Preparation and Morphology Studies of Nano Zinc Oxide Obtained Using Native and Modified Chitosans
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
2.2. Chemical Modification of Chitosan
2.3. Adsorption Studies
2.4. Synthesis of ZnO Using Native and Surface Modified Chitosan
| Precursors | ZnO samples prepared at various calcination temperatures of Zn-chitosan polymers | ||
| 450 °C | 650 °C | 850 °C | |
| CTS | ZnO-CTS-450 | ZnO-CTS-650 | ZnO-CTS-850 |
| CMC1 | ZnO-CMC1-450 | ZnO-CMC1-650 | ZnO-CMC1-850 |
| CMC2 | ZnO-CMC2-450 | ZnO-CMC2-650 | ZnO-CMC2-850 |
| CMC3 | ZnO-CMC3-450 | ZnO-CMC3-650 | ZnO-CMC3-850 |
| CMC4 | ZnO-CMC4-450 | ZnO-CMC4-650 | ZnO-CMC4-850 |
| CMC5 | ZnO-CMC5-450 | ZnO-CMC5-650 | ZnO-CMC5-850 |
| CMC6 | ZnO-CMC6-450 | ZnO-CMC6-650 | ZnO-CMC6-850 |
2.5. Characterization Techniques
3. Results and Discussion
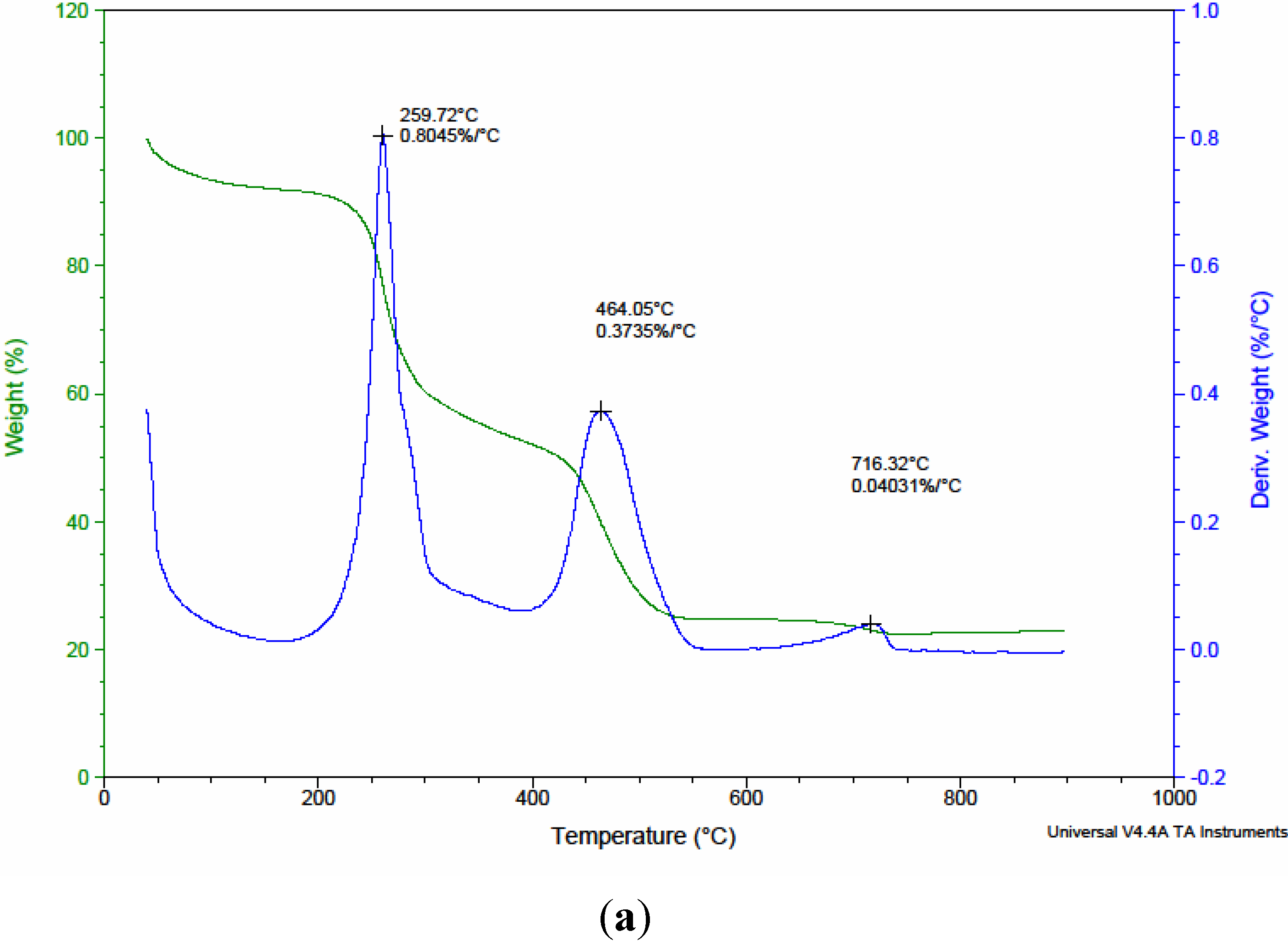
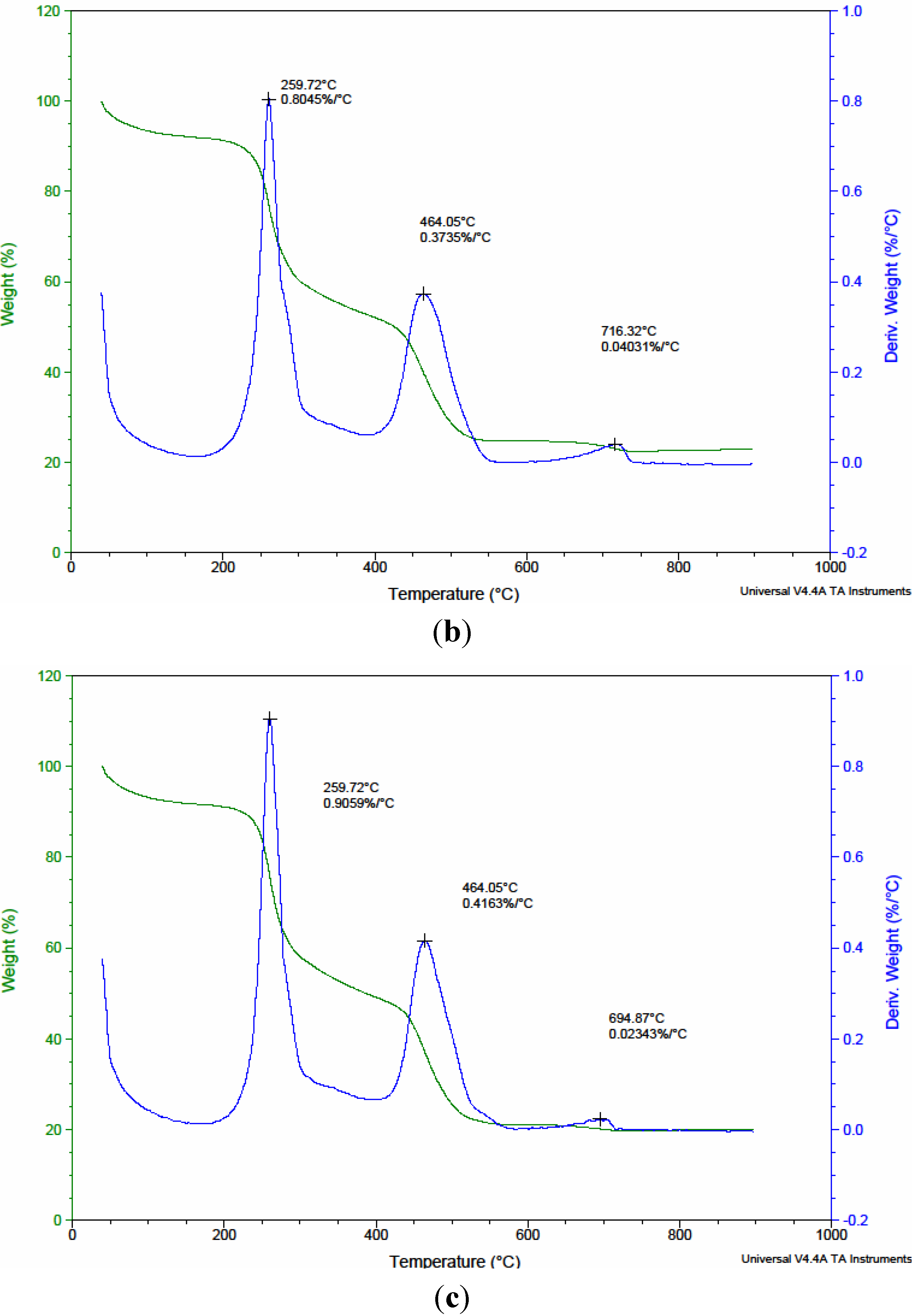
3.1. Characterization of Native and Surface Modified Chitosans

| Precursors | Residual weight (%) of precursors after pyrolysis | ||
|---|---|---|---|
| 450 °C | 650 °C | 850 °C | |
| CTS | 34.05 | 11.45 | 11.40 |
| CMC1 | 53.11 | 36.80 | 32.02 |
| CMC2 | 48.94 | 29.61 | 25.10 |
| CMC3 | 52.92 | 34.05 | 30.89 |
| CMC4 | 31.66 | 23.92 | 23.00 |
| CMC5 | 42.52 | 20.87 | 19.99 |
| CMC6 | 41.56 | 15.49 | 15.42 |
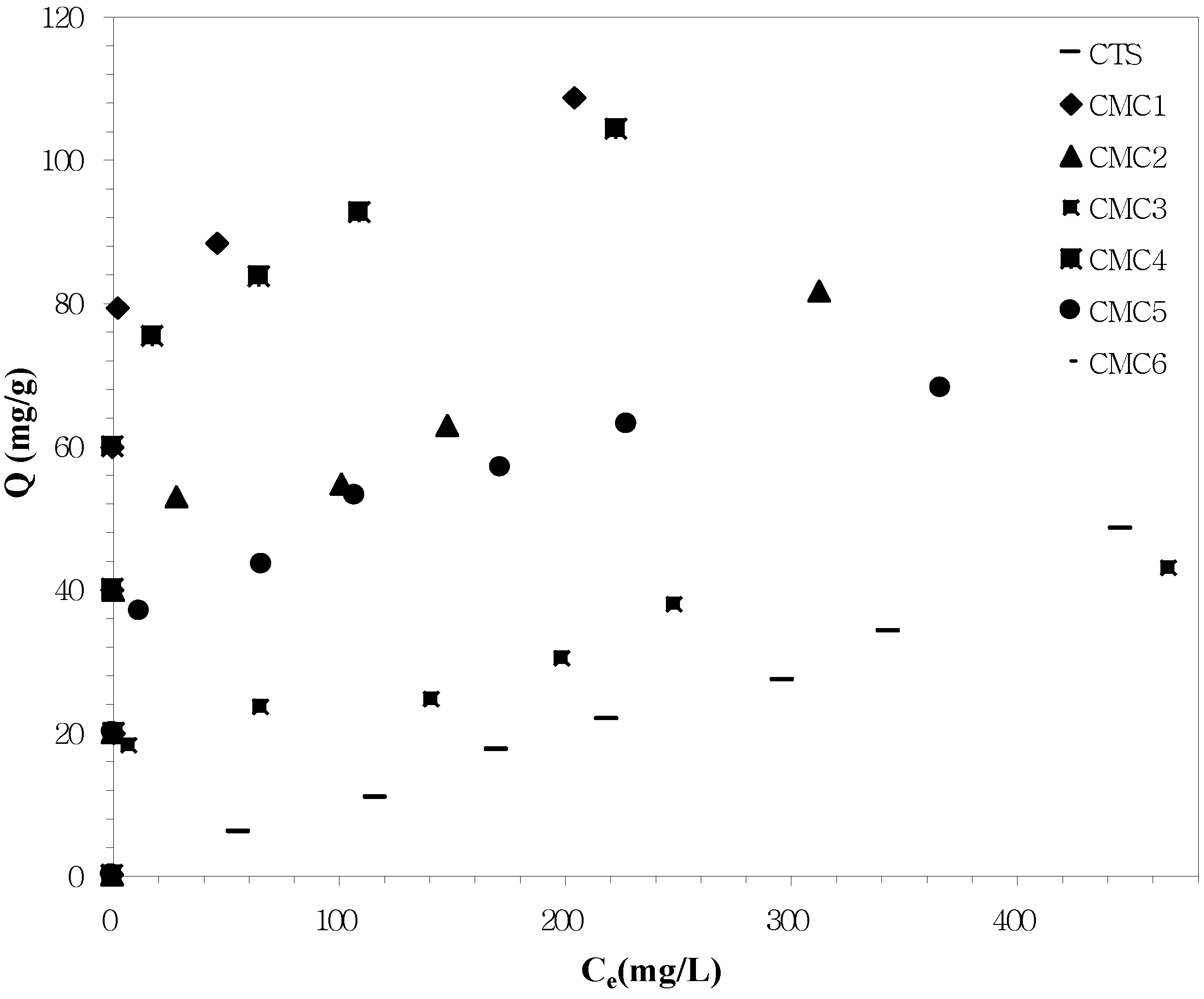
