Hydrogen Bonding-Mediated Microphase Separation during the Formation of Mesoporous Novolac-Type Phenolic Resin Templated by the Triblock Copolymer, PEO-b-PPO-b-PEO
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermal Analyses of Phenolic Resin/Block Copolymer Blends
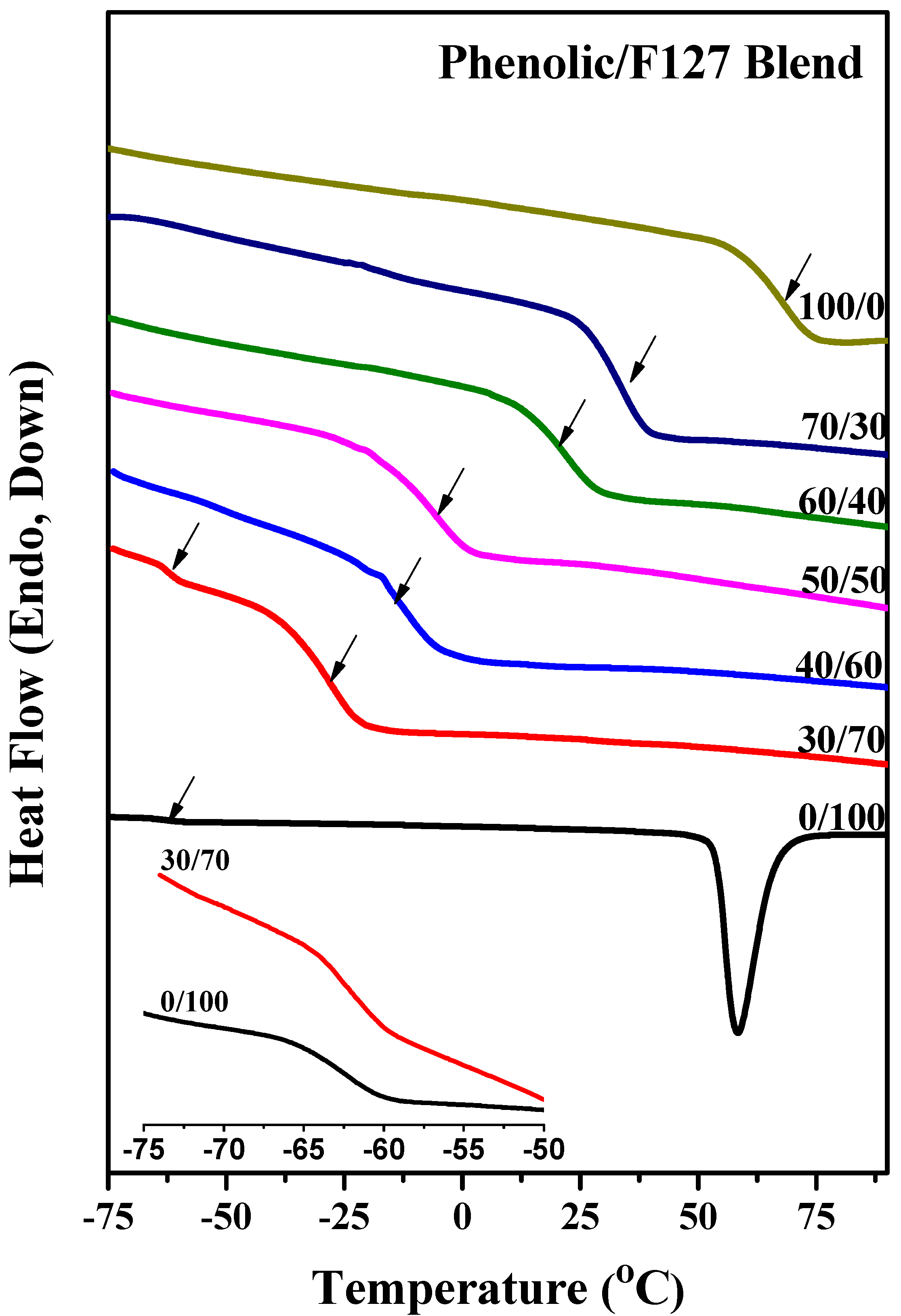
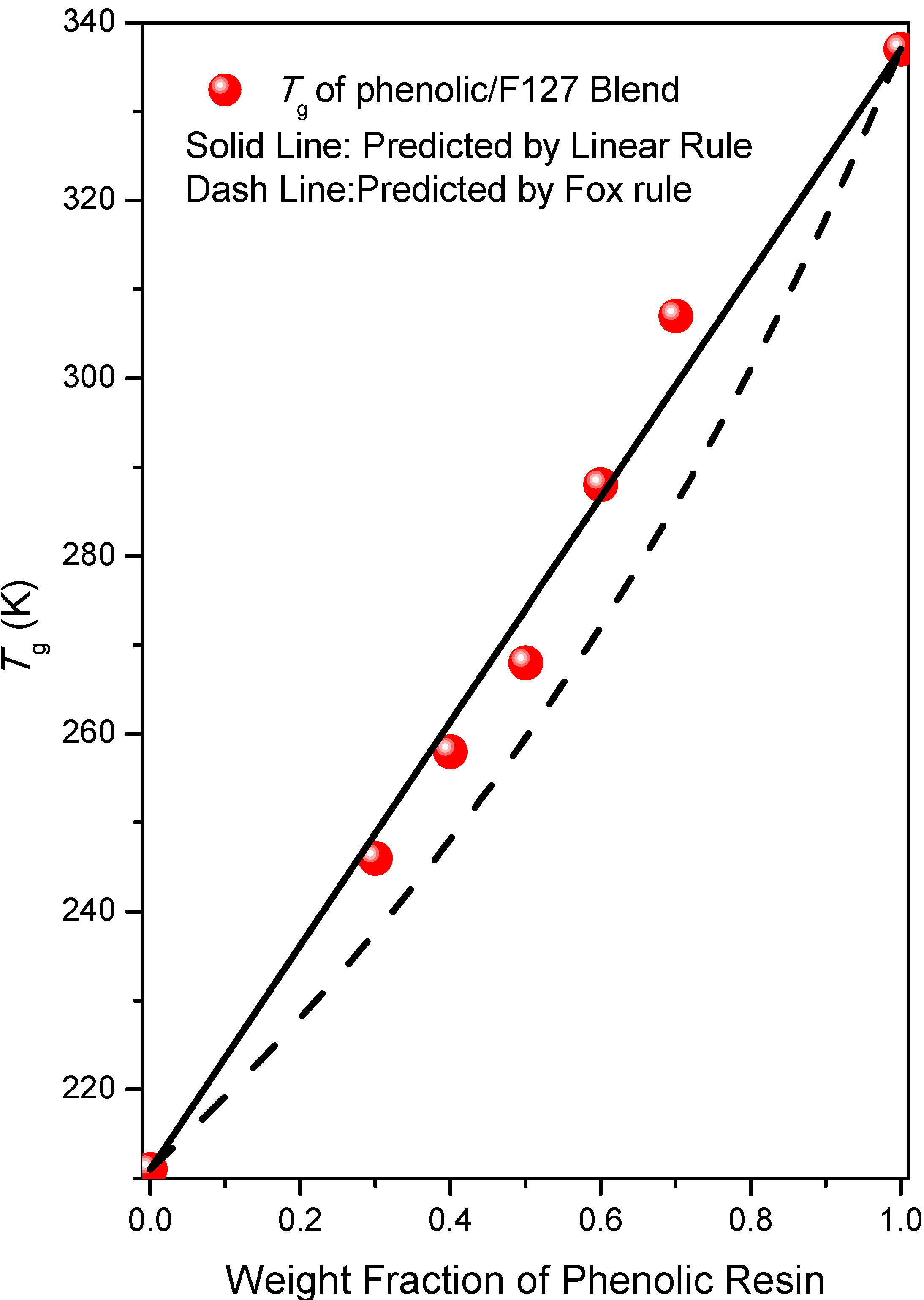
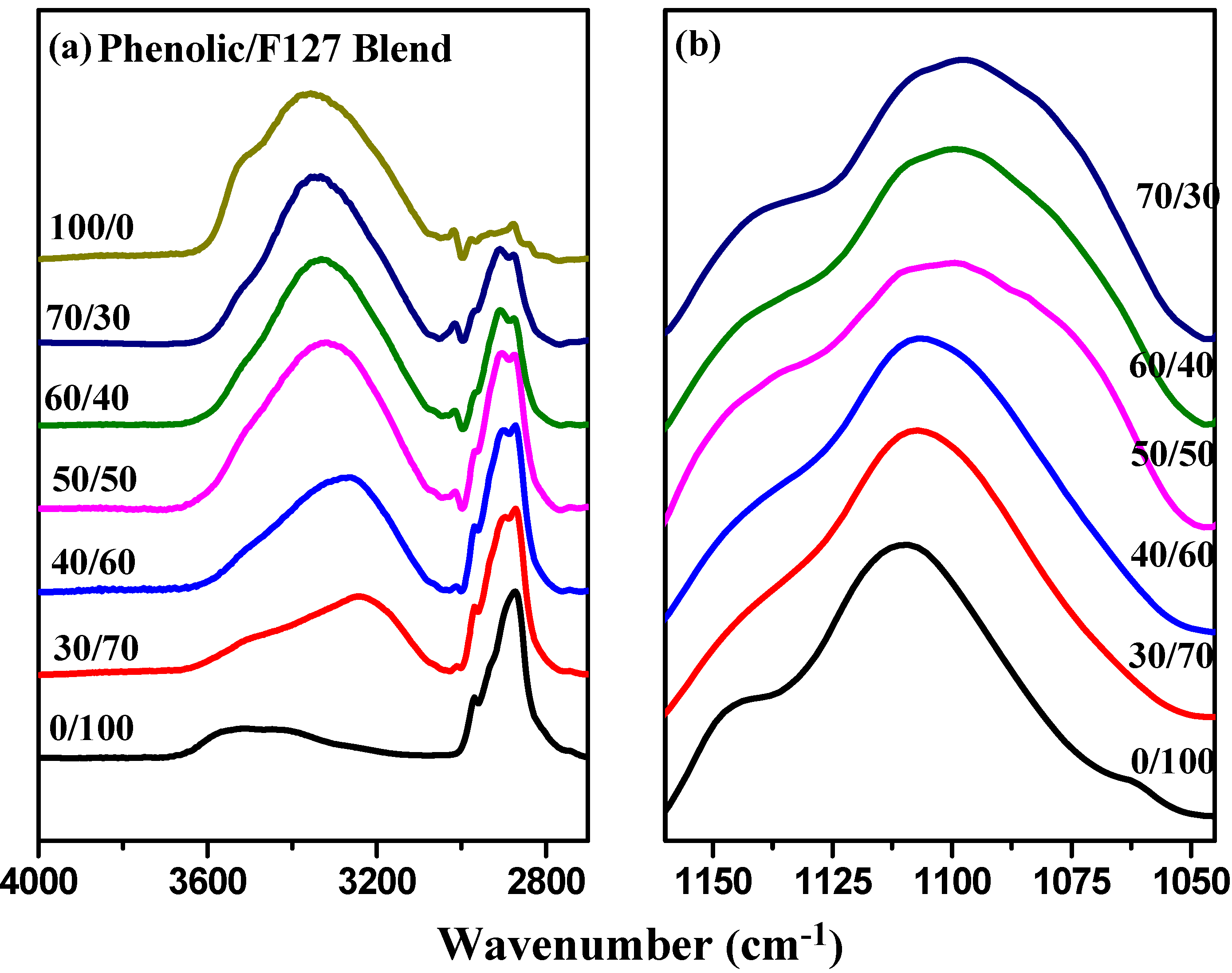
2.2. FTIR Spectroscopic Analyses of Phenolic Resin/Block Copolymer Blends
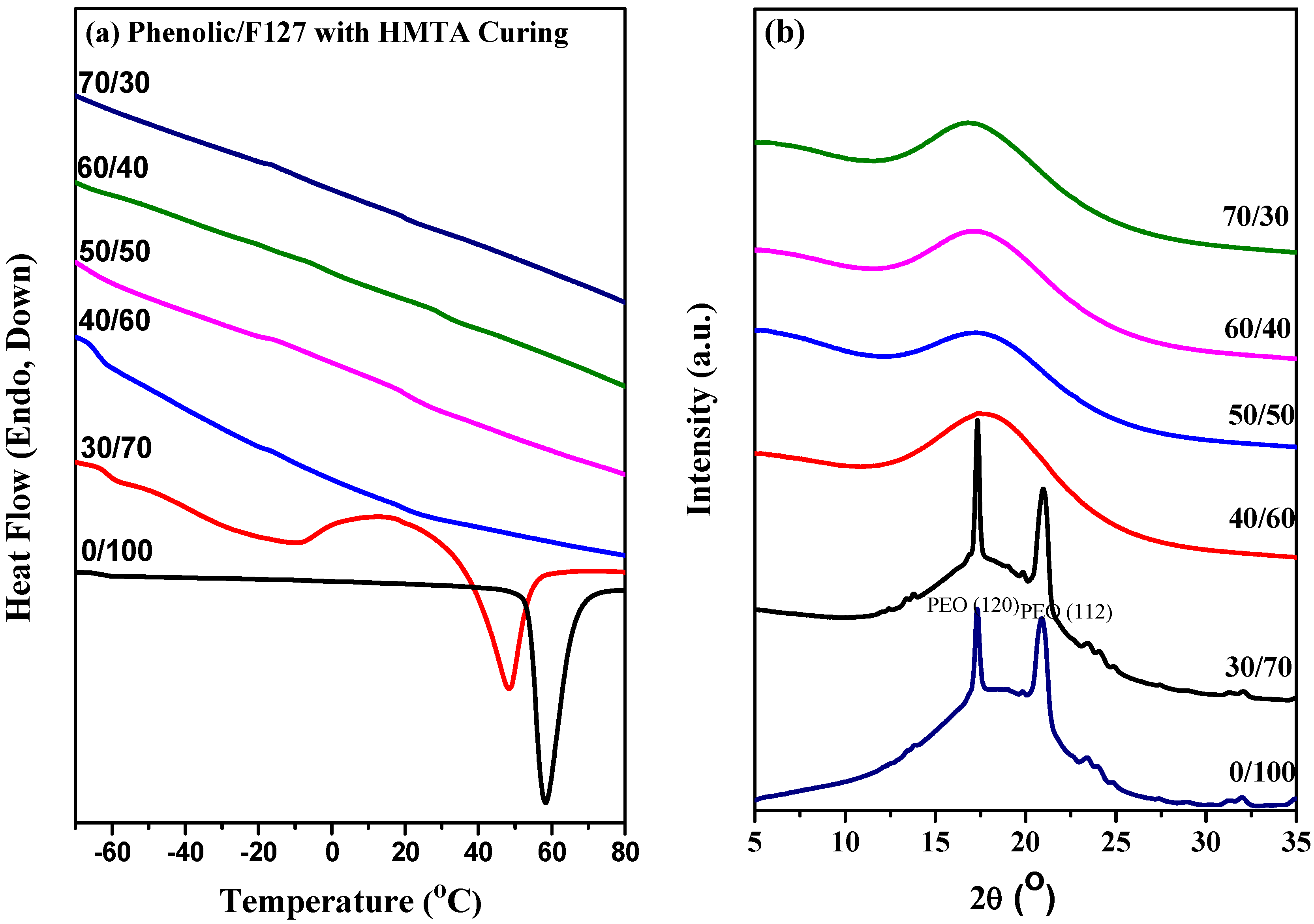
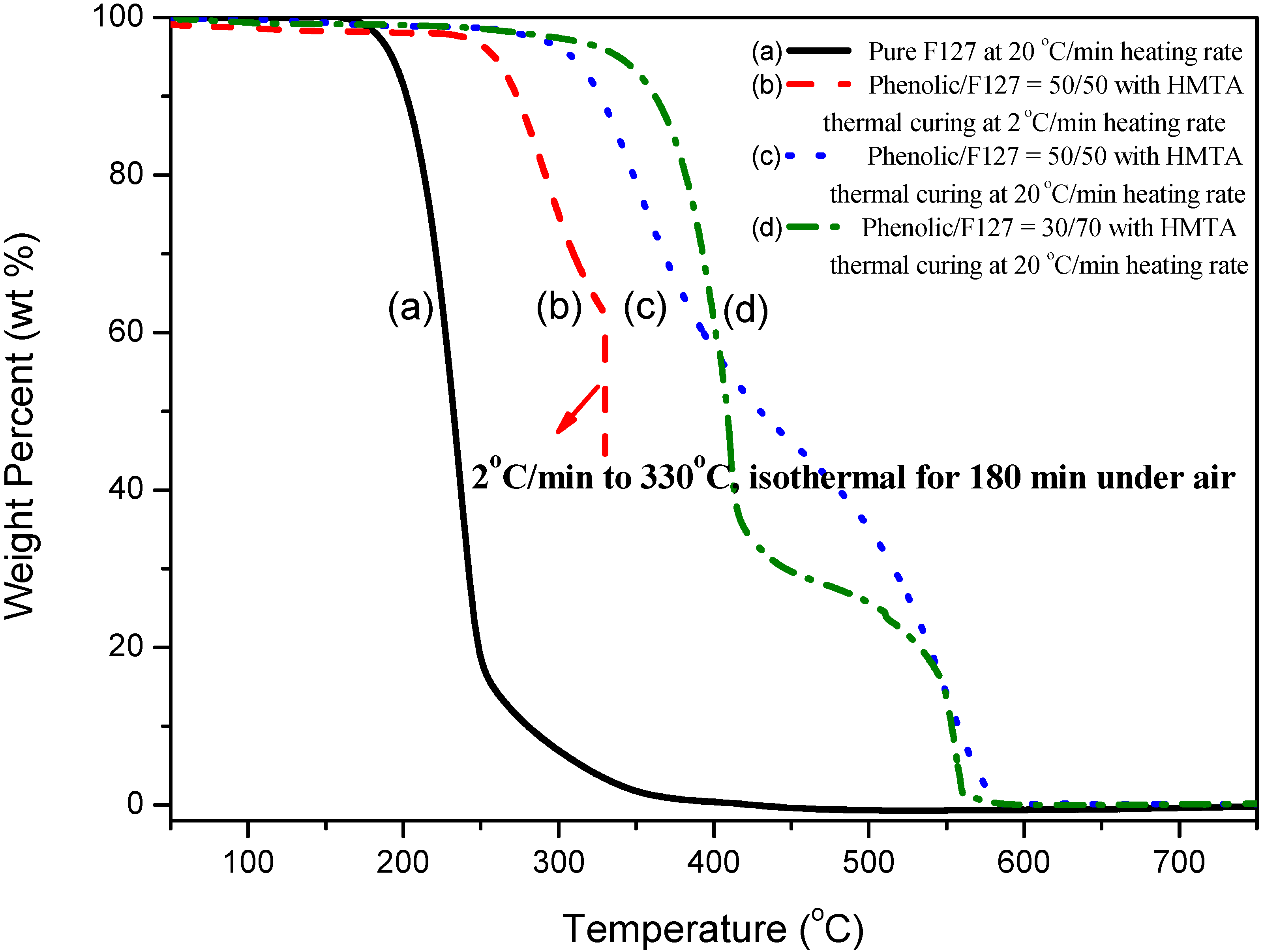
2.3. Thermal Analyses of Phenolic Resin/Block Copolymer Blends with HMTA Curing
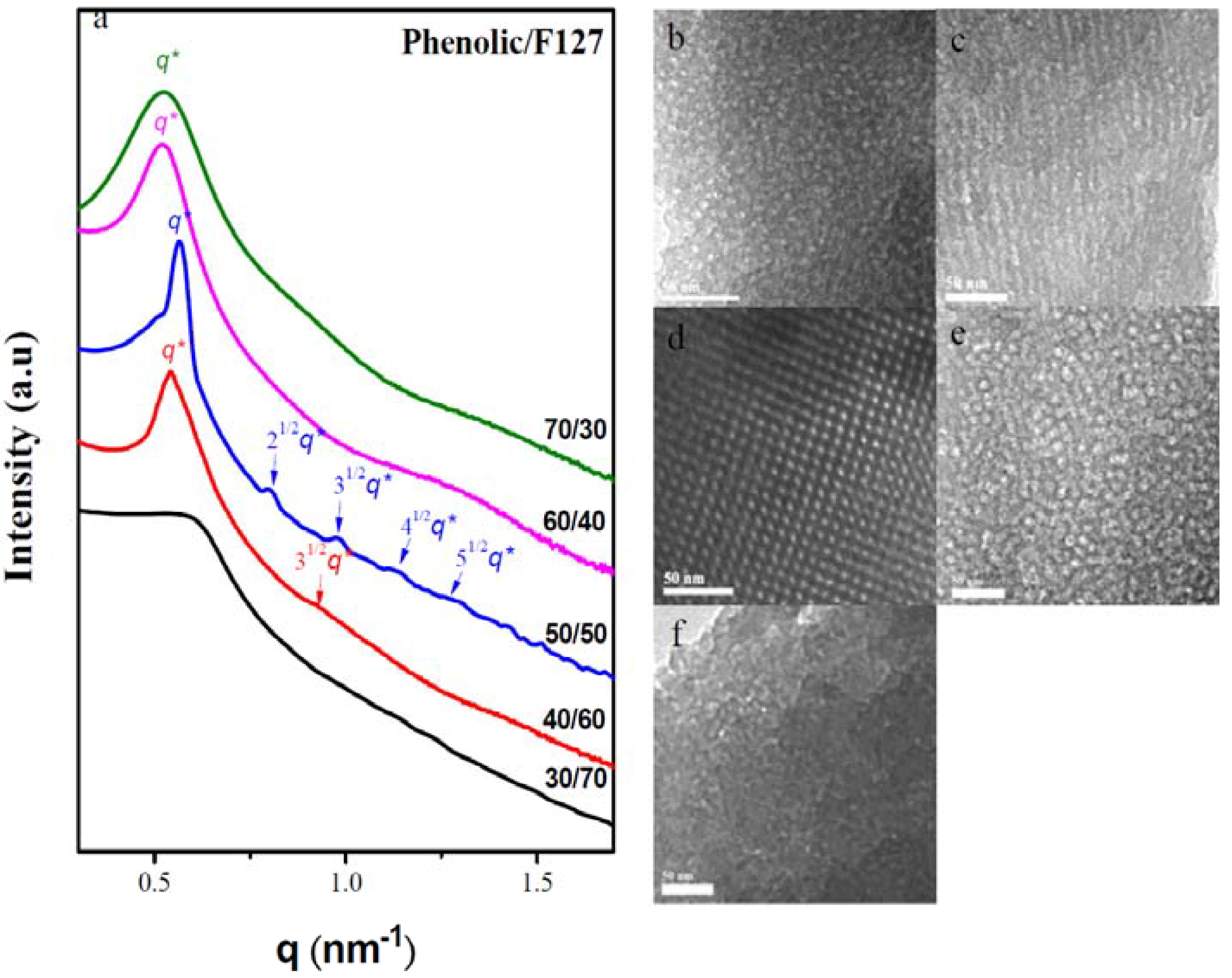
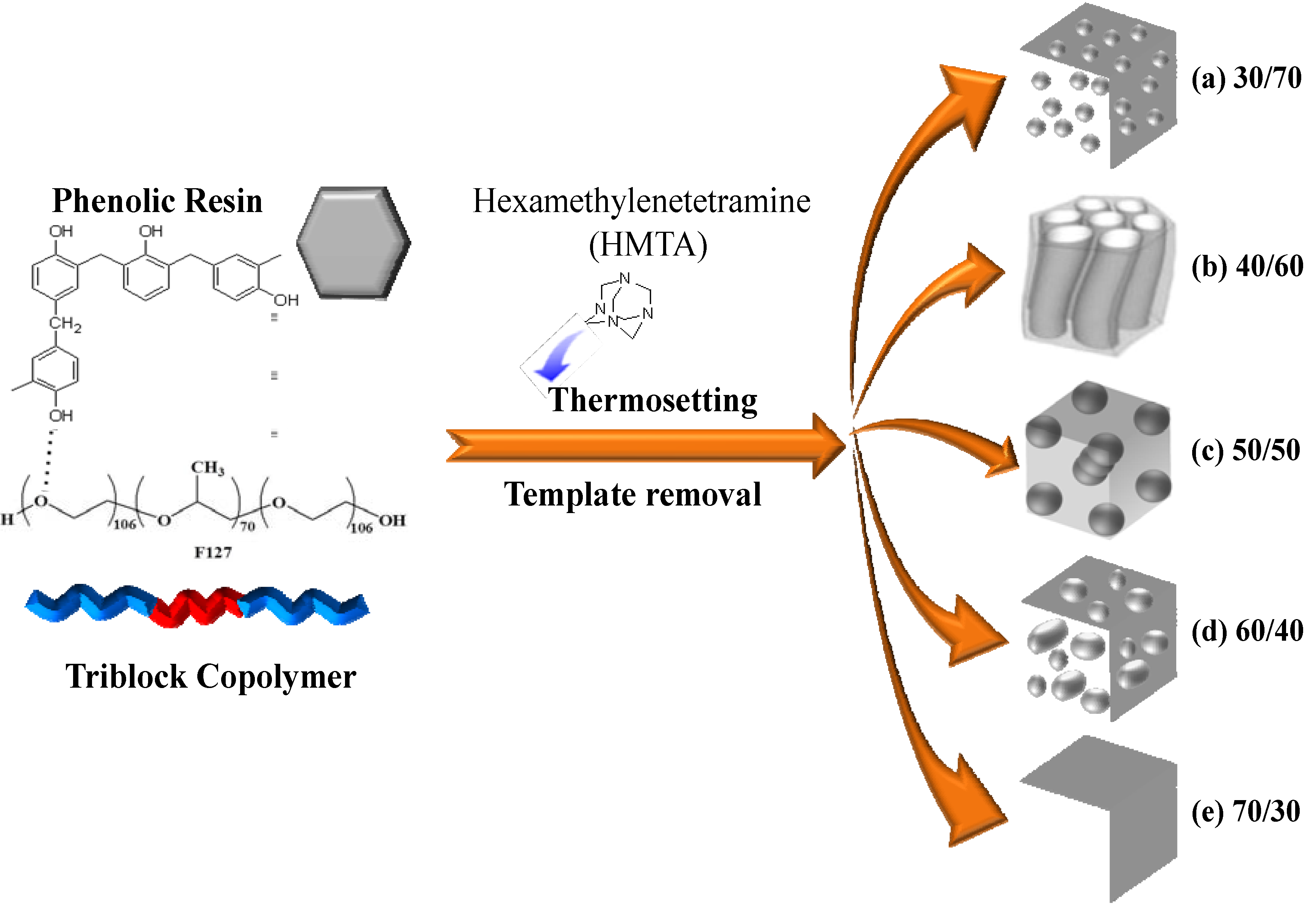
2.4. SAXS, TEM and BET Analyses of Mesoporous Phenolic Resins
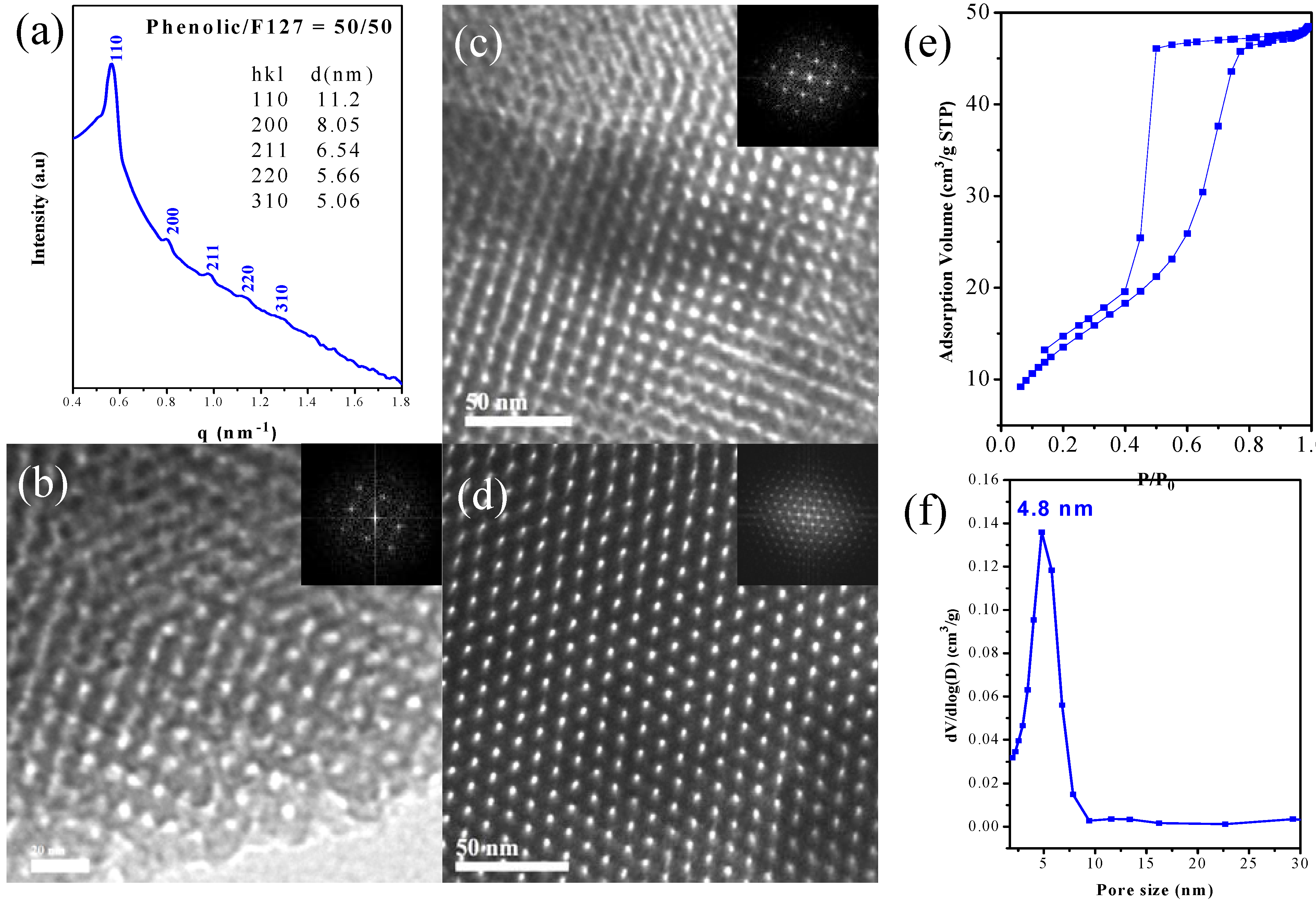
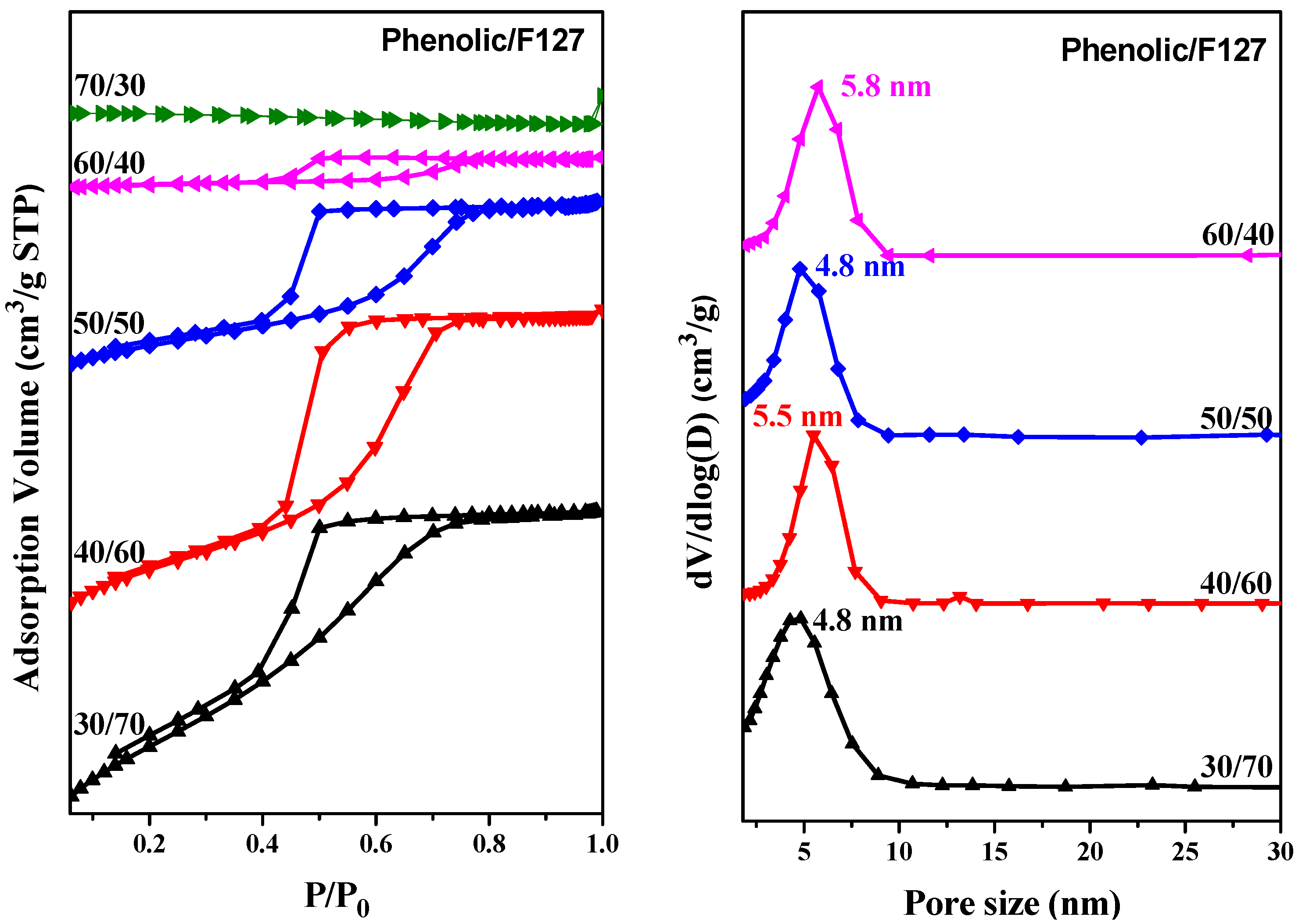
| Sample Phenolic/F127 | d (nm) a | Pore Size (nm) | SBET (m2/g) b | Pore Volume (cm3/g) |
|---|---|---|---|---|
| 30/70 | 10.6 | 4.8 | 155 | 0.15 |
| 40/60 | 11.6 | 5.5 | 82 | 0.11 |
| 50/50 | 11.2 | 4.8 | 52 | 0.08 |
| 60/40 | 12.1 | 5.8 | 10 | 0.01 |
| 70/30 | 12.1 | – | – | – |
3. Experimental Section
3.1. Materials
3.2. Phenolic/F127 Blends
3.3. Mesoporous Phenolic Resins
3.4. Characterization
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Corma, A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem. Rev. 1997, 97, 2373–2420. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Vinu, A.; Ji, O.; OOhmori, O.; Hill, J.P.; Acharya, S.; Koike, J.; Shiratori, S. A layered mesoporous carbon sensor based on nanopore-filling cooperative adsorption in the liquid phase. Angew. Chem. Int. Ed. 2008, 47, 7254–7257. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Balas, F.; Acros, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, F.; Fu, Y.; Jin, W.; Yang, P.; Zhao, D. Biomolecule separation using large pore mesoporous SBA-15 as a substrate in high performance liquid chromatography. Chem. Commun. 2002, 752–753. [Google Scholar]
