Chitosonic® Acid as a Novel Cosmetic Ingredient: Evaluation of its Antimicrobial, Antioxidant and Hydration Activities
Abstract
:1. Introduction
2. Results and Discussion
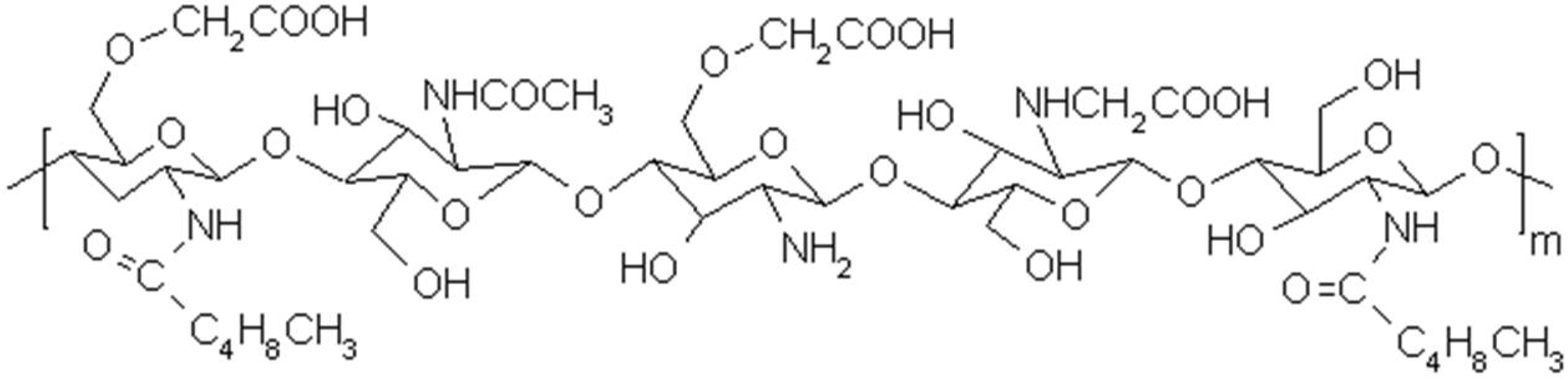
2.1. Characteristics of Chitosonic® Acid
2.1.1. Molecular Weight and Solubility of Chitosonic® Acid
| Characteristic | Value |
|---|---|
| Average molecular weight | ~160,000 g/mol |
| Solubility in water | ~5% |
| Zeta-potential | 29 mV (pH 5) |
| Isoelectric point | pH 4.6 |
| HLB value a | 36.5 |
2.1.2. Zeta potential, Isoelectric Point and HLB Value of Chitosonic® Acid
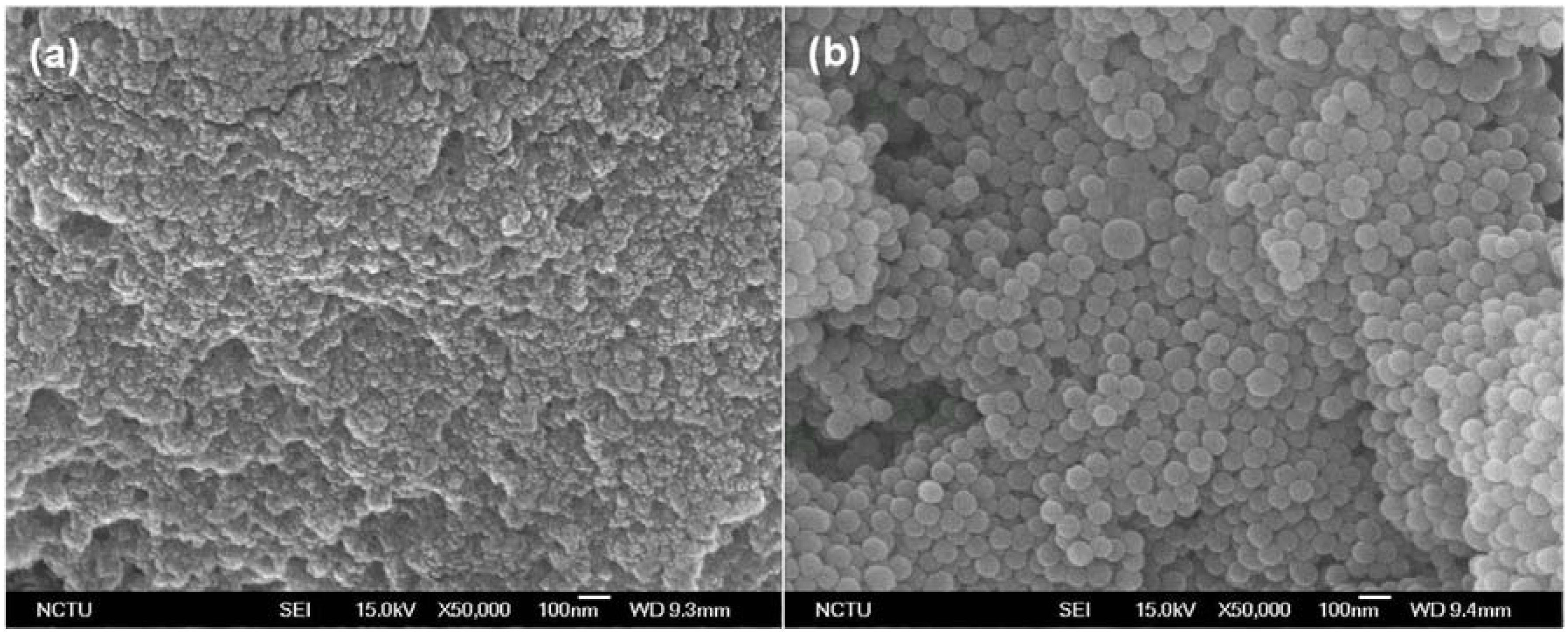
2.1.3. Morphological Evaluation of Chitosonic® Acid
2.2. Antimicrobial and Antioxidant Activities of Chitosonic® Acid
2.2.1. Antimicrobial Activity of Chitosonic® Acid
| Antimicrobial activity | ||
|---|---|---|
| Microorganism a | Type | Inhibition rate (%) |
| Escherichia coli | gram-negative bacterium | >99.99% |
| Pseudomonas aeruginosa | gram-negative bacterium | >99.99% |
| Propionibacterium acnes | gram-positive bacterium | 99.9% |
| Staphylococcus epidermidis | gram-positive bacterium | 87.3% |
| Staphylococcus aureus | gram-positive bacterium | 95.6% |
| Methicillin-resistant Staphylococcus aureus | gram-positive bacterium | 91.4% |
| Candida albicans | diploid fungus | 99.4% |
| Antioxidant activity | ||
| Ingredient | Free radical b | Scavenging rate (%) |
| 1% Arbutin | DPPH | 84% |
| 1% Hyaluronic acid | DPPH | 0% |
| 1% Chitosonic® Acid | DPPH | 66% |
| 4% Chitosonic® Acid | DPPH | 89% |
2.2.2. Antioxidant Activity of Chitosonic® Acid
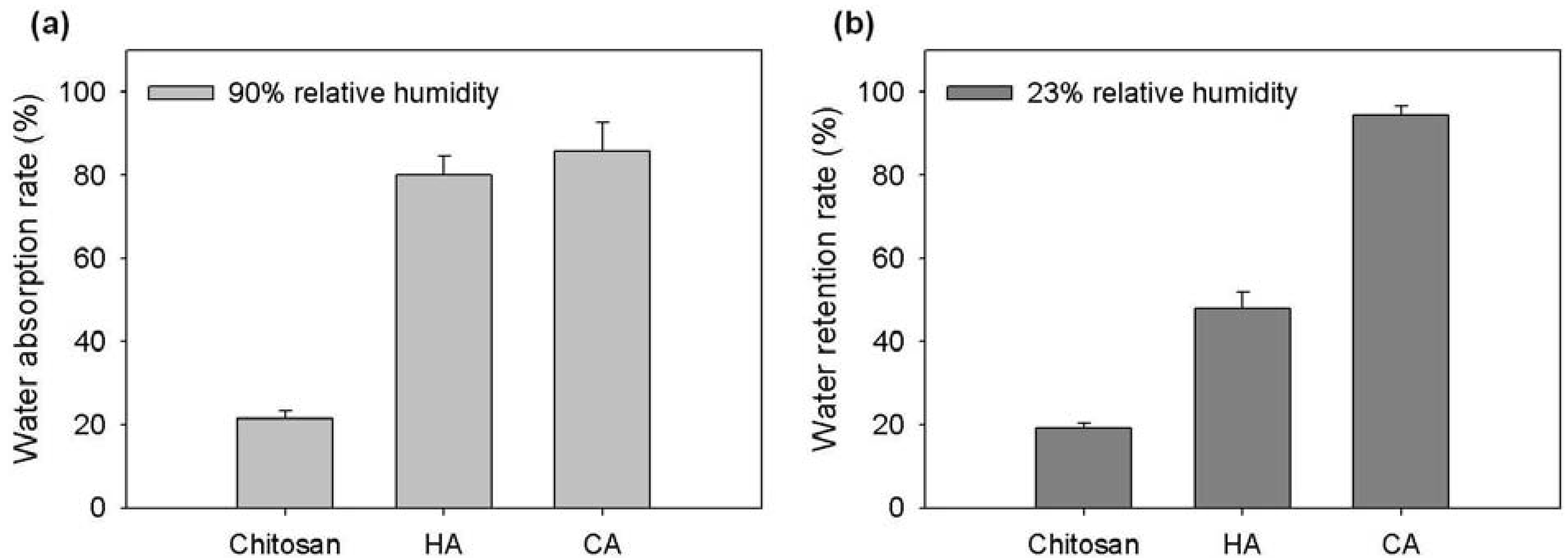
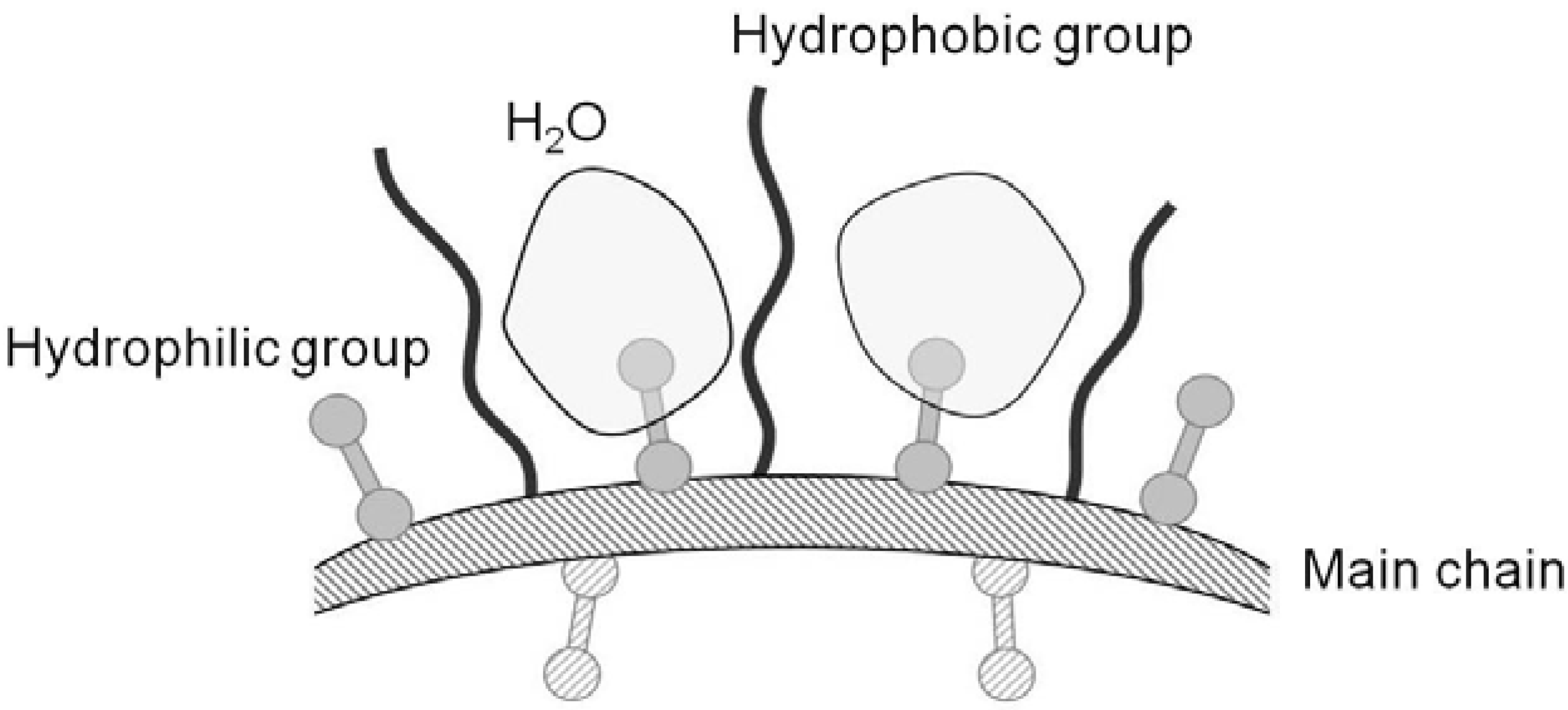
2.3. Hydration Activity of Chitosonic® Acid
2.4. Cytotoxicity of Chitosonic® Acid in Mouse Fibroblast L-929 Cells

3. Experimental Section
3.1. Materials