3.2. Zn2+ Adsorption Studies
| Precursors | R2 | K (capacity of the adsorbent for the adsorbate, mg/g) | n (constant) |
|---|---|---|---|
| CTS | 0.9918 | 9.85 | 1.0040 |
| CMC1 | 0.9547 | 69.78 | 12.9032 |
| CMC2 | 0.9333 | 32.36 | 7.0373 |
| CMC3 | 0.9438 | 5.29 | 2.9551 |
| CMC4 | 0.9556 | 51.17 | 7.8612 |
| CMC5 | 0.9828 | 24.00 | 5.9277 |
| CMC6 | 0.9522 | 20.20 | 6.7843 |
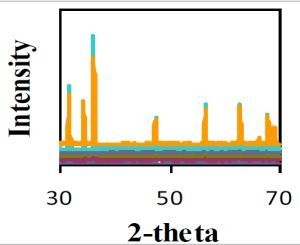
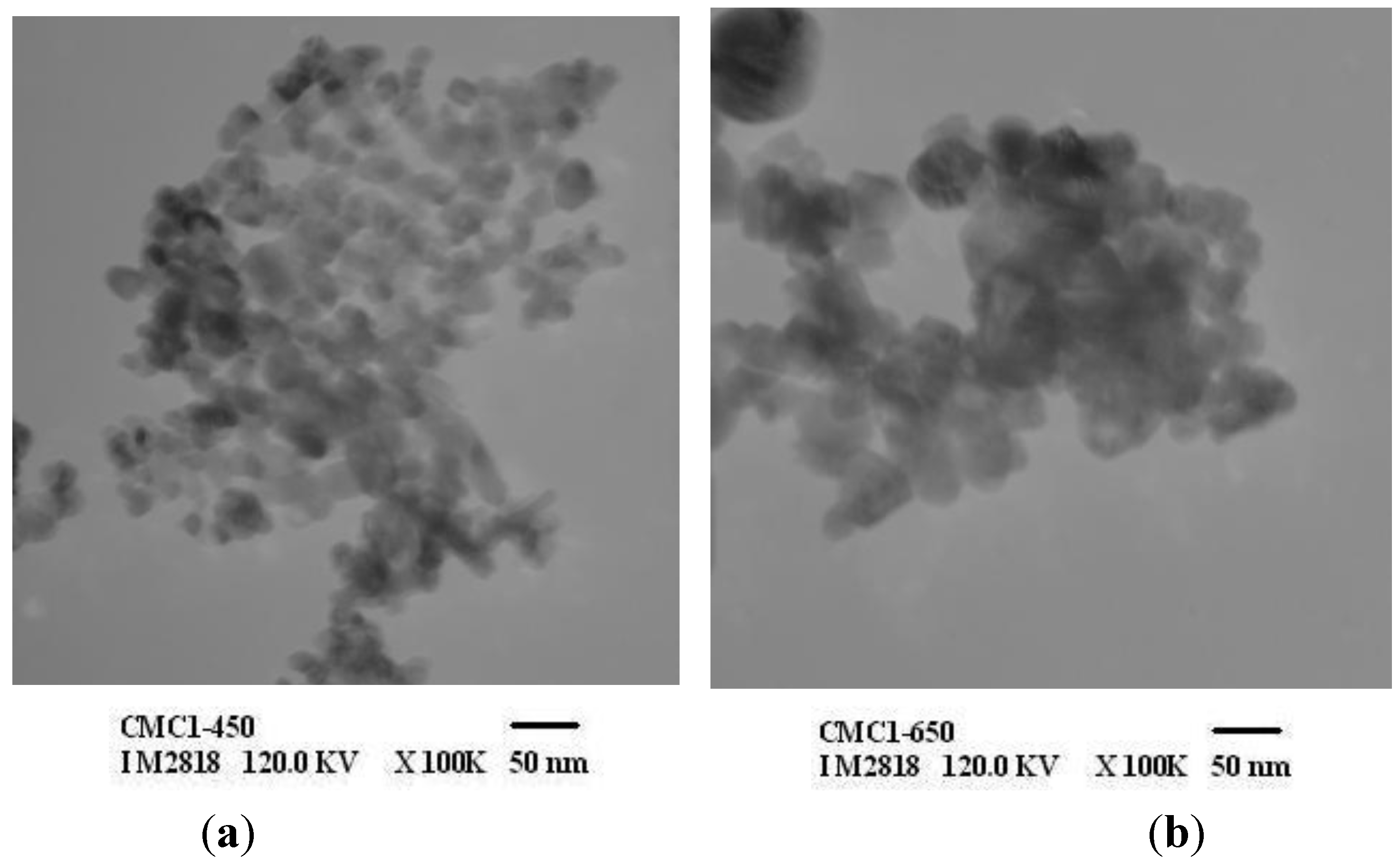
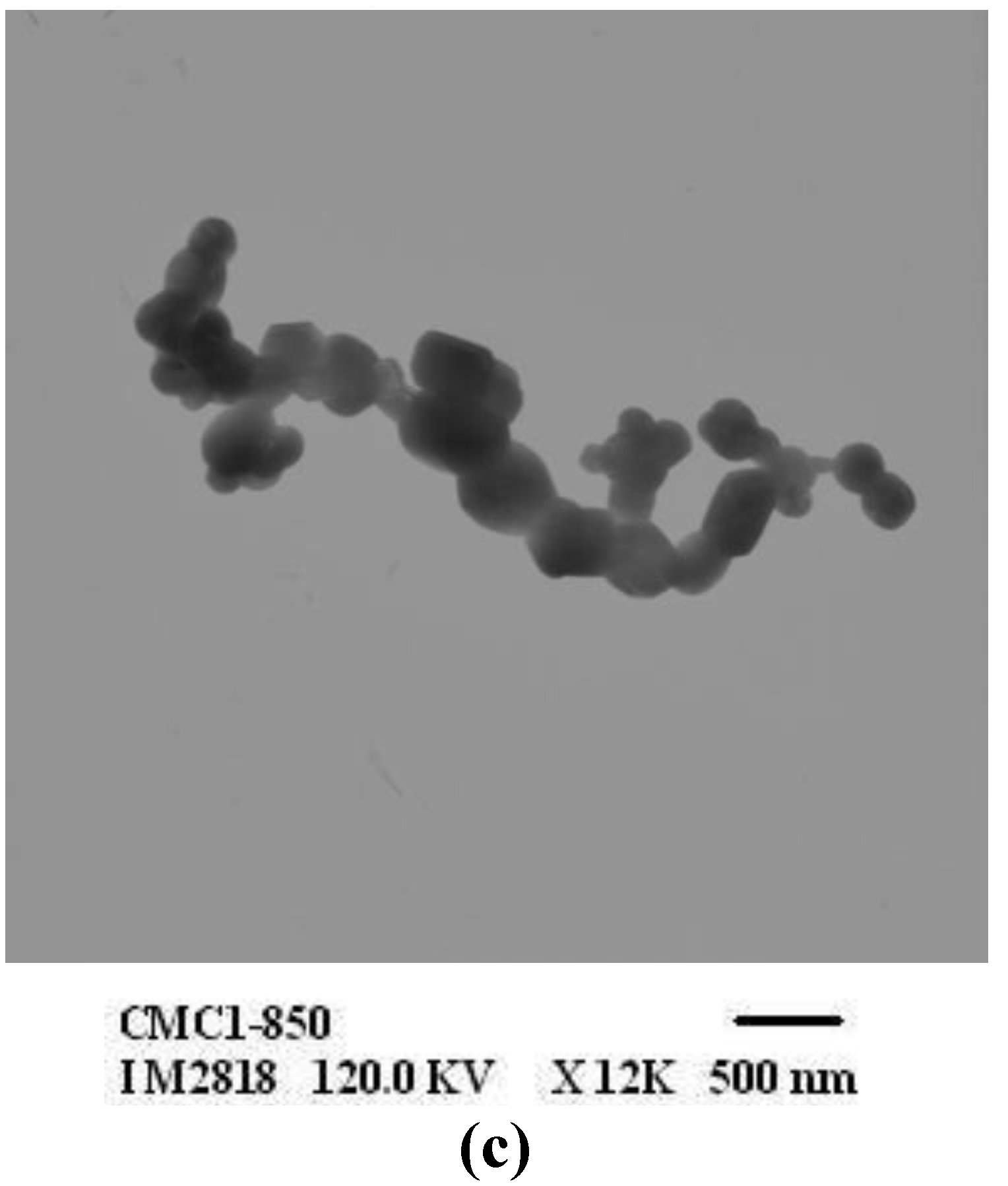
3.3. Characterization of ZnO
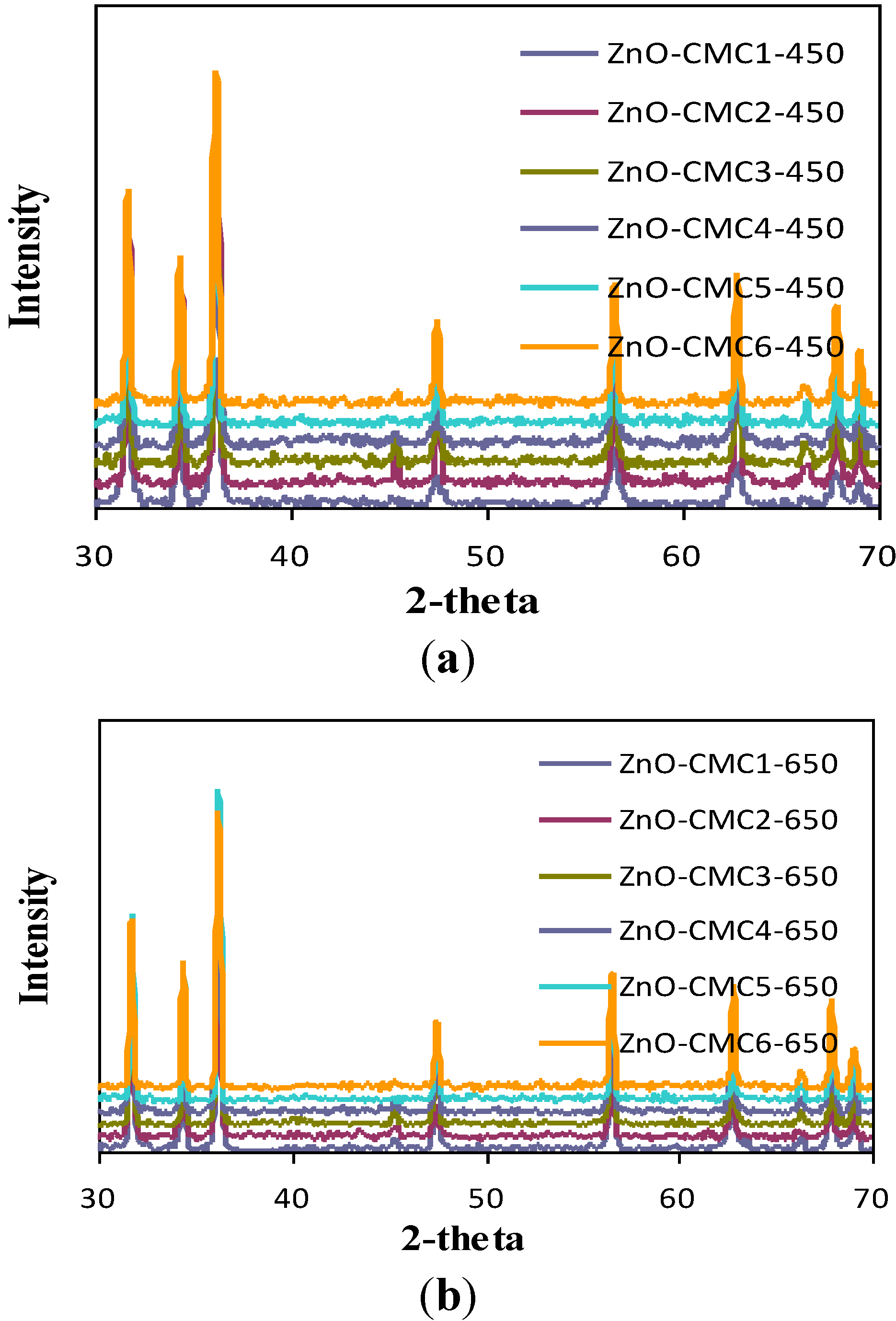
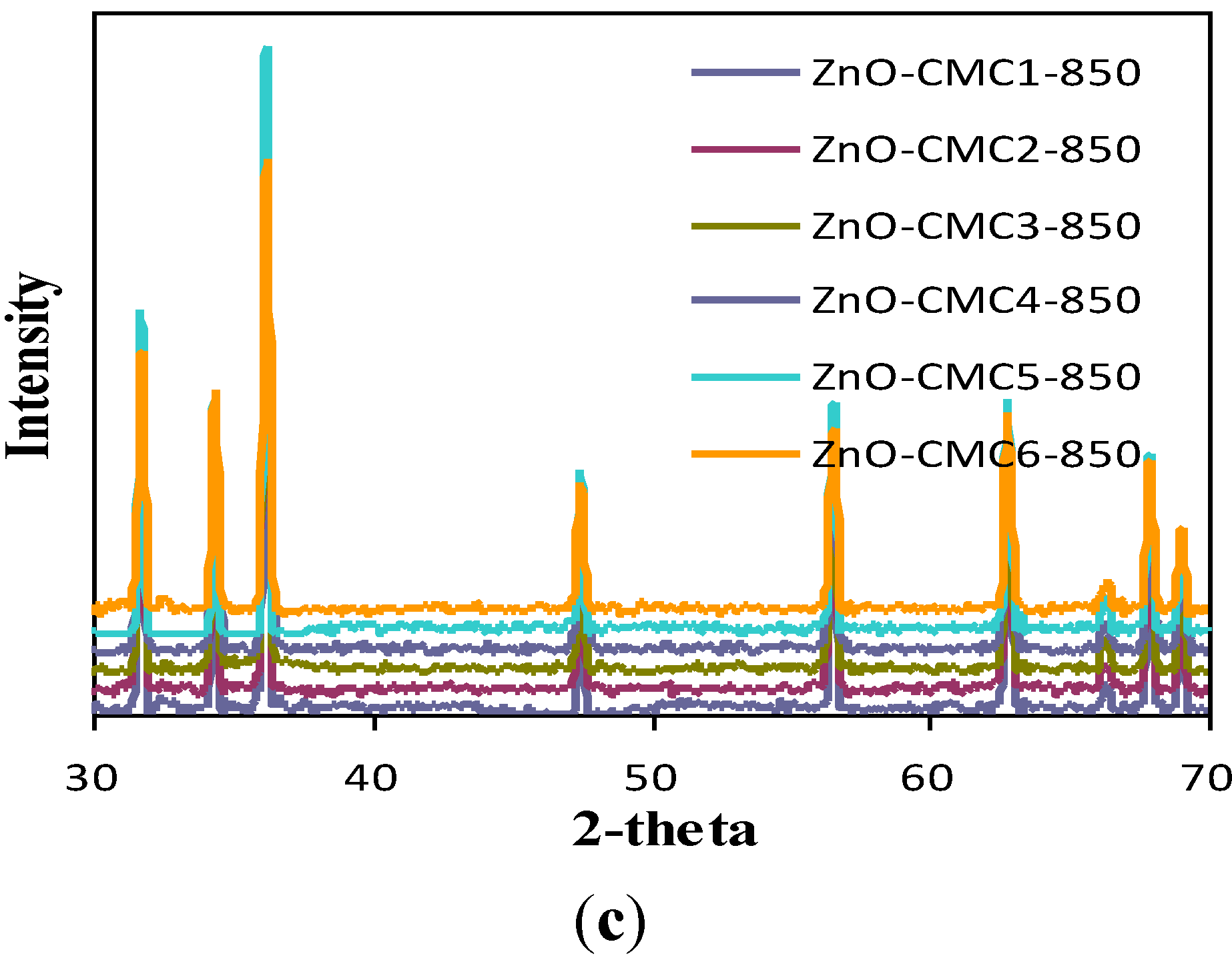

| ZnO samples | Particle size of ZnO (nm) | ||
|---|---|---|---|
| 450 °C | 650 °C | 850 °C | |
| ZnO-CTS | 31–42 | 74–193 | 121–292 |
| ZnO-CMC1 | 19–54 | 91–220 | 306–746 |
| ZnO-CMC2 | 59–107 | 72–198 | 300–835 |
| ZnO-CMC3 | 43–124 | 135–519 | 580–584 |
| ZnO-CMC4 | 26–69 | 65–683 | 207–636 |
| ZnO-CMC5 | 32–70 | 67–380 | 226–1069 |
| ZnO-CMC6 | 33.89 | 96–211 | 237–345 |
| Samples | BET surface area (m2/g) | Average pore diameter (nm) |
|---|---|---|
| ZnO-CTS-450 | 23.7664 | 9.70 |
| ZnO-CMC1-450 | 15.4485 | 221.40 |
| ZnO-CMC2-450 | 4.7763 | 106.29 |
| ZnO-CMC3-450 | 4.5767 | 161.62 |
| ZnO-CMC4-450 | 14.1519 | 212.03 |
| ZnO-CMC5-450 | 6.0641 | 119.69 |
| ZnO-CMC6-450 | 6.0966 | 131.10 |
| ZnO-CTS-650 | 11.9273 | 6.97 |
| ZnO-CMC1-650 | 5.8813 | 160.49 |
