Synthesis of New RE3+ Doped Li1+xTa1−xTixO3 (RE: Eu, Sm, Er, Tm, and Dy) Phosphors with Various Emission Colors
Abstract
:1. Introduction
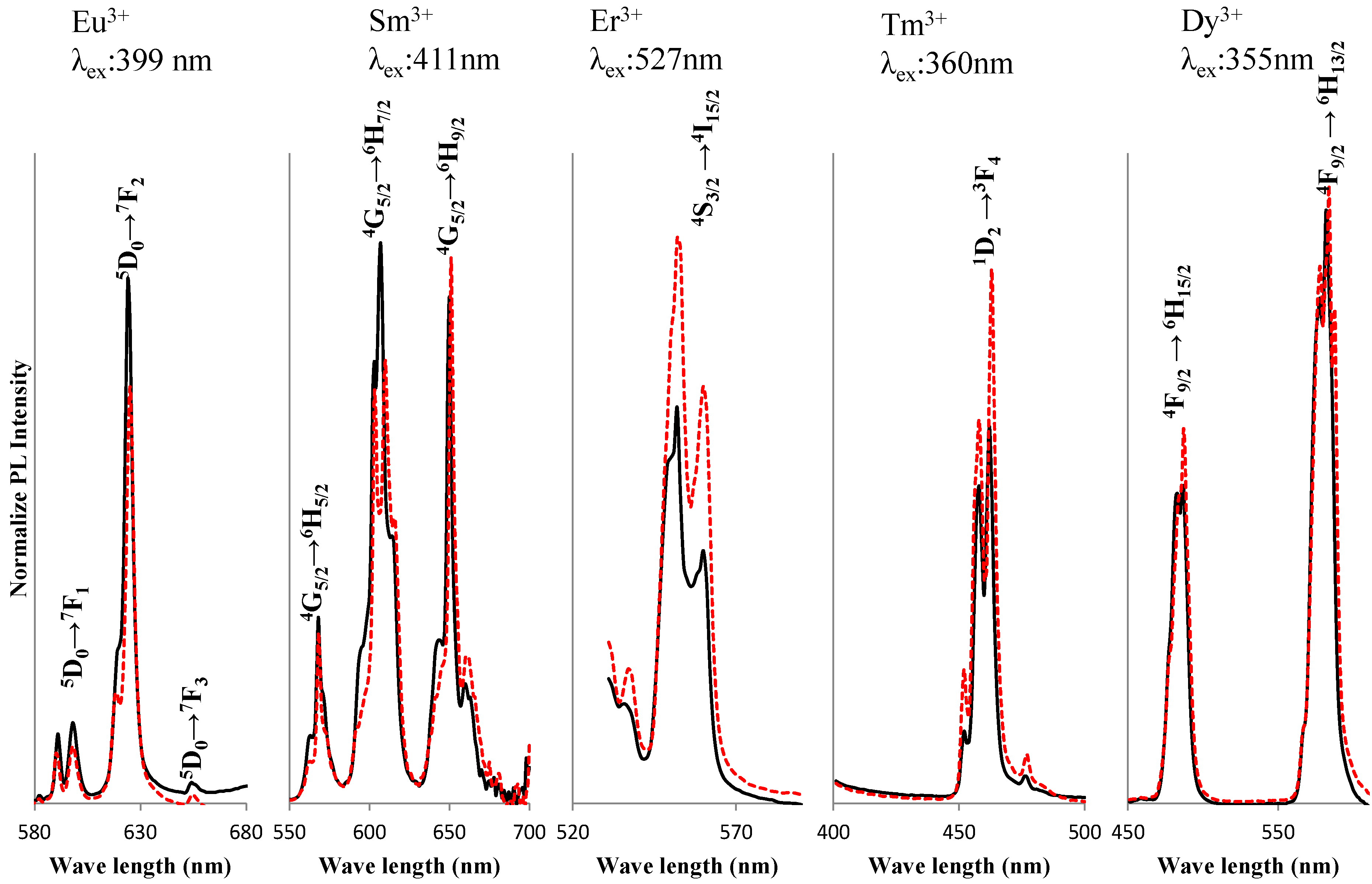
2. Results and Discussion
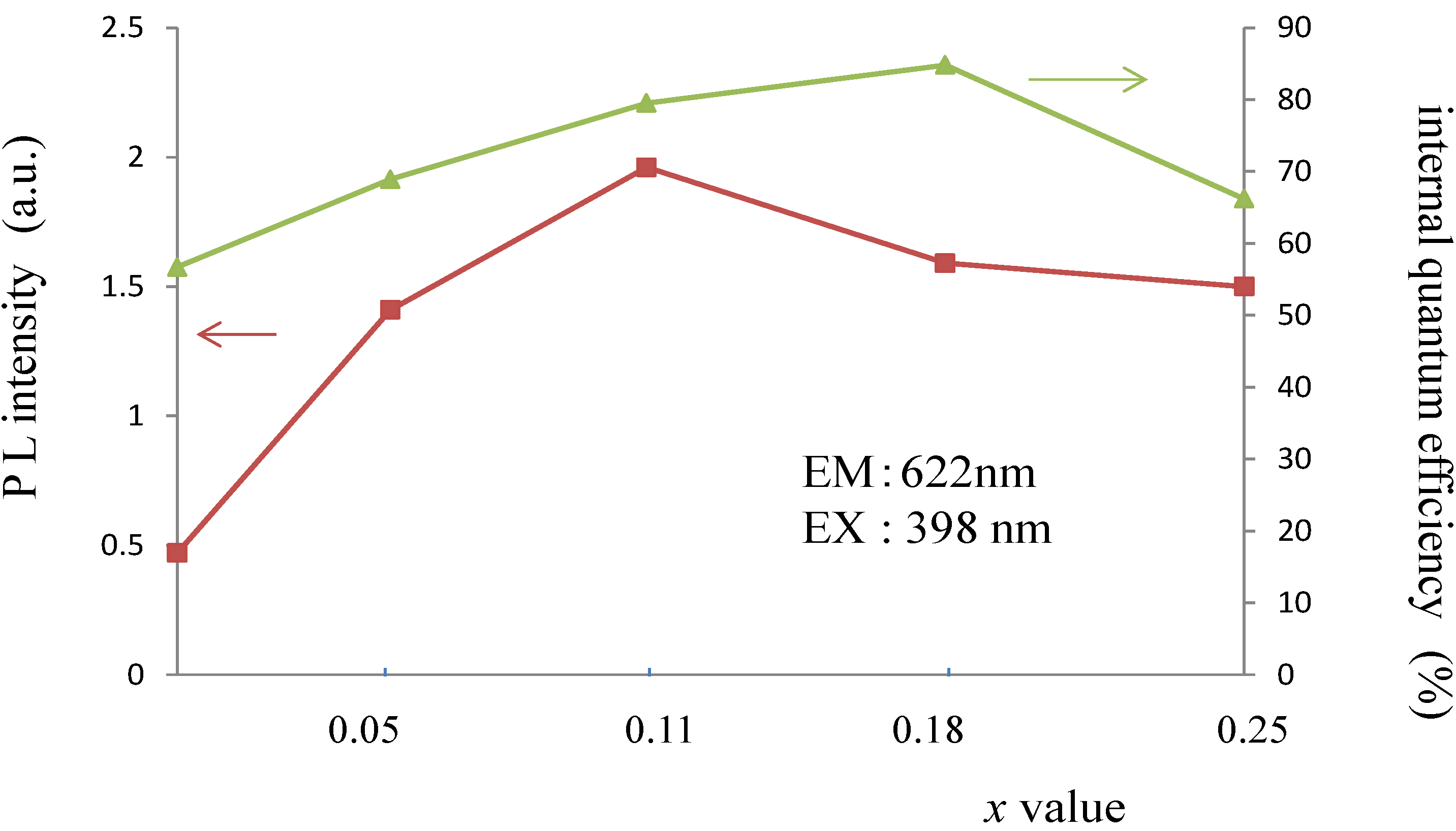
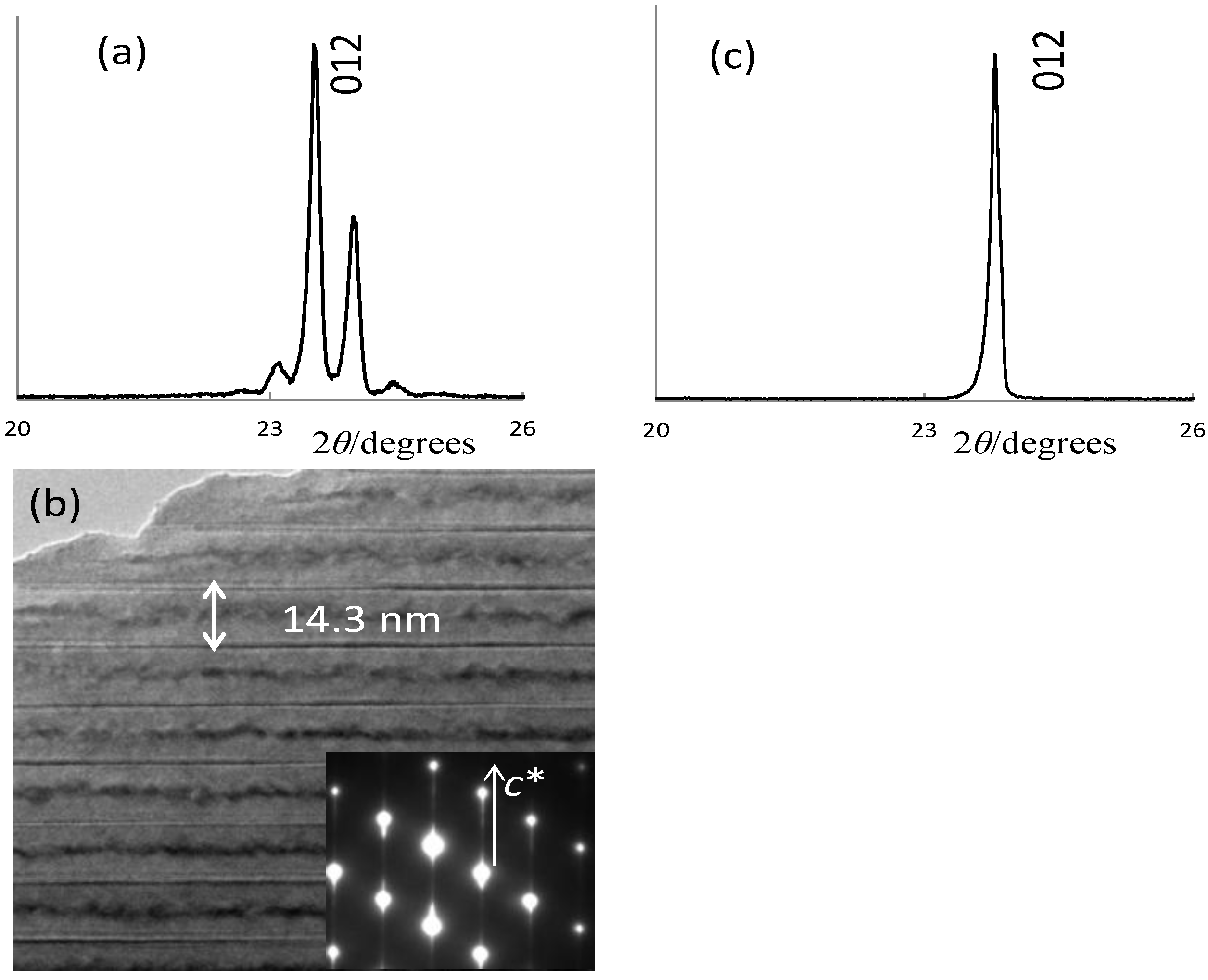
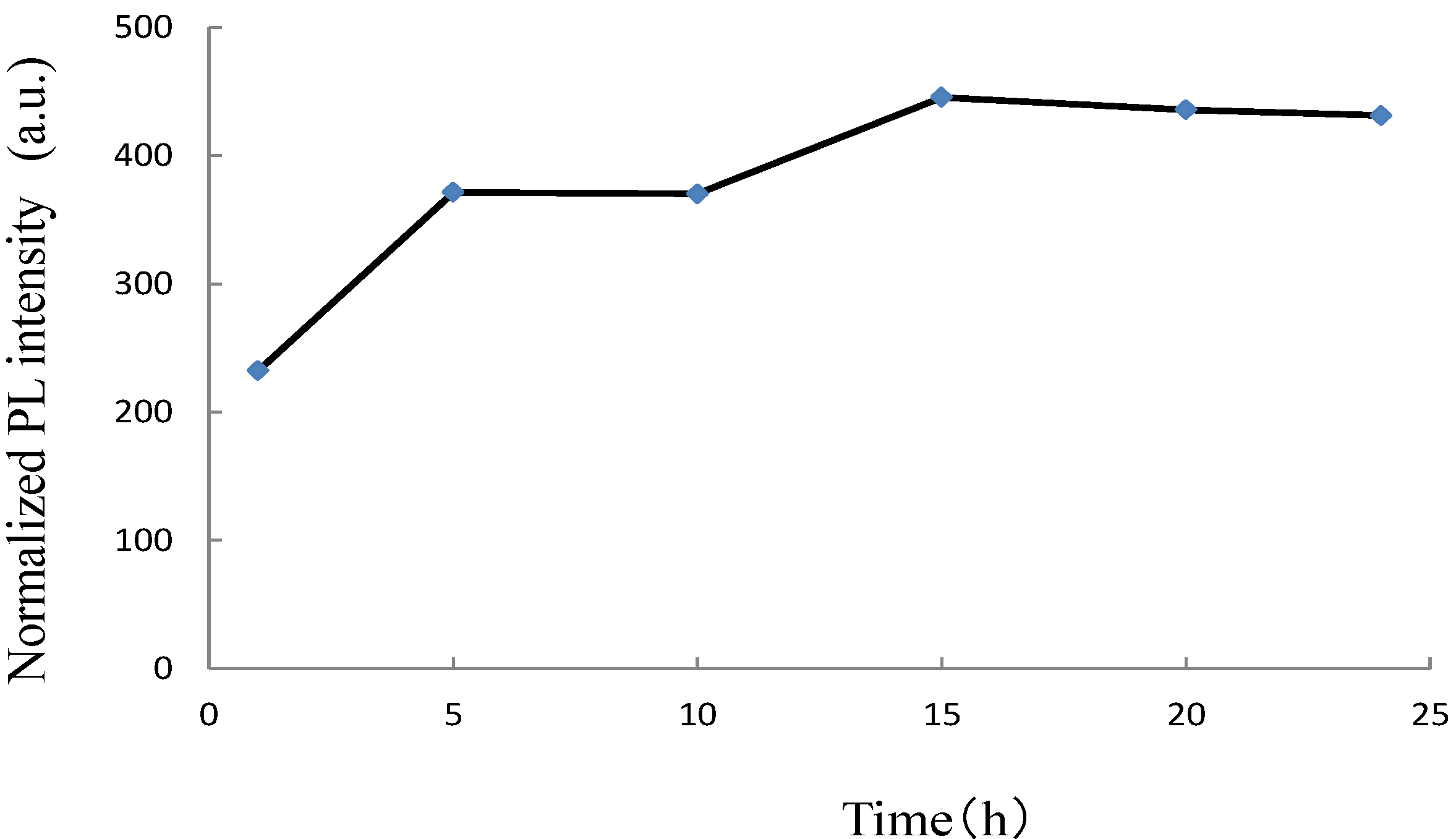
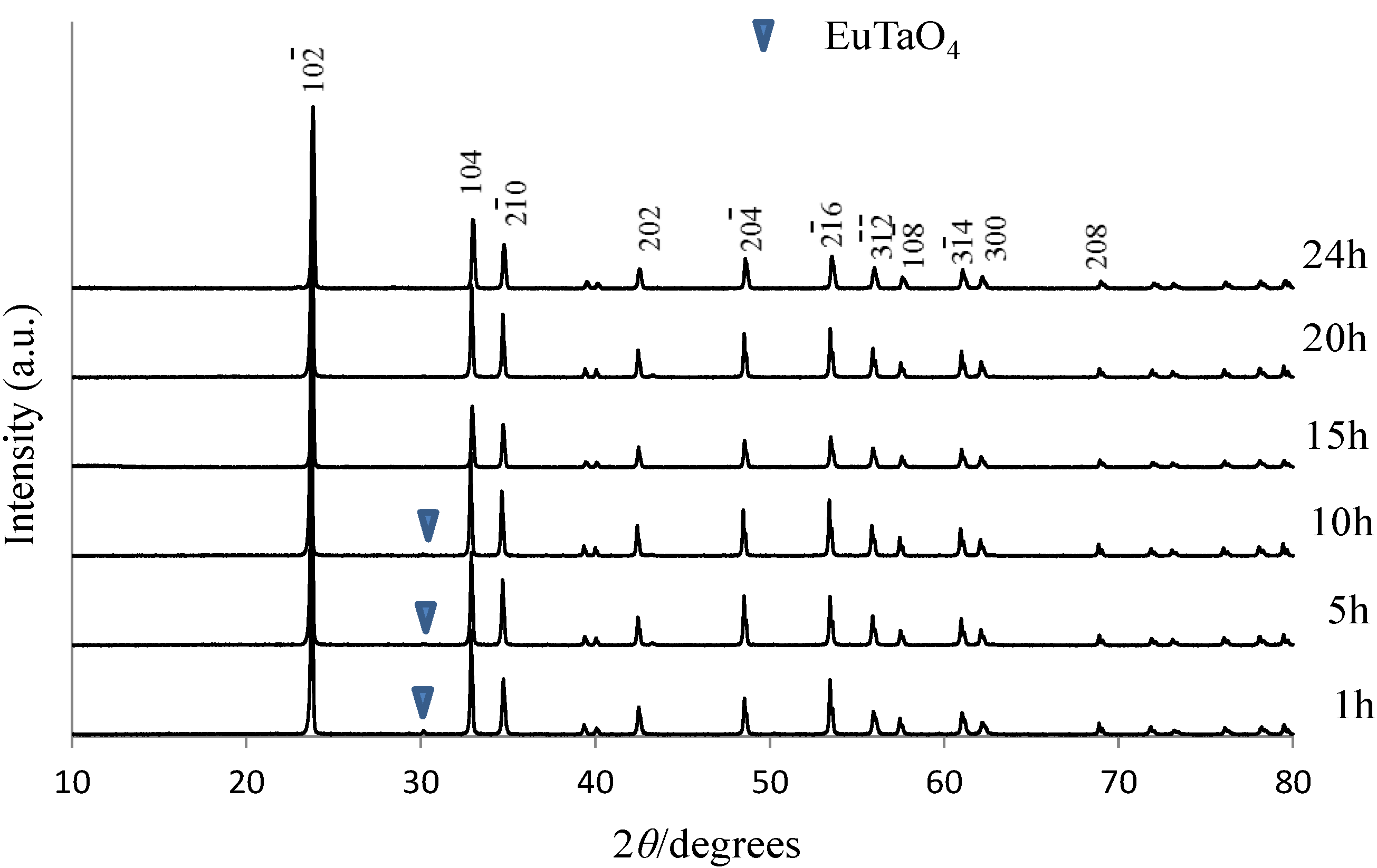

| Activator | LTT:RE3+ | LNT:RE3+ | ||
|---|---|---|---|---|
| X | Y | X | Y | |
| Eu | 0.673 | 0.327 | 0.676 | 0.324 |
| Sm | 0.622 | 0.378 | 0.630 | 0.370 |
| Er | 0.307 | 0.685 | 0.347 | 0.647 |
| Tm | 0.144 | 0.033 | 0.143 | 0.033 |
| Dy | 0.418 | 0.425 | 0.433 | 0.423 |
3. Experimental Procedure
4. Conclusions
Acknowledgments
References