3.2. Synthesis of Chitosonic® Acid
3.3. Characterization
3.3.1. Molecular Weight and Solubility
3.3.2. Zeta-potential, Isoelectric Point and Hydrophilic-Lipophilic Balance (HLB) Value
3.3.3. Morphological Evaluation by Scanning Electron Microscopy (SEM)
3.4. Antimicrobial Assay
3.5. Antioxidant Assay
3.6. Hydration Assay
3.7. Cytotoxicity Assay
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Cochran, S.; Brockman, T. A cosmetic ingredient innovation for the stabilization and delivery of volatile fluoroether with cosmetic applications. J. Cosmet. Sci. 2007, 58, 413–419. [Google Scholar] [PubMed]
- Yang, C.H.; Chen, Y.S.; Lai, J.S.; Hong, W.W.; Lin, C.C. Determination of the thermodegradation of deoxyArbutin in aqueous solution by high performance liquid chromatography. Int. J. Mol. Sci. 2011, 11, 3977–3987. [Google Scholar] [CrossRef]
- Patravale, V.B.; Mandawgade, S.D. Novel cosmetic delivery systems: An application update. Int. J. Cosmet. Sci. 2008, 30, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-H.; Chen, S.-Y.; Liu, D.-M.; Liu, T.-Y. Self-assembled hollow nanocapsule from amphiphatic carboxymethyl-hexanoyl chitosan as drug carrier. Macromolecules 2008, 41, 6511–6516. [Google Scholar] [CrossRef]
- Garcia-Fuentes, M.; Alonso, M.J. Chitosan-based drug nanocarriers: Where do we stand? J. Control. Release 2012, 161, 496–504. [Google Scholar] [CrossRef] [PubMed]
- No, H.K.; Meyers, S.P.; Prinyawiwatkul, W.; Xu, Z. Applications of chitosan for improvement of quality and shelf life of foods: A review. J. Food Sci. 2007, 72, R87–R100. [Google Scholar] [CrossRef] [PubMed]
- Kumirska, J.; Weinhold, M.X.; Thöming, J.; Stepnowski, P. Biomedical activity of chitin/chitosan based materials—Influence of physicochemical properties apart from molecular weight and degree of N-acetylation. Polymers 2011, 3, 1875–1901. [Google Scholar] [CrossRef]
