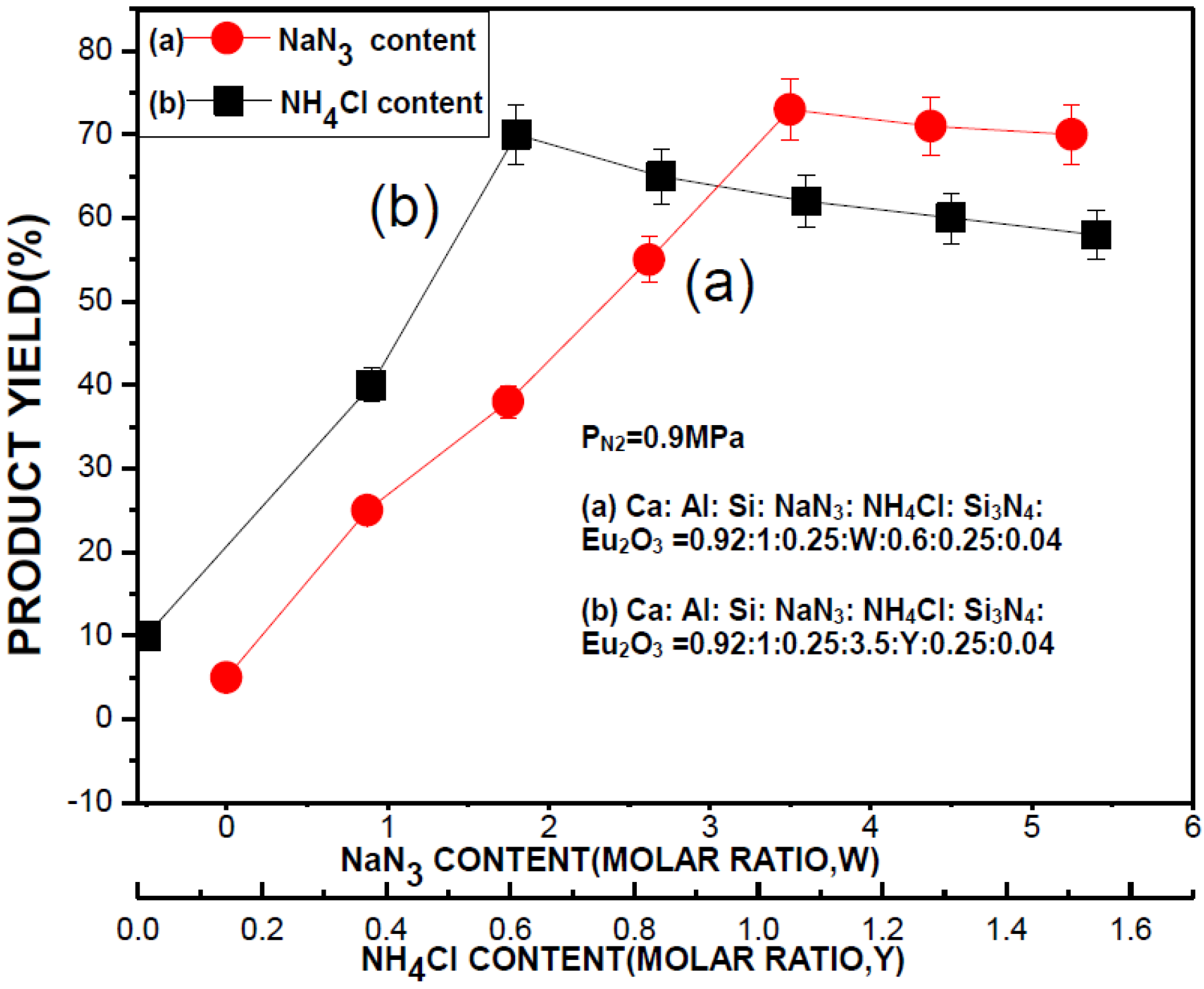
2.2.2. Effects of Si3N4
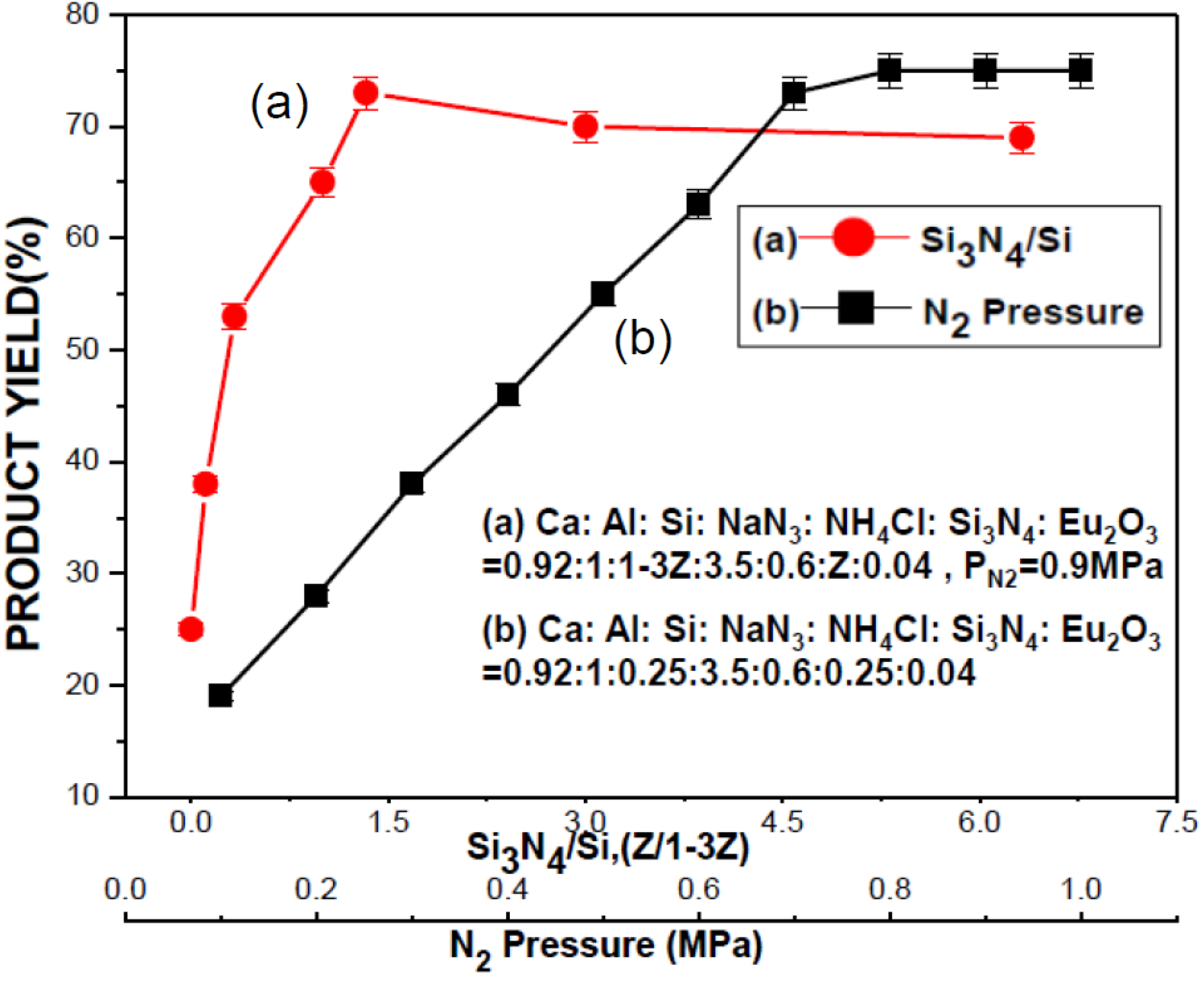
2.2.3. Effect of N2 Pressure
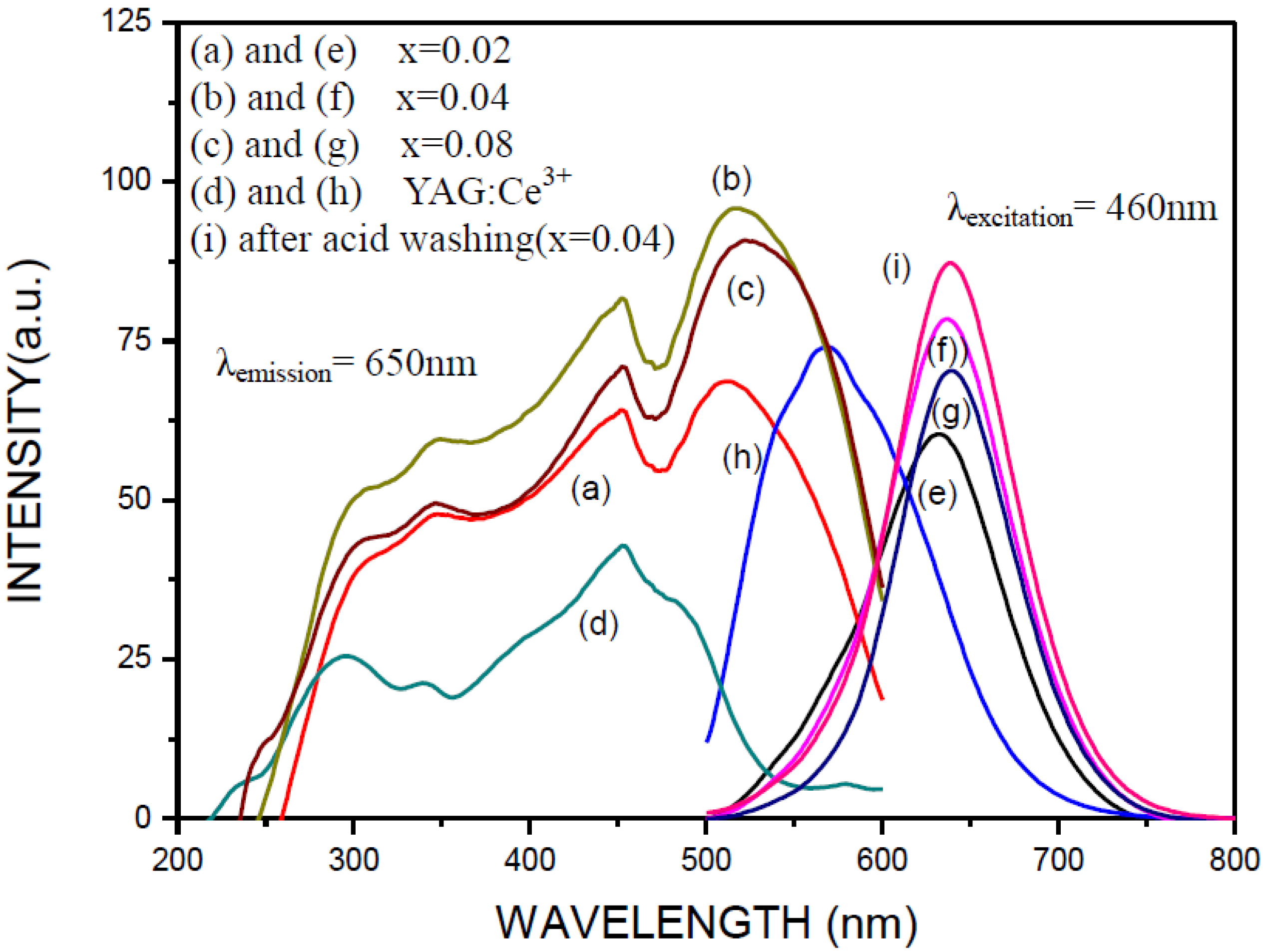
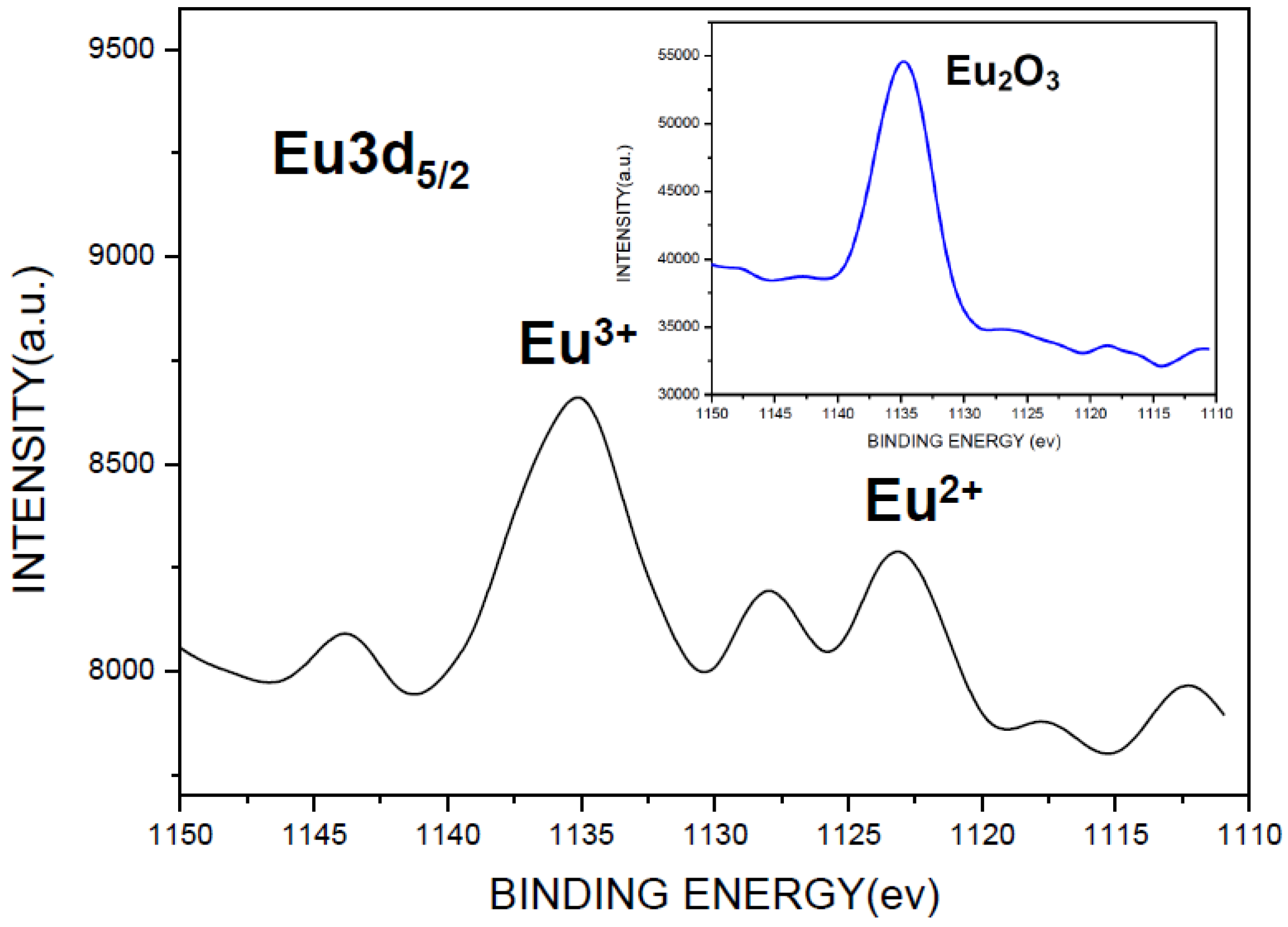
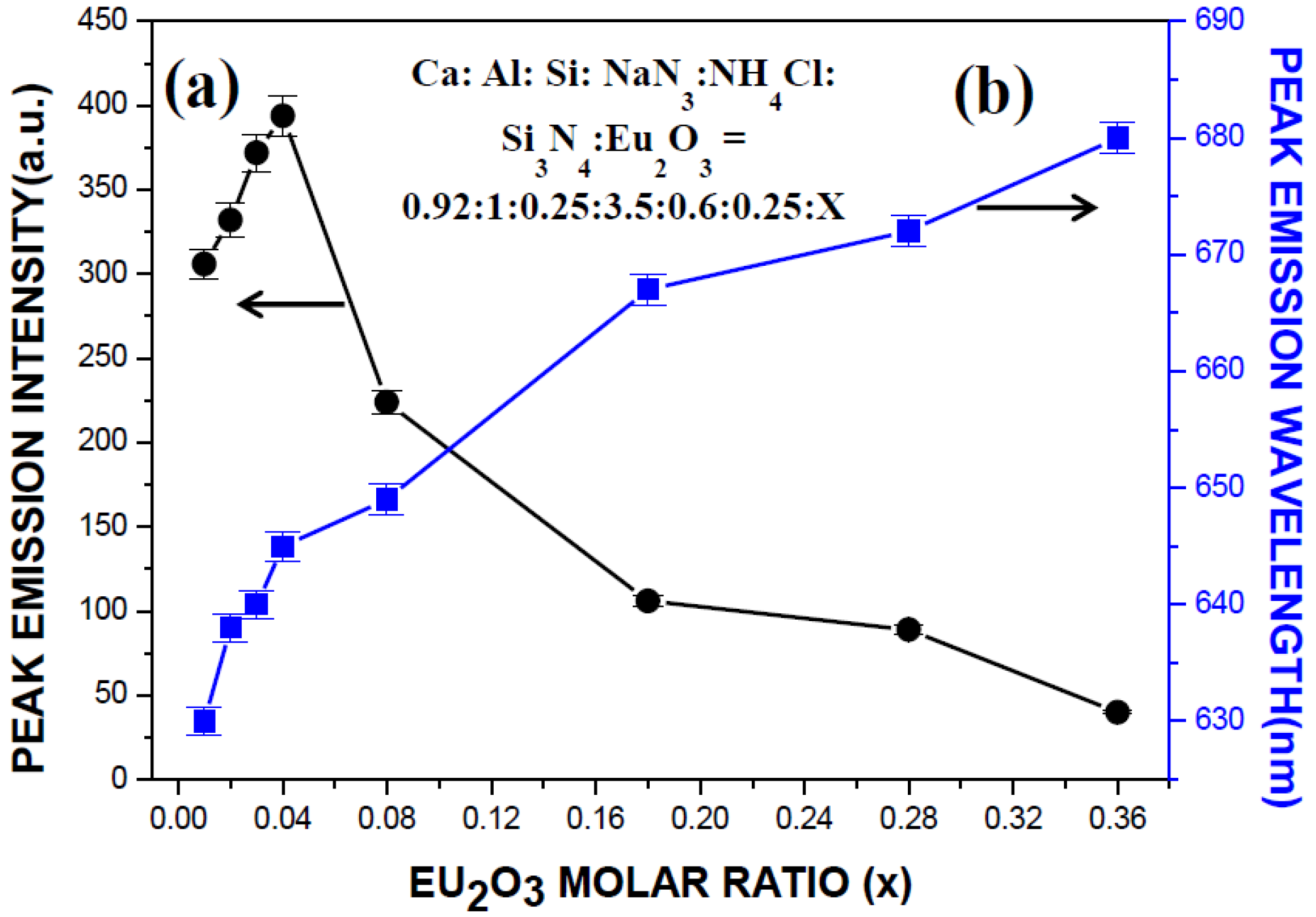
2.3. Photoluminescence Properties of CaAlSiN3:Eu2+ Phosphor
3. Experimental Section
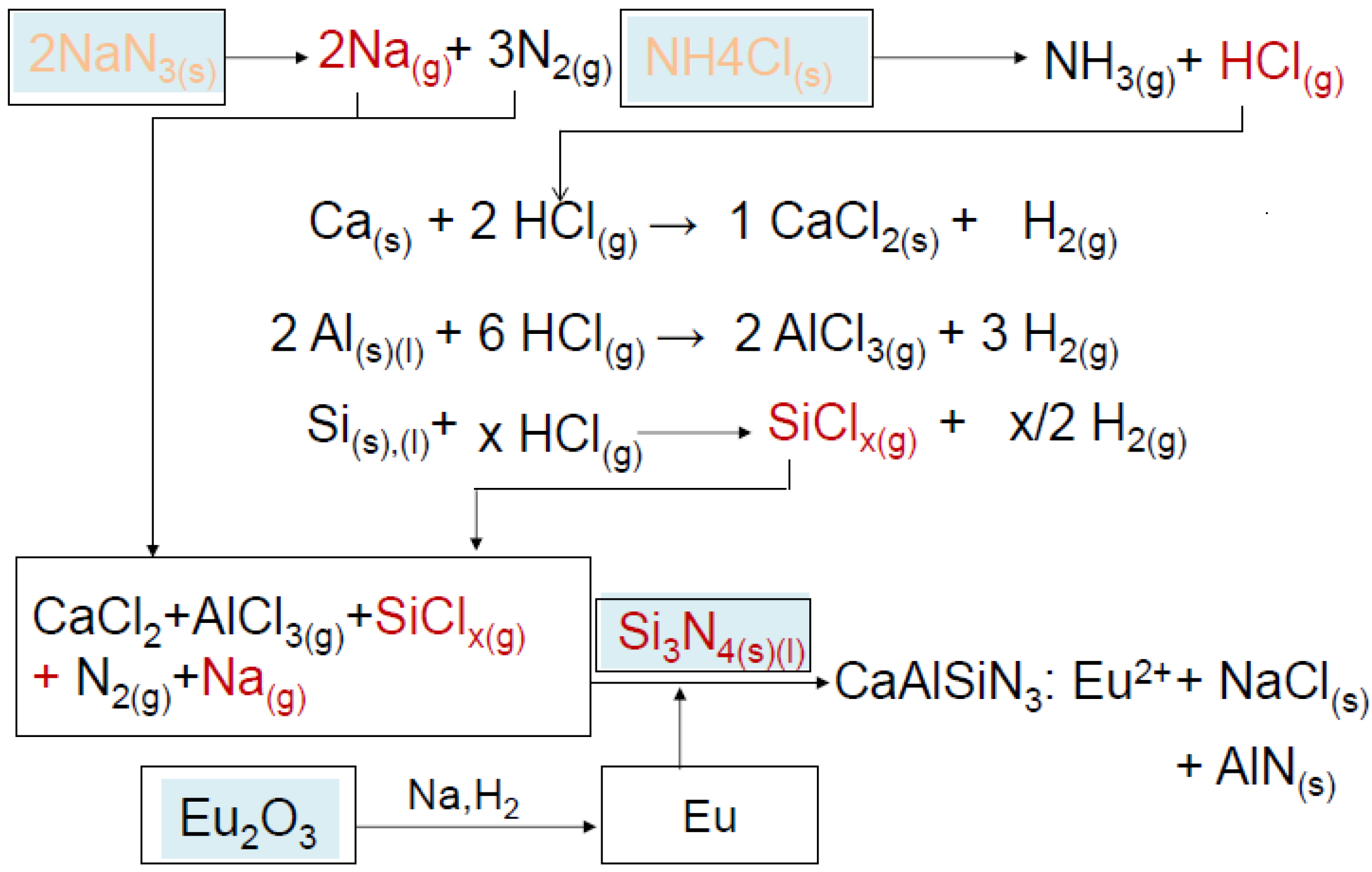
3.1. Development of the Process
3.2. Description of Experiment
| Reagent | Particle Size (um) | Purity (%) | Source |
|---|---|---|---|
| Si | 1–5 | 99 | Alfa Aesar |
| Ca | <963 | 99.5 | Alfa Aesar |
| Eu2O3 | 2.5–6 | 99.99 | Seedchem Company |
| NaN3 | d50 ≅ 150 | 99 | Johnson Matthey |
| NH4Cl | – | 99 | Panreac |
| Al | d50 ≅ 3 | 99 | First Chemical Work |
| α-Si3N4 | d50 ≅ 10 | 95 | Alfa Aesar |
| Mg | d50 ≅ 20 | 99 | Nihon Shiyaku |
| Fe3O4 | d50 ≅ 102 | 99 | Nihon Shiyaku |
| N2 | Gas | 99 | Yun Shan |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smet, P.F.; Parmentier, A.B.; Poelman, D. Selecting conversion phosphors for white light-emitting diodes. J. Electrochem. Soc. 2011, 158, R37–R54. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, S.; Mukai, T.; Senoh, M. Candela-class high-brightness InGaN/AlGaN double heterostructure blue-light-emitting diodes. Appl. Phys. Lett. 1994, 64, 1687–1689. [Google Scholar] [CrossRef]