- Villafuerte-Castrejon, M.E.; Gracia, J.A.; Cisneros, E.; Valenzuela, R.; West, A.R. New rutile solid solutions Ti1−4xLixM3xO2:M = Nb, Ta, Sb. J. Brit. Ceram. Soc. 1984, 83, 143–145. [Google Scholar]
- Villafuerte-Castrejon, M.E.; Aragon-Pina, A.; Valenzuela, R.; West, A.R. Compound and solid-solution formation in the system Li2O-Nb2O5-TiO2. J. Solid State Ceram. 1987, 71, 103–108. [Google Scholar] [CrossRef]
- Smith, R.I.; West, A.R. Characterization of an incommensurate LiTiNb oxide. Mater. Res. Bull. 1992, 27, 277–285. [Google Scholar] [CrossRef]
- Hayashi, H.; Nakano, H.; Suzumura, K.; Urabe, K.; West, A.R. Superstructure in LiTiNb oxides. Forth Ceram. Soc. 1995, 2, 391–398. [Google Scholar]
- Hayashi, H.; Urabe, K.; Niihara, K. Preparation of stoichiometric crystalline Li(Nb,Ti)O3 solid solutions by sol-gel processing with metal alkoxides. Key Eng. Mater. 1999, 501, 161–163. [Google Scholar]