- Freitas, R.; Spin-Neto, R.; Spolidório, L.C.; Campana-Filho, S.P.; Marcantonio, R.; Marcantonio, J.E. Different molecular weight chitosan-based membranes for tissue regeneration. Materials 2011, 4, 380–389. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, Y.; Cai, J.; Ma, Y.; Wang, B.; Xia, K.; He, X. Self-assembly and fractal feature of chitosan and Its conjugate with metal Ions: Cu (II)/Ag (I). Int. J. Mol. Sci. 2007, 8, 1–12. [Google Scholar] [CrossRef]
- Liu, T.Y.; Liu, T.Y.; Chen, S.Y.; Chen, S.C.; Liu, D.M. Effect of hydroxyapatite nanoparticles on ibuprofen release from carboxymethyl-hexanoyl chitosan/O-hexanoyl chitosan hydrogel. J. Nanosci. Nanotechnol. 2006, 6, 2929–2935. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Koh, J. Physiochemical, optical and biological activity of chitosan-chromone derivative for biomedical applications. Int. J. Mol. Sci. 2012, 13, 6102–6116. [Google Scholar] [CrossRef] [PubMed]
- Masotti, A.; Ortaggi, G. Chitosan micro- and nanospheres: Fabrication and applications for drug and DNA delivery. Mini Rev. Med. Chem. 2009, 9, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Hurler, J.; Škalko-Basnet, N. Potentials of chitosan-based delivery systems in wound therapy: Bioadhesion study. J. Funct. Biomater. 2012, 3, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Jeong, Y.I.; Choi, K.C. Hair dye-incorporated poly-gamma-glutamic acid/glycol chitosan nanoparticles based on ion-complex formation. Int. J. Nanomed. 2011, 6, 2879–2888. [Google Scholar]
- Liu, K.H.; Chen, B.R.; Chen, S.Y.; Liu, D.M. Self-assembly behavior and doxorubicin-loading capacity of acylated carboxymethyl chitosans. J. Phys. Chem. B 2009, 113, 11800–11807. [Google Scholar] [CrossRef] [PubMed]