- Rohwer, L.S.; Srivastava, A.M. Development of Phosphors for LEDs. Electrochem. Soc. Interface 2003, 36–39. [Google Scholar]
- Taso, J.Y. Light Emitting Diodes (LEDs) for General Illumination Update; Optoelectronics Industry Development Association: Washington, DC, USA, 2002. [Google Scholar]
- Chung, S.L.; Huang, S.C.; Chou, W.C.; Tangguh, W. Phosphors based on nitridosilicates: Synthesis methods and luminescent properties. Curr. Opin. Chem. Eng. 2014, 3, 62–67. [Google Scholar] [CrossRef]
- Li, Y.Q.; van Steen, J.E.J.; van Krevel, J.W.H.; Botty, G.; Delsing, A.C.A.; DiSalvo, F.J.; de With, G.; Hintzen, H.T. Luminescence properties of red-emitting M2Si5N8:Eu2+ (M = Ca, Sr, Ba) LED conversion phosphors. J. Alloy. Compd. 2006, 417, 273–279. [Google Scholar] [CrossRef]
- Piao, X.; Horikawa, T.; Hanzawa, H.; Machida, K.I. Photoluminescence properties of Ca2Si5N8:Eu2+ nitride phosphor prepared by carbothermal reduction and nitridation method. Chem. Lett. 2006, 35, 334–335. [Google Scholar] [CrossRef]
- Watanabe, H.; Yamane, H.; Kijima, N. Crystal structure and luminescence of Sr0.99Eu0.01AlSiN3. J. Solid State Chem. 2008, 181, 1848–1852. [Google Scholar] [CrossRef]