- Farber, L.; Levin, I.; Borisevichi, A.; Grey, I.E.; Roth, R.S.; Davies, P.K. Structural study of Li1+x−yNb1−x−3yTix+4yO3 solid solutions. J. Solid State Chem. 2002, 166, 81–90. [Google Scholar] [CrossRef]
- Borisevich, A.Y.; Davies, P.K. Crystalline structure and dielectric properties of Li1+x−yNb1−x−3yTix+4yO3 M-phase solid solutions. J. Am. Ceram. Soc. 2002, 85, 573–578. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Hayashi, H.; Sekino, T.; Nakayama, T.; Kondo, T.; Wada, M.; Adachi, T.; Niihara, K. Microstructure and dielectric properties of sintered Li-Nb-Ti-O solid solution ceramics having superstructure. Mater. Res. Innov. 2003, 7, 74–79. [Google Scholar]
- Hayashi, H.; Nakano, H. Evolution and preparation of Li1+x−yNb1−x−3yTix+4yO3 solid solution with superstructure as new phosphor. J. Alloy. Compd. 2010, 502, 360–364. [Google Scholar] [CrossRef]
- Hayashi, H.; Nakano, H.; Jones, M.I. Microstructure and luminescence of Eu-doped Li1+x−yNb1−x−3yTix+4yO3 solid solutions with superstructure. J. Ceram. Soc. Jpn. 2010, 118, 226–230. [Google Scholar] [CrossRef]
- Nakano, H.; Saji, T.; Yuasa, M.; Shoji, M.; Mamoru, M. Rapid synthesis and structural analysis of Li-Nb-Ti-O solid solutions with superstructure by millimeter-wave heating. J. Ceram. Soc. Jpn. 2011, 119, 808–812. [Google Scholar] [CrossRef]
- Naknao, H.; Ozono, K.; Saji, T.; Miyake, S.; Hiroyuki, H. Rapid synthesis of Eu3+-doped LNT (Li-Nb-Ti-O) phosphor by millimeter-wave heating. Opt. Mater. 2012, in press. [Google Scholar]
- Nakano, H.; Suehrio, S.; Saji, T.; Miyake, S. Synthesis of a rare-erath doped LNT (Li-Nb-Ti-O) phosphor by millimemetr-wave heating. J. Alloy. Compd. 2013, 475–479. [Google Scholar] [CrossRef]
