Mechanism of Generation of ZnO Microstructures by Microwave-Assisted Hydrothermal Approach
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Discussion of ZnO Microstructure Morphology

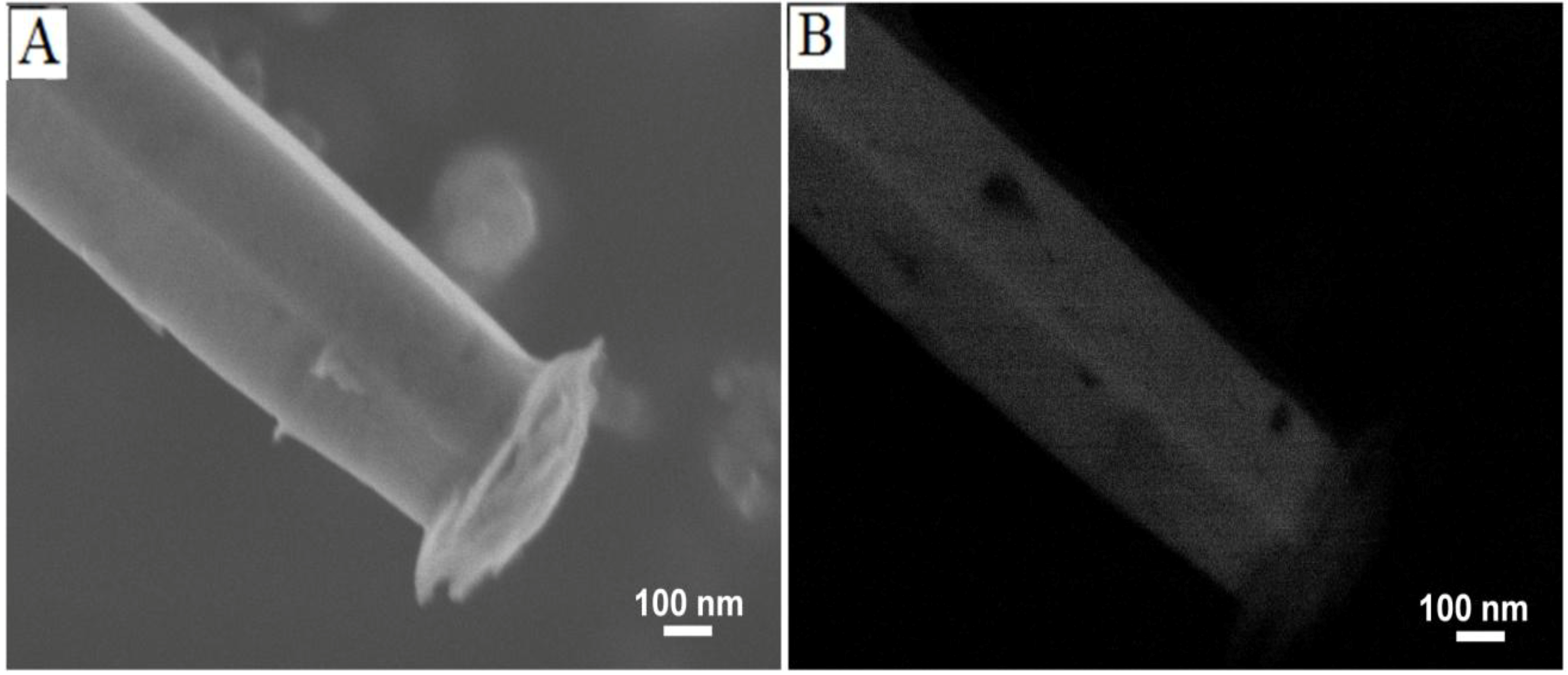
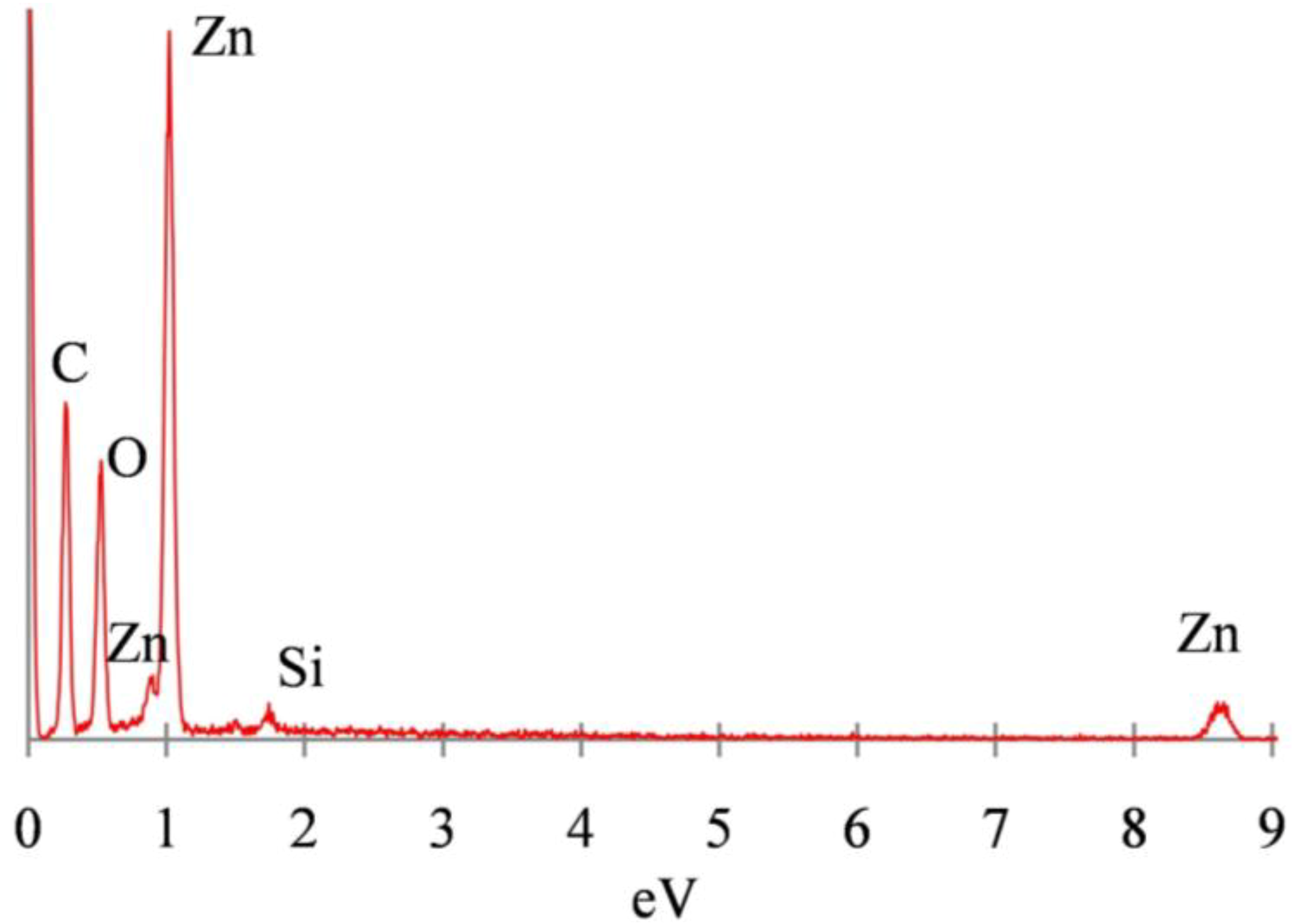
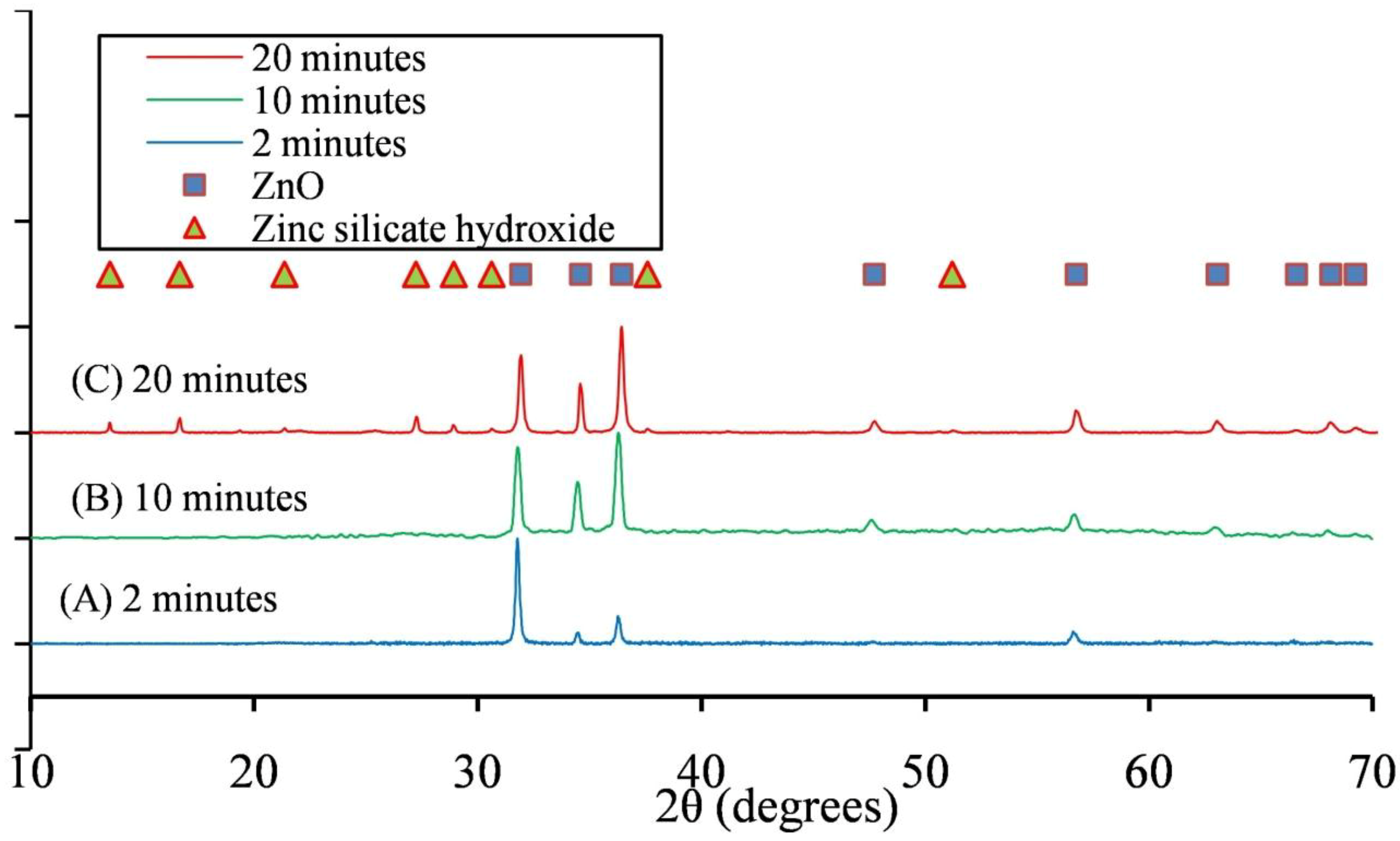
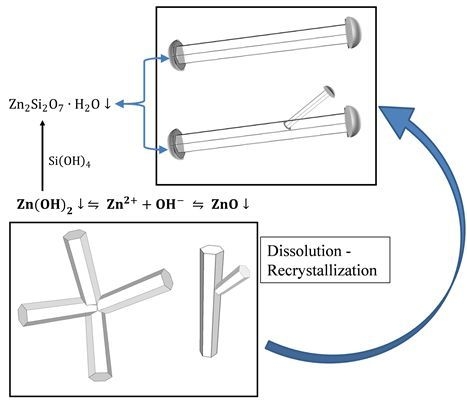
3.2. Mechanism of ZnO Microstructure Generation
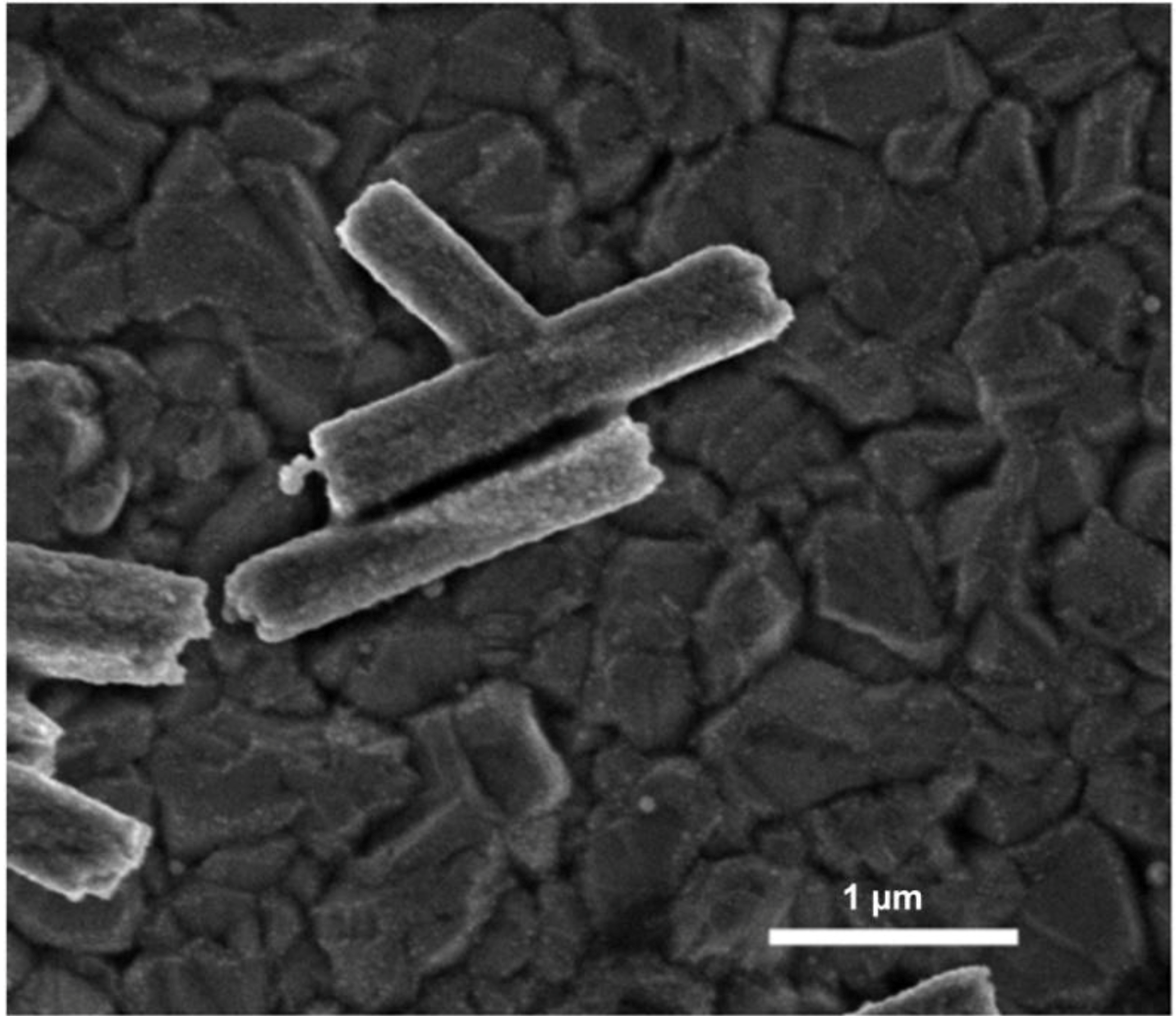
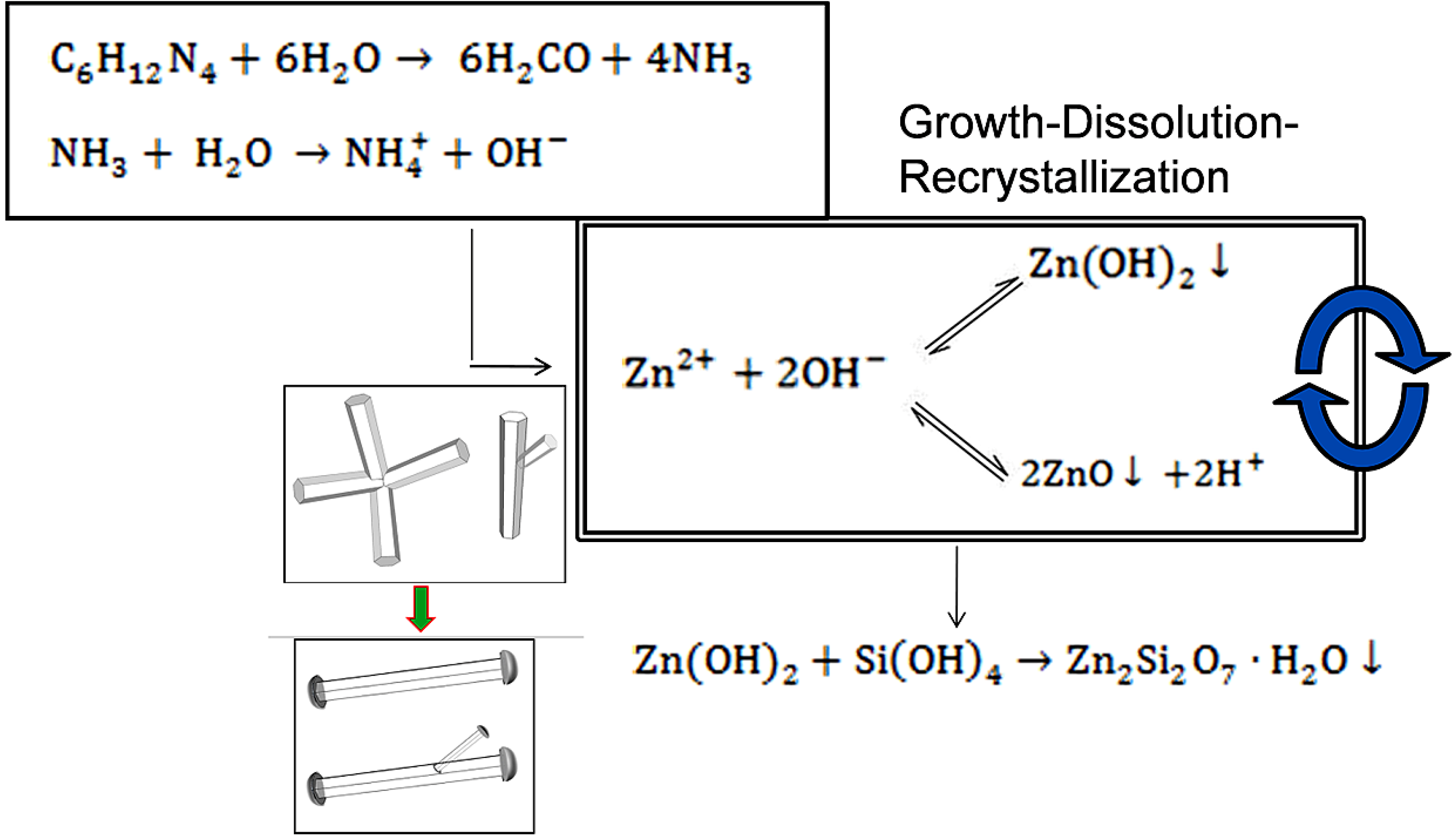
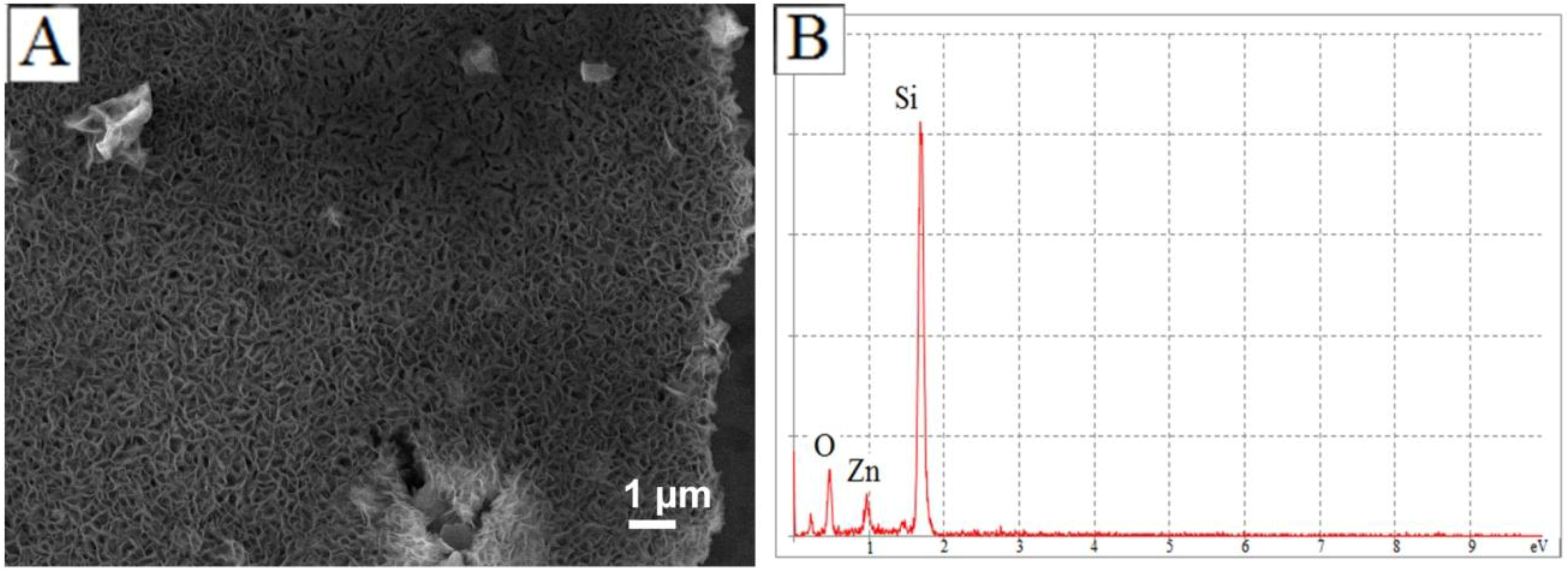
4. Conclusions
Supplementary Files
Supplementary File 1References
- Pan, Z.W. Nanobelts of Semiconducting Oxides. Science 2001, 291, 1947–1949. [Google Scholar] [CrossRef]
- Wang, X.D.; Summers, C.J.; Wang, Z.L. Large-scale hexagonal-patterned growth of aligned ZnO nanorods for nano-optoelectronics and nanosensor arrays. Nano Lett. 2004, 4, 423–426. [Google Scholar] [CrossRef]