| ZnO-CMC2-650 | 2.9608 | 108.93 |
| ZnO-CMC5-650 | 2.3485 | 8.96 |
| ZnO-CMC6-650 | 3.1625 | 10.59 |
4. Conclusions
Acknowledgment
Conflicts of Interest
References
- Nakayama, V.; Pauzauskie, P.J.; Radenovic, A.; Onorato, R.M.; Saykally, R.J.; Liphardt, J.; Yang, P.D. Tunable nanowire nonlinear optical probe. Nature 2007, 447, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Tomchenko, A.A.; Harmer, G.P.; Marquis, B.T.; Allen, J.W. Semiconducting metal oxide sensor array for the selective detection of combustion gases. Sens. Actuators B 2003, 93, 126–134. [Google Scholar] [CrossRef]
- Chen, S.H.; Nickel, U.; Ren, X.M. Fluorescence of ZnO ultrafine particles quenched by naphthothiacarbocyanine dye in ethanol: the effect of water. J. Colloid Interface Sci. 1995, 176, 286–292. [Google Scholar] [CrossRef]
- Marcì, G.; Augugliaro, V.; López-Muñoz, M.J.; Martín, C.; Palmisano, L.; Rives, V.; Schiavello, M.; Tilley, R.J.D.; Venezia, A.M. Preparation characterization and photocatalytic activity of polycrystalline ZnO/TiO2 systems. 2. Surface, bulk characterization, and 4-nitrophenol photodegradation in liquid-solid regime. J. Phys. Chem. B 2001, 105, 1033–1040. [Google Scholar] [CrossRef]
- Ghosh, A.; Das, S.K.; Biswas, J.R.; Tripathi, H.S.; Banerjee, G. The effect of ZnO addition on the densification and properties of magnesium aluminate spinel. Ceram. Int. 2000, 26, 605–608. [Google Scholar] [CrossRef]