- Gryk, D.; Dyl, D.; Ryba-Romanowski, W.; Grinberg, M. Spectral properties of LiTaO3:Pr3+ under high hydrostatic pressure. J. Phys. Condens. Matter 2005, 17, 5383–5395. [Google Scholar] [CrossRef]
- Kostritskii, S.M.; Maring, D.B.; Tavlykasev, R.F.; Ramaswamy, R.V. Energy transfer upconversion in Er-doped LiTaO3. Appl. Phys. Lett. 2000, 76, 2161–2163. [Google Scholar] [CrossRef]
- Ryba-Romanowski, W.; Golab, S.; Dominiak-Dzik, G.; Paltnikov, M.N.; Sidorov, N.V. Influence of temperature on luminescence of terbium ions in LiNbO3. J. Appl. Lett. 2001, 78, 3610–3611. [Google Scholar] [CrossRef]
- Gasparotto, G.; Cebin, M.A.; Goes, M.S.; Lima, S.A.M.; Davolos, M.R.; Varela, J.A.; Paiva-Santos, C.O.; Zaghete, M.A. Correlation between the spectroscopic and structural properties with the occupation of Eu3+ sites in powdered Eu3+-doped LiTaO3 prepared by the pechini method. J. Appl. Phys. 2009, 106, 3506–3509. [Google Scholar] [CrossRef]
- Sokolska, I.; Ryba-Romanowski, W.; Golab, S.; Baba, M.; Swirkowicz, M.; Lukasiewicz, T. Spectroscopy of LiTaO3:Tm3+ crystals. J. Phys. Chem. Solids 2000, 61, 1573–1581. [Google Scholar]
- Nakano, H.; Ozono, K.; Hayashi, H.; Fujihara, S. Synthesis and luminescent properties of a new Eu3+-doped Li1+x (Ta1−zNbz)1−xTixO3 red phosphor. J. Am. Ceram. Soc. 2012, 2795–2797. [Google Scholar] [CrossRef]
- Uchida, T.; Suehiro, S.; Asaka, T.; Nakano, H.; Fukuda, K. Syntheses and crystal structures of Li(Ta0.89Ti0.11)O2.945 and (Li0.977Eu0.023)(Ta0.89Ti0.11)O2.968. Powder Diffr. 2013, in press. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nakano, H.; Suehiro, S.; Furuya, S.; Hayashi, H.; Fujihara, S. Synthesis of New RE3+ Doped Li1+xTa1−xTixO3 (RE: Eu, Sm, Er, Tm, and Dy) Phosphors with Various Emission Colors. Materials 2013, 6, 2768-2776. https://doi.org/10.3390/ma6072768
Nakano H, Suehiro S, Furuya S, Hayashi H, Fujihara S. Synthesis of New RE3+ Doped Li1+xTa1−xTixO3 (RE: Eu, Sm, Er, Tm, and Dy) Phosphors with Various Emission Colors. Materials. 2013; 6(7):2768-2776. https://doi.org/10.3390/ma6072768
Chicago/Turabian StyleNakano, Hiromi, Shiho Suehiro, Shohei Furuya, Hiroyuki Hayashi, and Shinobu Fujihara. 2013. "Synthesis of New RE3+ Doped Li1+xTa1−xTixO3 (RE: Eu, Sm, Er, Tm, and Dy) Phosphors with Various Emission Colors" Materials 6, no. 7: 2768-2776. https://doi.org/10.3390/ma6072768