- Vayssieres, L. Growth of arrayed nanorods and nanowires of ZnO from aqueous solutions. Adv. Mater. 2003, 15, 464–466. [Google Scholar] [CrossRef]
- Tarat, A.; Majithia, R.; Brown, R.A.; Penny, M.W.; Meissner, K.E.; Maffeis, T.G.G. Maffeis synthesis of nanocrystalline ZnO nanobelts via pyrolytic decomposition of zinc acetate nanobelts and their gas sensing behavior. Surf. Sci. 2012, 606, 715–721. [Google Scholar] [CrossRef]
- Li, Y.; Meng, G.W.; Zhang, L.D.; Phillipp, F. Ordered semiconductor ZnO nanowire arrays and their photoluminescence properties. Appl. Phys. Lett. 2000, 76, 2011–2013. [Google Scholar] [CrossRef]
- Weintraub, B.; Zhou, Z.; Li, Y.; Deng, Y. Solution synthesis of one-dimensional ZnO nanomaterials and their applications. Nanoscale 2010, 2, 1573–1587. [Google Scholar] [CrossRef]
- Greene, L.E.; Law, M.; Goldberger, J.; Kim, F.; Johnson, J.C.; Zhang, Y.; Saykally, R.J.; Yang, P. Low-temperature wafer-scale production of ZnO nanowire arrays. Angew. Chem. Int. Ed. 2003, 42, 3031–3034. [Google Scholar] [CrossRef]
- Greene, L.E.; Yuhas, B.D.; Law, M.; Zitoun, D.; Yang, P. Solution-grown Zinc oxide nanowires. Inorg. Chem. 2006, 45, 7535–7543. [Google Scholar] [CrossRef]
- Vayssieres, L.; Keis, K.; Lindquist, S.-E.; Hagfeldt, A. Purpose-built anisotropic metal oxide material: 3D highly oriented microrod array of ZnO. J. Phys. Chem. B 2001, 105, 3350–3352. [Google Scholar] [CrossRef]
- Sun, X.M.; Chen, X.; Deng, Z.X.; Li, Y.D. A CTAB-assisted hydrothermal orientation growth of ZnO nanorods. Mater. Chem. Phys. 2003, 78, 99–104. [Google Scholar] [CrossRef]
- Gao, X.; Li, X.; Yu, W. Flowerlike ZnO Nanostructures via Hexamethylenetetramine-assisted thermolysis of Zinc−Ethylenediamine complex. J. Phys. Chem. B 2005, 109, 1155–1161. [Google Scholar] [CrossRef]