- Wu, Y.L.; Tok, A.I.Y.; Boey, F.Y.C.; Zeng, X.T.; Zhang, X.H. Surface modification of ZnO nanocrystals. Appl. Surf. Sci. 2007, 253, 5473–5479. [Google Scholar] [CrossRef]
- Bae, S.Y.; Seo, H.W.; Park, J. Vertically aligned sulfur-doped ZnO nanowires synthesized via chemical vapor deposition. J. Phys. Chem. B 2004, 108, 5206–5210. [Google Scholar] [CrossRef]
- Chang, P.C.; Fan, Z.Y.; Wang, D.W.; Tseng, W.Y.; Chiou, W.A.; Hong, J.; Lu, J.G. ZnO nanowires synthesized by vapor trapping CVD method. Chem. Mater. 2004, 16, 5133–5137. [Google Scholar] [CrossRef]
- Choi, K.S.; Lichtenegger, H.C.; Stucky, G.D.; McFarland, E.W. Electrochemical synthesis of nanostructured ZnO films utilizing self-assembly of surfactant molecules at solid-liquid interfaces. J. Am. Chem. Soc. 2002, 124, 12402–12403. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhai, J.; Liu, H.; Song, Y.L.; Jiang, L.; Zhu, D.B. Electrochemical deposition of conductive superhydrophobic zinc oxide thin films. J. Phys. Chem. B 2003, 107, 9954–9957. [Google Scholar] [CrossRef]
- Wang, Y.C.; Leu, I.C.; Hon, M.H. Effect of colloid characteristics on the fabrication of ZnO nanowire arrays by electrophoretic deposition. J. Mater. Chem. 2002, 12, 2439–2444. [Google Scholar] [CrossRef]