- Hoppe, H.A.; Lutz, H.; Morys, P.; Schnick, W.; Seilmeier, A. Luminescence in Eu2+-doped Ba2Si5N8 fluorescence, thermoluminescence, and upconversion. J. Phys. Chem. Solids 2000, 61, 2001–2006. [Google Scholar] [CrossRef]
- Li, H.L.; Xie, R.J.; Hirosaki, N.; Takeda, T.; Zhou, G.H. Synthesis and luminescence properties of orange-red-emitting M2Si5N8:Eu2+ (M = Ca, Sr, Ba) light-emitting diode conversion phosphors by a simple nitridation of MSi2. Int. J. Appl. Ceram. Technol. 2009, 6, 459–464. [Google Scholar] [CrossRef]
- Zeuner, M.; Hintze, F.; Schnick, W. Low temperature precursor route for highly efficient spherically shaped LED-phosphors M2Si5N8:Eu2+ (M = Eu, Sr, Ba). Chem. Mater. 2008, 21, 336–342. [Google Scholar] [CrossRef]
- Kim, Y.S.; Choi, S.W.; Park, J.H.; Bok, E.; Kim, B.K.; Hong, S.H. Redemitting (Sr,Ca)AlSiN3:Eu2+ phosphors synthesized by spark plasma sintering. ECS J. Solid State Sci. Technol. 2013, 2, R3021–R3025. [Google Scholar] [CrossRef]
- Yang, J.J.; Wang, T.; Chen, D.C.; Chen, G.D.; Liu, Q.L. An investigation of Eu2+-doped CaAlSiN3 fabricated by an alloy-nitridation method. Mater. Sci. Eng. B 2012, 177, 1596–1604. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, W.J.; Rainwater, B.; Liu, L.; Zhang, H.; Liu, Y.; Guo, X.; Zhou, J.; Xie, E. M2Si5N8:Eu2+-based (M = Ca, Sr) red-emitting phosphors fabricated by nitrate reduction process. Opt. Mater. 2011, 33, 1585–1590. [Google Scholar] [CrossRef]