- Jo, E.; Lim, M.C.; Kim, H.N.; Park, H.J.; Kim, Y.R.; Jeong, U. Microfluidic channels fabricated on mesoporous electrospun fiber mats: A facile route to microfluidic chips. J. Polym. Sci. Polym. Phys. 2011, 49, 89–95. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Hernandez, J.; Checot, F.; Gnanou, Y.; Lecommandoux, S. Toward “smart” nano-objects by self-assembly of block copolymers in solution. Prog. Polym. Sci. 2005, 30, 691–724. [Google Scholar] [CrossRef]
- Hamley, I.U. The Physics of Block Copolymers; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Foerster, S.; Antonietti, M. Amphiphilic block copolymers in structure-controlled nanomaterial hybrids. Adv. Mater. 1998, 10, 195–217. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pispas, S.; Floudas, G.A. Block Copolymers Synthetic Strategies, Physical Properties, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Li, J.G.; Kuo, S.W. Phase behavior of mesoporous nanostructures templated by amphiphilic crystalline–crystalline diblock copolymers of poly(ethylene oxide-b-ε-caprolactone). RSC Adv. 2011, 1, 1822–1833. [Google Scholar] [CrossRef]
- Li, J.G.; Lin, Y.D.; Kuo, S.W. From microphase separation to self-organized mesoporous phenolic resin through competitive hydrogen bonding with double crystalline diblock copolymers of poly(ethylene oxide-b-ε-caprolactone). Macromolecules 2011, 44, 9295–9309. [Google Scholar] [CrossRef]
- Gorka, J.; Zawislak, A.; Choma, J.; Jaroniec, M. KOH activation of mesoporous carbons obtained by soft-templating. Carbon 2008, 46, 1159–1161. [Google Scholar] [CrossRef]