- Gao, X.P.; Zheng, Z.F.; Zhu, H.Y.; Pan, G.L.; Bao, J.L.; Wu, F.; Song, D.Y. Rotor-like ZnO by epitaxial growth under hydrothermal conditions. Chem. Commun. 2004, 12, 1428–1429. [Google Scholar] [CrossRef]
- Kuo, C.; Kuo, T.J.; Huang, M.H. Hydrothermal synthesis of ZnO microspheres and hexagonal microrods with sheetlike and platelike nanostructures. J. Phys. Chem. B 2005, 109, 20115–20121. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Samulski, E.T. Hydrothermal synthesis of one-dimensional ZnO nanostructures with different aspect ratios. Chem. Commun. 2004, 8, 986–987. [Google Scholar] [CrossRef]
- Tokumoto, M.S.; Pulcinelli, S.H.; Santilli, C.V.; Briois, V. Catalysis and temperature dependence on the formation of ZnO nanoparticles and of Zinc acetate derivatives prepared by the sol-gel route. J. Phys. Chem. B 2003, 107, 568–574. [Google Scholar] [CrossRef]
- Werde, K.V.; Mondelaers, D.; Vanholand, G.; Nelis, D.; Van Bael, M.K.; Mullens, J.; Van Poucke, L.C. Thermal decomposition of the ammonium zinc acetate citrate precursor for aqueous chemical solution deposition of ZnO. J. Mater. Sci. 2002, 37, 81–88. [Google Scholar] [CrossRef]
- Abdullah, M.; Okuyama, K. Zinc oxide nanoparticles prepared by a simple heating: Effect of polymer addition and polymer absence on the morphology. PROC ITB Eng. Sci. 2004, 36, 141–153. [Google Scholar] [CrossRef]