- Merzhanov, A.G.; Borovinskaya, I.P. A new class of combustion processes. Combust. Sci. Technol. 1975, 10, 195–201. [Google Scholar] [CrossRef]
- Chung, S.L.; Chou, W.C. Combustion synthesis of Ca2Si5N8:Eu2+ phosphors and their luminescent properties. J. Am. Ceram. Soc. 2013, 96, 2086–2092. [Google Scholar] [CrossRef]
- Hu, Y.S.; Zhuang, W.D.; He, H.Q.; Liu, R.H.; Chen, G.T.; Liu, Y.H.; Huang, X.W. High temperature stability of Eu2+-activated nitride red phosphors. J. Rare Earth 2014, 32, 12–16. [Google Scholar] [CrossRef]
- Li, J.; Watanabe, T.; Wada, H.; Setoyama, T.; Yoshimura, M. Low-temperature crystallization of Eu-doped red-emitting CaAlSiN3 from alloy-derived ammonometallates. Chem. Mater. 2007, 19, 3592–3594. [Google Scholar] [CrossRef]
- Xie, R.J.; Hirosaki, N.; Sakuma, K.; Kimura, N. White light-emitting diodes (LEDs) using (oxy)nitride phosphors. J. Phys. D Appl. Phys. 2008, 41. [Google Scholar] [CrossRef]
- Kubus, M.; Meyer, H.J.; Anorg, Z. A Low-temperature synthesis route for CaAlSiN3 doped with Eu2+. Allg. Chem. 2013, 639, 669–672. [Google Scholar] [CrossRef]