- Song, L.; Feng, D.; Fredin, N.J.; Yager, K.G.; Jones, R.L.; Wu, Q.; Zhao, D.; Vogt, B.D. Challenges in fabrication of mesoporous carbon films with ordered cylindrical pores via phenolic oligomer self-assembly with triblock copolymers. ACS Nano 2010, 4, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Hu, X.; Hong, Y.; Guan, J.; Shen, J. Photografting of poly(hydroxylethyl acrylate) onto porous polyurethane scaffolds to improve their endothelial cell compatibility. J. Biomater. Sci. Polym. Ed. 2003, 14, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Zalusky, A.S.; Olayo-Valles, R.; Taylor, C.J.; Hillmyer, M.A. Mesoporous polystyrene monoliths. J. Am. Chem. Soc. 2001, 123, 1519–1520. [Google Scholar] [CrossRef]
- Pitet, L.M.; Amendt, M.A.; Hillmyer, M.A. Nanoporous linear polyethylene from a block polymer precursor. J. Am. Chem. Soc. 2010, 132, 8230–8231. [Google Scholar] [CrossRef] [PubMed]
- Hillmyer, M.A.; Lipic, P.M.; Hajduk, D.A.; Almdal, K.; Bates, F.S. Self-assembly and polymerization of epoxy resin-amphiphilic block copolymer nanocomposites. J. Am. Chem. Soc. 1997, 119, 2749–2750. [Google Scholar] [CrossRef]
- Lipic, P.M.; Bates, F.S.; Hillmyer, M.A. Nanostructured thermosets from self-assembled amphiphilic block copolymer/epoxy resin mixtures. J. Am. Chem. Soc. 1998, 120, 8963–8970. [Google Scholar] [CrossRef]
- Guo, Q.; Thomann, R.; Gronski, W. Phase behavior, crystallization, and hierarchical nanostructures in self-organized thermoset blends of epoxy resin and amphiphilic poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) triblock copolymers. Macromolecules 2002, 35, 3133–3144. [Google Scholar] [CrossRef]
- Meng, F.; Zheng, S.; Li, H.; Liang, Q.; Liu, T. Formation of ordered nanostructures in epoxy thermosets: A mechanism of reaction-induced microphase separation. Macromolecules 2006, 39, 5072–5080. [Google Scholar] [CrossRef]