- Shen, X.; Liang, Y.; Zhai, Y.; Ning, Z. Shape-controllable synthesis of ultrafine ZnO powders of fifferent morphologies. J. Mater. Sci. Technol. 2013, 29, 44–48. [Google Scholar] [CrossRef]
- Tian, Y.; Li, J.; Xiong, H.; Dai, J. Controlled synthesis of ZnO hollow microspheres via precursor-template method and its gas sensing property. Appl. Surf. Sci. 2012, 258, 8431–8438. [Google Scholar] [CrossRef]
- Pant, H.R.; Park, C.H.; Pokharel, P.; Tijing, L.D.; Lee, D.S.; Kim, C.S. ZnO micro-flowers assembled on reduced graphene sheets with high photocatalytic activity for removal of pollutants. Powder Technol. 2013, 235, 853–858. [Google Scholar] [CrossRef]
- Pant, H.R.; Pant, B.; Sharma, R.; Amarjargal, A.; Kim, H.J.; Park, C.H.; Tijing, L.D.; Kima, C.S. Antibacterial and photocatalytic properties of Ag/TiO2/ZnO nano-flowers prepared by facile one-pot hydrothermal process. Ceram. Int. 2013, 39, 1503–1510. [Google Scholar] [CrossRef]
- Odunola, O.A. Spectroscopic and magnetic properties of Zn(II), Cd(II) and Hg(II) carboxylates. Synth. React. Inorg. Met. Org. Chem. 1993, 23, 1241–1249. [Google Scholar] [CrossRef]
- Tang, L.-G.; Hon, D.N.-S. Chelation of chitosan derivatives with zinc ions. II. Association complexes of Zn2+ onto O,N-Carboxymethyl chitosan. J. Appl. Polym. Sci. 2001, 79, 1476–1485. [Google Scholar] [CrossRef]