- Chung, S.L.; Chang, C.W.; Cadete Santos Aires, F.J. Reaction mechanism in combustion synthesis of α-Si3N4 powder using NaN3. J. Mater. Res. 2008, 23, 2720–2726. [Google Scholar] [CrossRef]
- Lin, C.C.; Zheng, Y.S.; Chen, H.Y.; Ruan, C.H.; Xiao, G.W.; Liu, R.S. Improving optical properties of white LED fabricated. J. Electrochem. Soc. 2010, 157, H900–H903. [Google Scholar] [CrossRef]
- Hu, W.W.; Cai, C.; Zhu, Q.Q.; Xu, X.; Hao, L.Y.; Agathopoulos, S. Preparation of high performance CaAlSiN3:Eu2+ phosphors with the aid of BaF2 flux. J. Alloy. Comp. 2014, 613, 226–231. [Google Scholar] [CrossRef]
- Cai, C.; Qian, J.; Zhang, B.; Hu, W.; Hao, L.; Xu, X.; Wang, Y. Synthesis of red-emitting CaAlSiN3:Eu2+ phosphors through a cost-effective synthetic route. ECS J. Solid State Sci. Technol. 2014, 3, R169–R172. [Google Scholar] [CrossRef]
- Lei, B.; Machida, K.I.; Horikawa, T.; Hanzawa, H. Synthesis and photoluminescence properties of CaAlSiN3:Eu2+ nanocrystals. Chem. Lett. 2010, 39, 104–105. [Google Scholar] [CrossRef]
- Piao, X.; Machida, K.I.; Horikawa, T.H.; Hanzawa, Y.; Shimomura, Y.; Kijima, N. Preparation of CaAlSiN3:Eu2+ phosphors by the self-propagating high-temperature synthesis and their luminescent properties. Chem. Mater. 2007, 19, 4592–4599. [Google Scholar] [CrossRef]