- Perugini, P.; Genta, I.; Pavanetto, F.; Conti, B.; Scalia, S.; Baruffini, A. Study on glycolic acid delivery by liposomes and microspheres. Int. J. Pharm. 2000, 196, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J. The promise of chitosan microspheres in drug delivery systems. Expert Opin. Drug Deliv. 2007, 4, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Chen, S.Y.; Lin, Y.L.; Liu, D.M. Synthesis and characterization of amphiphatic carboxymethyl-hexanoyl chitosan hydrogel: Water-retention ability and drug encapsulation. Langmuir 2006, 22, 9740–9745. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Fahmy, M.M. Synthesis and antimicrobial activity of some novel cross-linked chitosan hydrogels. Int. J. Mol. Sci. 2012, 13, 11194–11209. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Kim, M.M. Applications of chitin and its derivatives in biological medicine. Int. J. Mol. Sci. 2010, 11, 5152–5164. [Google Scholar] [CrossRef] [PubMed]
- Hisatomi, E.; Matsui, M.; Kubota, K.; Kobayashi, A. Antioxidative activity in the pericarp and seed of Japanese pepper (Xanthoxylum piperitum DC). J. Agric. Food Chem. 2000, 48, 4924–4928. [Google Scholar] [CrossRef] [PubMed]
- Aytekin, A.O.; Morimura, S.; Kida, K. Synthesis of chitosan—Caffeic acid derivatives and evaluation of their antioxidant activities. J. Biosci. Bioeng. 2011, 111, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Yasufuku, T.; Anraku, M.; Kondo, Y.; Hata, T.; Hirose, J.; Kobayashi, N.; Tomida, H. Useful extend-release chitosan tablets with high antioxidant activity. Pharmaceutics 2010, 2, 245–257. [Google Scholar] [CrossRef]
- Zhang, H.; Neau, S.H. In vitro degradation of chitosan by a commercial enzyme preparation: Effect of molecular weight and degree of deacetylation. Biomaterials 2001, 22, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-C.; Chou, C.-C.; Li, C.-F. Preparation, water solubility and rheological property of the N-alkylated mono or disaccharide chitosan derivatives. Food Res. Int. 2002, 35, 707–713. [Google Scholar] [CrossRef]