- Muylaert, I.; Verberckmoes, A.; Decker, J.D.; Voort, P.V.D. Ordered mesoporous phenolic resins: Highly versatile and ultra stable support materials. Adv. Colloid Interface Sci. 2012, 175, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Nishiyama, N.; Egashira, Y.; Ueyama, K. Synthesis of ordered mesoporous carbons with channel structure from an organic-organic nanocomposite. Chem. Commun. 2005, 2125–2127. [Google Scholar]
- Maiez-Tribut, S.; Pascault, J.P.; Soule, E.R.; Borrajo, J.; Williams, R.J.J. Nanostructured epoxies based on the self-assembly of block copolymers: A new miscible block that can be tailored to different epoxy formulations. Macromolecules 2007, 40, 1268–1273. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, S. Reaction-induced microphase separation in epoxy thermosets containing poly(ε-caprolactone)-block-poly(n-butyl acrylate) diblock copolymer. Macromolecules 2007, 40, 2548–2558. [Google Scholar] [CrossRef]
- Ritzenthaler, S.; Court, F.; David, I.; Girard-Reydet, E.; Leibler, I.; Pascault, J.P. ABC triblock copolymers/epoxy−diamine blends. 1. keys to achieve nanostructured thermosets. Macromolecules 2002, 35, 6245–6254. [Google Scholar] [CrossRef]
- Fan, W.; Wang, L.; Zheng, S. Nanostructures in thermosetting blends of epoxy resin with polydimethylsiloxane-block-poly(ε-caprolactone)-block-polystyrene ABC triblock copolymer. Macromolecules 2009, 42, 327–336. [Google Scholar] [CrossRef]
- Meng, F.; Xu, Z.; Zheng, S. Microphase separation in thermosetting blends of epoxy resin and poly(ε-caprolactone)-block-polystyrene block copolymers. Macromolecules 2008, 41, 1411–1420. [Google Scholar] [CrossRef]