- Tam, K.H.; Cheung, C.K.; Leung, Y.H.; Djurišić, A.B.; Ling, C.C.; Beling, C.D.; Fung, S.; Kwok, W.M.; Chan, W.K.; Phillips, D.L.; et al. Defects in ZnO nanorods prepared by a hydrothermal method. J. Phys. Chem. B 2006, 110, 20865–20871. [Google Scholar] [CrossRef]
- Unalan, H.E.; Hiralal, P.; Rupesinghe, N.; Dalal, S.; Milne, W.I.; Amaratunga, G.A.J. Rapid synthesis of aligned zinc oxide nanowires. Nanotechnology 2008, 19, 255608:1–255608:5. [Google Scholar] [CrossRef]
- Ma, M.-G.; Zhu, Y.-J.; Cheng, G.-F.; Huang, Y.-H. Microwave synthesis and characterization of ZnO with various morphologies. Mater. Lett. 2008, 62, 507–510. [Google Scholar] [CrossRef]
- Qurashi, A.; Tabet, N.; Faiz, M.; Yamzaki, T. Ultra-fast microwave synthesis of ZnO nanowires and their dynamic response toward hydrogen gas. Nanoscale Res. Lett. 2009, 4, 948–954. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, Y.; Wang, S. Sonochemical and microwave-assisted synthesis of linked single-crystalline ZnO rods. Mater. Chem. Phys. 2004, 88, 421–426. [Google Scholar] [CrossRef]
- De Moura, A.; Lima, R.; Moreira, M.; Volanti, D.; Espinosa, J.; Orlandi, M.; Pizani, P.; Varela, J.; Longo, E. ZnO architectures synthesized by a microwave-assisted hydrothermal method and their photoluminescence properties. Solid State Ion. 2010, 181, 775–780. [Google Scholar]
- Ivanov, V.K.; Shaporev, A.S.; Sharikov, F.Y.; Baranchikov, A.Y. Hydrothermal and microwave-assisted synthesis of nanocrystalline ZnO photocatalysts. Superlattices Microstruct. 2007, 42, 421–424. [Google Scholar] [CrossRef]