- Mercier, F.; Alliot, C.; Bion, L.; Thromat, N.; Toulhoat, P. XPS study of Eu(III) coordination compounds: Core levels binding energies in solid mixed-oxo-compounds EumXxOy. J. Electron. Spectrosc. 2006, 150, 21–26. [Google Scholar] [CrossRef]
- Lacanilao, A.; Wallez, G.; Mazerolles, L.; Dubot, P.; Binet, L.; Pavageau, B.; Servant, L.; Buissette, V.; Mercier, T.L. Structural analysis of thermal degradation and regeneration in blue phosphor BaMgAl10O17:Eu2+ based upon cation diffusion. Solid. State Ionics 2013, 253, 32–38. [Google Scholar] [CrossRef]
- Kang, J.G.; Jung, Y.; Min, B.K.; Sohn, Y. Full characterization of Eu(OH)3 and Eu2O3 nanorods. Appl. Surf. Sci. 2014, 314, 158–165. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, V.; Som, S.; Duvenhage, M.M.; Ntwaeaborwa, O.M.; Swart, H.C. Effect of Eu doping on the photoluminescence properties of ZnO nanophosphors for red emission applications. Appl. Surf. Sci. 2014, 308, 419–430. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, S.; Li, Q.; Zhang, X.; Li, T.; Li, P.; Yang, Z.; Guo, Q. Luminescent properties of Ba2SiO4:Eu3+ for white light emitting diodes. Phys. B. Condens. Matter 2013, 411, 110–113. [Google Scholar] [CrossRef]
- Dexter, D.L. A theory of sensitized luminescence in solids. J. Chem. Phys. 1953, 21, 836–850. [Google Scholar]