- Mijovic, J.; Shen, M.; Sy, J.W. Dynamics and morphology in nanostructured thermoset network/block copolymer blends during network formation. Macromolecules 2000, 33, 5235–5244. [Google Scholar] [CrossRef]
- Kosonen, H.; Ruokolainen, J.; Nyholm, P.; Ikkala, O. Self-organized cross-linked phenolic thermosets: Thermal and dynamic mechanical properties of novolac/block copolymer blends. Polymer 2001, 42, 9481–9486. [Google Scholar] [CrossRef]
- Kosonen, H.; Ruokolainen, J.; Nyholm, P.; Ikkala, O. Self-organized thermosets: Blends of hexamethyltetramine cured novolac with poly(2-vinylpyridine)-block-poly(isoprene). Macromolecules 2001, 34, 3046–3049. [Google Scholar] [CrossRef]
- Valkama, S.; Nykanen, A.; Kosonen, H.; Ramani, R.; Tuomisto, F.; Engelhardt, P.; ten Brinke, G.; Ikkala, O.; Ruokolainen, J. Hierarchical porosity in self-assembled polymers: Post-modification of block copolymer–phenolic resin complexes by pyrolysis allows the control of micro- and mesoporosity. Adv. Funct. Mater. 2007, 17, 183–190. [Google Scholar] [CrossRef]
- Hu, D.; Xu, Z.; Zeng, K.; Zheng, S. From self-organized novolac resins to ordered nanoporous carbons. Macromolecules 2010, 43, 2960–2969. [Google Scholar] [CrossRef]
- Li, J.G.; Chung, C.Y.; Kuo, S.W. Transformations and enhanced long-range ordering of mesoporous phenolic resin templated by poly(ethylene oxide-b-ε-caprolactone) block copolymers blended with star poly(ethylene oxide)-functionalized silsesquioxane (POSS). J. Mater. Chem. 2012, 22, 18583–18595. [Google Scholar] [CrossRef]
- Li, J.G.; Chang, Y.H.; Lin, Y.S.; Kuo, S.W. Templating amphiphilic poly(ethylene oxide-b-ε-caprolactone) diblock copolymers provides ordered mesoporous silicas with large tunable pores. RSC Adv. 2012, 2, 12973–12982. [Google Scholar] [CrossRef]