- Govender, K.; Boyle, D.S.; Kenway, P.B.; O’Brien, P. Understanding the factors that govern the deposition and morphology of thin films of ZnO from aqueous solution. J. Mater. Chem. 2004, 14, 2575–2591. [Google Scholar] [CrossRef]
- Goldstein, J.; Newbury, D.E.; Joy, D.C.; Lyman, C.E.; Echlin, P.; Lifshin, E.; Sawyer, L.; Michael, J.R. Scanning Electron Microscopy and X-Ray Microanalysis; Springer: New York, NY, USA, 2003; pp. 75–97. [Google Scholar]
- Verges, M.A.; Mifsud, A.; Serna, C.J. Formation of rod-like zinc oxide microcrystals in homogeneous solutions. J. Chem. Soc. Faraday Trans. 1990, 86, 959–963. [Google Scholar] [CrossRef]
- Oliveira, A.P.A.; Hochepied, J.-F.; Grillon, F.; Berger, M.-H. Controlled precipitation of Zinc oxide particles at room temperature. Chem. Mater. 2003, 15, 3202–3207. [Google Scholar] [CrossRef]
- Liu, B.; Zeng, H.C. Hydrothermal synthesis of ZnO nanorods in the diameter regime of 50 nm. J. Am. Chem. Soc. 2003, 125, 4430–4431. [Google Scholar] [CrossRef]