- Blasse, G. Energy transfer between inequivalent Eu2+ ions. J. Solid State Chem. 1986, 62, 207–211. [Google Scholar] [CrossRef]
- Li, Y.Q.; Delsing, A.C.; de With, A.G.; Hintzen, H.T. Luminescence properties of Eu2+-activated alkaline-earth silicon-oxynitride MSi2O2-δN2+2/3δ (M = Ca, Sr, Ba): A promising class of novel LED conversion phosphors. Chem. Mater. 2005, 17, 3242–3248. [Google Scholar] [CrossRef]
- Avci, N.; Korthout, K.; Newton, M.A.; Smet, P.F.; Poelman, D. Valence states of europium in CaAl2O4:Eu phosphors. Opt. Mater. Exp. 2012, 2, 321–330. [Google Scholar] [CrossRef]
- Jang, B.Y.; Park, J.S. Luminescence properties of Eu2O3-doped Ca2Si5N8 phosphors. J. Ceram. Process. Res. 2009, 10, 844–847. [Google Scholar]
- Xie, R.J.; Hirosaki, N.; Suehiro, T.; Xu, F.F.; Mamoru, M. A simple, efficient synthetic route to Sr2Si5N8:Eu2+-based red phosphors for white light-emitting diodes. Chem. Mater. 2006, 18, 5578–5583. [Google Scholar] [CrossRef]
- Lee, W.C.; Tu, C.L.; Weng, C.U.; Chung, S.L. A novel process for combustion synthesis of AlN powder. J. Mater. Res. 1995, 10, 774–778. [Google Scholar] [CrossRef]
- Chung, S.L.; Yu, W.L.; Lin, C.N. A self-propagating high temperature synthesis method for synthesis of AlN powder. J. Mater. Res. 1999, 14, 1928–1933. [Google Scholar] [CrossRef]