- Chu, W.C.; Li, J.G.; Kuo, S.W. From flexible to mesoporous polybenzoxazine resins templated by poly(ethylene oxide-b-ε-caprolactone) copolymer through reaction induced microphase separation mechanism. RSC Adv. 2013, 3, 6485–6498. [Google Scholar] [CrossRef]
- Li, J.G.; Chu, W.C.; Jeng, U.S.; Kuo, S.W. In situ monitoring of the reaction-induced self-assembly of phenolic resin templated by diblock copolymers. Macromol. Chem. Phys. 2013, 214, 2115–2123. [Google Scholar] [CrossRef]
- Kosonen, H.; Valkama, S.; Nykänen, A.; Toivanen, M.; ten Brinke, G.; Ruokolainen, J.; Ikkala, O. Functional porous structures based on the pyrolysis of cured templates of block copolymer and phenolic resin. Adv. Mater. 2006, 18, 201–205. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, C.; Yu, T.; Liu, F.; Zhang, F.; Wan, Y.; Zhang, L.; Wang, C.; Tu, B.; Webley, P.A.; Wang, H.; Zhao, D. Facile synthesis of hierarchically porous carbons from dual colloidal crystal/block copolymer template approach. Chem. Mater. 2007, 19, 3271–3277. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, F.; Meng, Y.; Tu, B.; Zhao, D. One-step synthesis of ordered mesoporous carbonaceous spheres by an aerosol-assisted self-assembly. Chem. Commun. 2007, 2867–2869. [Google Scholar]
- Huang, Y.; Cai, H.; Feng, D.; Gu, D.; Deng, Y.; Tu, B.; Wang, H.; Webley, P.A.; Zhao, D. One-step hydrothermal synthesis of ordered mesostructured carbonaceous monoliths with hierarchical porosities. Chem. Commun. 2008, 2641–2643. [Google Scholar]
- Huang, Y.; Yang, J.; Cai, H.; Zhai, Y.; Feng, D.; Deng, Y.; Tu, B.; Zhao, D. A curing agent method to synthesize ordered mesoporous carbons from linear novolac phenolic resin polymers. J. Mater. Chem. 2009, 19, 6536–6541. [Google Scholar] [CrossRef]
- Kosonen, H.; Ruokolainen, J.; Torkkeli, M.; Serimaa, R.; Nyholm, P.; Ikkala, O. Micro- and macrophase separation in phenolic resol resin/PEO-PPO-PEO block copolymer blends: Effect of hydrogen-bonded PEO length. Macromol. Chem. Phys. 2002, 203, 388–392. [Google Scholar] [CrossRef]