- Orafidiya, L.O.; Oladimeji, F.A. Determination of the required HLB values of some essential oils. Int. J. Pharm. 2002, 237, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.; Soriano, E.; Hernandez-Munoz, P.; Gavara, R. Migration of antimicrobial silver from composites of polylactide with silver zeolites. J. Food Sci. 2010, 75, E186–E193. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Wu, P.S.; Liang, D.W.; Kwan, C.C.; Chen, Y.S. Quality, antioxidative ability, and cell proliferation-enhancing activity of fermented black soybean broths with various supplemental culture medium. J. Food Sci. 2011, 77, C95–C101. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.-H.; Chen, Y.-S.; Hung, M.-S.; Lee, S.-M.; Lin, C.-C. The enhancement effect of Salvia miltiorrhiza on melanin production of B16F10 melanoma cells. J. Med. Plants Res. 2012, 6, 4338–4342. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, S.-M.; Liu, K.-H.; Liu, Y.-Y.; Chang, Y.-P.; Lin, C.-C.; Chen, Y.-S. Chitosonic® Acid as a Novel Cosmetic Ingredient: Evaluation of its Antimicrobial, Antioxidant and Hydration Activities. Materials 2013, 6, 1391-1402. https://doi.org/10.3390/ma6041391
Lee S-M, Liu K-H, Liu Y-Y, Chang Y-P, Lin C-C, Chen Y-S. Chitosonic® Acid as a Novel Cosmetic Ingredient: Evaluation of its Antimicrobial, Antioxidant and Hydration Activities. Materials. 2013; 6(4):1391-1402. https://doi.org/10.3390/ma6041391
Chicago/Turabian StyleLee, Shu-Mei, Kun-Ho Liu, Yen-Yu Liu, Yen-Po Chang, Chih-Chien Lin, and Yi-Shyan Chen. 2013. "Chitosonic® Acid as a Novel Cosmetic Ingredient: Evaluation of its Antimicrobial, Antioxidant and Hydration Activities" Materials 6, no. 4: 1391-1402. https://doi.org/10.3390/ma6041391