- McBride, R.A.; Kelly, J.M.; McCormack, D.E. Growth of well-defined ZnO microparticles by hydroxide ion hydrolysis of zinc salts. J. Mater. Chem. 2003, 13, 1196–1201. [Google Scholar] [CrossRef]
- Sun, Y.; Zou, R.; Tian, Q.; Wu, J.; Chen, Z.; Hu, J. Hydrothermal synthesis, growth mechanism, and properties of three-dimensional micro/nanoscaled hierarchical architecture films of hemimorphite zinc silicate. CrystEngComm 2011, 13, 2273–2280. [Google Scholar] [CrossRef]
- Li, Z.; Khimyak, Y.; Taubert, A. Lessons from a “Failed” experiment: Zinc silicates with complex morphology by reaction of Zinc acetate, the ionic liquid precursor (ILP) tetrabutylammonium hydroxide (TBAH), and glass. Materials 2008, 1, 3–24. [Google Scholar] [CrossRef]
- Cook, L.M. Chemical processes in glass polishing. J. Non-Cryst. Solids 1990, 120, 152–171. [Google Scholar] [CrossRef]
- Iler, R.K. Chemistry of Silica–Solubility, Polymerization, Colloid and Surface Properties and Biochemistry; John Wiley & Sons: Hoboken, NJ, USA, 1979. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Majithia, R.; Speich, J.; Meissner, K.E. Mechanism of Generation of ZnO Microstructures by Microwave-Assisted Hydrothermal Approach. Materials 2013, 6, 2497-2507. https://doi.org/10.3390/ma6062497
Majithia R, Speich J, Meissner KE. Mechanism of Generation of ZnO Microstructures by Microwave-Assisted Hydrothermal Approach. Materials. 2013; 6(6):2497-2507. https://doi.org/10.3390/ma6062497
Chicago/Turabian StyleMajithia, Ravish, Jeffrey Speich, and Kenith E. Meissner. 2013. "Mechanism of Generation of ZnO Microstructures by Microwave-Assisted Hydrothermal Approach" Materials 6, no. 6: 2497-2507. https://doi.org/10.3390/ma6062497