- Serman, C.J.; Xu, Y.; Painter, P.C.; Coleman, M.M. Poly(vinyl phenol)—polyether blends. Polymer 1991, 32, 516–522. [Google Scholar] [CrossRef]
- Kulinski, Z.; Piorkowska, E.; Gadzinowska, K.; Stasiak, M. Plasticization of Poly(l-lactide) with Poly(propylene glycol). Biomacromolecules 2006, 7, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, C.; Liu, H.Z.; Wang, J.; Liang, X.F.; Zheng, L.; Ma, J.H. Thermodynamic analysis of micellization in PEO–PPO–PEO block copolymer solutions from the hydrogen bonding point of view. Mol. Simul. 2006, 32, 409–418. [Google Scholar] [CrossRef]
- Kuo, S.W. Hydrogen-bonding in polymer blends. J. Polym. Res. 2008, 15, 459–486. [Google Scholar] [CrossRef]
- Kuo, S.W.; Lin, C.L.; Chang, F.C. Phase behavior and hydrogen bonding in ternary polymer blends of phenolic resin/poly(ethylene oxide)/poly(ε-caprolactone). Macromolecules 2002, 35, 278–285. [Google Scholar] [CrossRef]
- Zhang, H.; Bhagwagar, D.E.; Graf, J.F.; Paitner, P.C.; Coleman, M.M. The effect of hydrogen bonding on the phase behaviour of ternary polymer blends. Polymer 1994, 35, 5379–5397. [Google Scholar] [CrossRef]
- Chen, W.C.; Kuo, S.W.; Lu, C.H.; Chang, F.C. Self-assembly structures through competitive interactions of crystalline−amorphous diblock copolymer/homopolymer blends: Poly(ε-caprolactone-b-4-vinyl pyridine)/Poly(vinyl phenol). Macromolecules 2009, 42, 3580–3590. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.S.; Feng, J.L.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Meng, Y.; Gu, D.; Zhang, F.; Shi, Y.; Cheng, L.; Feng, D.; Wu, Z.; Chen, Z.; Wan, Y.; Stein, A.; Zhao, D. A family of highly ordered mesoporous polymer resin and carbon structures from organic-organic self-assembly. Chem. Mater. 2006, 18, 4447–4464. [Google Scholar] [CrossRef]