- Farag, S.; Kareem, S.S.A.A. Different natural biomasses for lead cation removal. Carbohyd. Polym. 2009, 78, 263–267. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Function. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Tang, L.-G.; Hon, D.N.-S. Chelation of chitosan derivatives with zinc ions. II. Association complexes of Zn2+ onto O,N-carboxymethyl chitosan. J. Appl. Polym. Sci. 2001, 79, 1476–1485. [Google Scholar] [CrossRef]
- Zhao, X.; Kato, K.; Fukumoto, Y.; Nakamae, K. Synthesis of bioadhesive hydrogels from chitin derivatives. Int. J. Adhes. Adhes. 2001, 21, 227–232. [Google Scholar] [CrossRef]
- Desai, K.; Kit, K.; Li, J.; Zivanovic, S. Morphological and surface properties of electrospun chitosan nanofibers. Biomacromolecules 2008, 9, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.; Schlaak, M.; Strasdeit, H. Adsorption of nickel(II), zinc(II) and cadmium(II) by new chitosan derivatives. React. Funct. Polym. 2000, 44, 289–298. [Google Scholar] [CrossRef]
- Gu, F.; Wang, S.F.; Lu, M.K.; Zhou, G.J.; Xu, D.; Yuan, D.R. Structure evaluation and highly enhanced luminescence of Dy3+-doped ZnO nanocrystals by Li+ doping via combustion method. Langmuir 2004, 20, 3528–3531. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhou, R.; Zheng, C.; Sun, Q.; Lv, Y.; Li, C.; Hou, X. Size-controllable synthesis of spherical ZnO nanoparticles: Size- and concentration-dependent resonant light scattering. Microchem. J. 2012, 100, 61–65. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Bi, S.; Luo, G. Preparation of ZnO nanoparticles using the direct precipitation method in a membrane dispersion micro-structured reactor. Powder Tech. 2010, 202, 130–136. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, S.; Kim, D.; Hwang, S.; Jeon, M.; Moon, J. ZnO nanoparticles with controlled shapes and sizes prepared using a simple polyol synthesis. Superlattice Microstruct. 2008, 43, 330–339. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Thirumavalavan, M.; Huang, K.-L.; Lee, J.-F. Preparation and Morphology Studies of Nano Zinc Oxide Obtained Using Native and Modified Chitosans. Materials 2013, 6, 4198-4212. https://doi.org/10.3390/ma6094198
Thirumavalavan M, Huang K-L, Lee J-F. Preparation and Morphology Studies of Nano Zinc Oxide Obtained Using Native and Modified Chitosans. Materials. 2013; 6(9):4198-4212. https://doi.org/10.3390/ma6094198
Chicago/Turabian StyleThirumavalavan, Munusamy, Kai-Lin Huang, and Jiunn-Fwu Lee. 2013. "Preparation and Morphology Studies of Nano Zinc Oxide Obtained Using Native and Modified Chitosans" Materials 6, no. 9: 4198-4212. https://doi.org/10.3390/ma6094198
APA StyleThirumavalavan, M., Huang, K.-L., & Lee, J.-F. (2013). Preparation and Morphology Studies of Nano Zinc Oxide Obtained Using Native and Modified Chitosans. Materials, 6(9), 4198-4212. https://doi.org/10.3390/ma6094198