- Lin, C.N.; Chung, S.L. Combustion synthesis of aluminum nitride powder using additives. J. Mater. Res. 2001, 16, 2200–2208. [Google Scholar] [CrossRef]
- Lin, C.N.; Chung, S.L. Combustion synthesis method for synthesis of aluminum nitride powder using aluminum containers. J. Mater. Res. 2001, 16, 3518–3525. [Google Scholar] [CrossRef]
- Lin, C.N.; Chung, S.L. Combustion synthesis method for synthesis of aluminum nitride powder using aluminum containers (II). J. Mater. Res. 2004, 19, 3037–3045. [Google Scholar]
- Moreno, L.A. Absolute quantum yield measurement of powder samples. J. Vis. Exp. 2012, 63. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, S.-L.; Huang, S.-C. Combustion Synthesis and Photoluminescence Properties of Red-Emitting CaAlSiN3:Eu2+ Phosphor for White-LEDs. Materials 2014, 7, 7828-7842. https://doi.org/10.3390/ma7127828
Chung S-L, Huang S-C. Combustion Synthesis and Photoluminescence Properties of Red-Emitting CaAlSiN3:Eu2+ Phosphor for White-LEDs. Materials. 2014; 7(12):7828-7842. https://doi.org/10.3390/ma7127828
Chicago/Turabian StyleChung, Shyan-Lung, and Shu-Chi Huang. 2014. "Combustion Synthesis and Photoluminescence Properties of Red-Emitting CaAlSiN3:Eu2+ Phosphor for White-LEDs" Materials 7, no. 12: 7828-7842. https://doi.org/10.3390/ma7127828