- Harkin, W.D.; Jura, G. Surfaces of solids. XIII. A vapor adsorption method for the determination of the area of a solid without the assumption of a molecular area, and the areas occupied by nitrogen and other molecules on the surface of a solid. J. Am. Chem. Soc. 1944, 66, 1366–1373. [Google Scholar] [CrossRef]
- Kuo, S.W.; Lin, H.C.; Huang, W.J.; Huang, C.F.; Chang, F.C. Hydrogen bonding interactions and miscibility between phenolic resin and octa(acetoxystyryl) polyhedral oligomeric silsesquioxane (AS-POSS) nanocomposites. J. Polym. Sci. Polym. Phys. 2006, 44, 673–686. [Google Scholar] [CrossRef]
- Kuo, S.W.; Lin, C.L.; Chang, F.C. The study of hydrogen bonding and miscibility in poly(vinylpyridines) with phenolic resin. Polymer 2002, 43, 3943–3949. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chu, W.-C.; Chiang, S.-F.; Li, J.-G.; Kuo, S.-W. Hydrogen Bonding-Mediated Microphase Separation during the Formation of Mesoporous Novolac-Type Phenolic Resin Templated by the Triblock Copolymer, PEO-b-PPO-b-PEO. Materials 2013, 6, 5077-5093. https://doi.org/10.3390/ma6115077
Chu W-C, Chiang S-F, Li J-G, Kuo S-W. Hydrogen Bonding-Mediated Microphase Separation during the Formation of Mesoporous Novolac-Type Phenolic Resin Templated by the Triblock Copolymer, PEO-b-PPO-b-PEO. Materials. 2013; 6(11):5077-5093. https://doi.org/10.3390/ma6115077
Chicago/Turabian StyleChu, Wei-Cheng, Shih-Fan Chiang, Jheng-Guang Li, and Shiao-Wei Kuo. 2013. "Hydrogen Bonding-Mediated Microphase Separation during the Formation of Mesoporous Novolac-Type Phenolic Resin Templated by the Triblock Copolymer, PEO-b-PPO-b-PEO" Materials 6, no. 11: 5077-5093. https://doi.org/10.3390/ma6115077




