Vanadium Pentoxide-Based Composite Synthesized Using Microwave Water Plasma for Cathode Material in Rechargeable Magnesium Batteries
Abstract
:1. Introduction
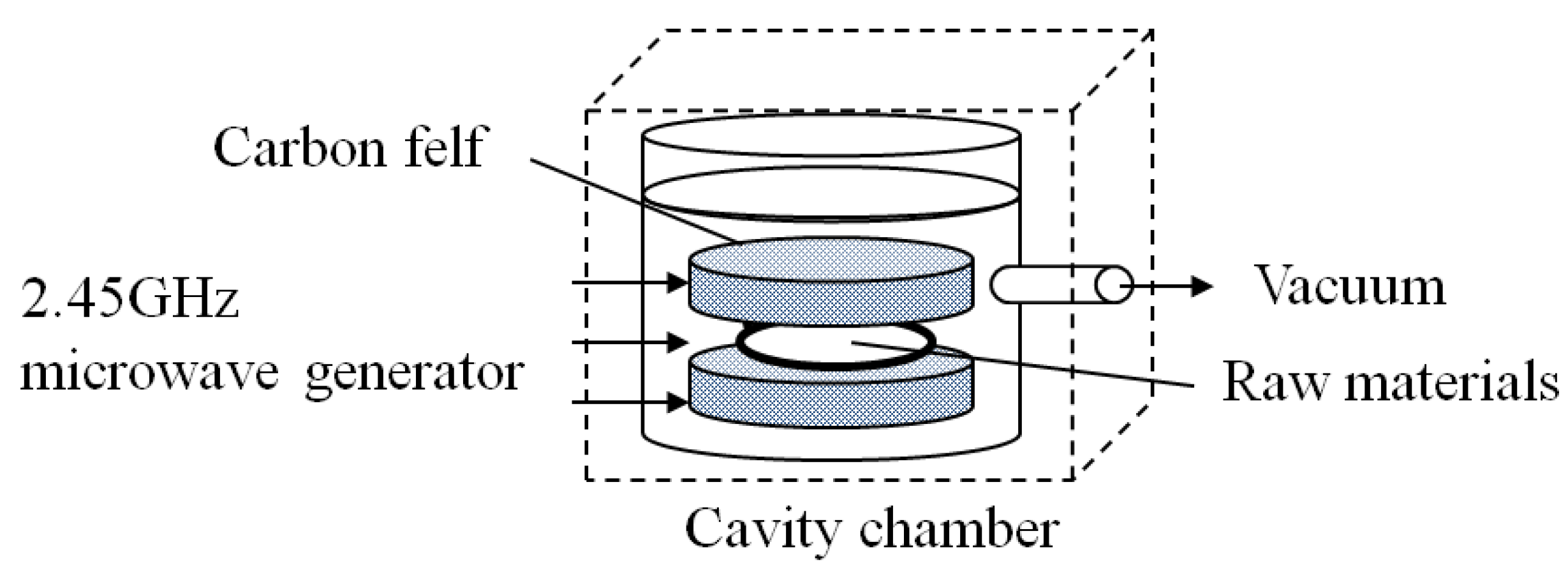

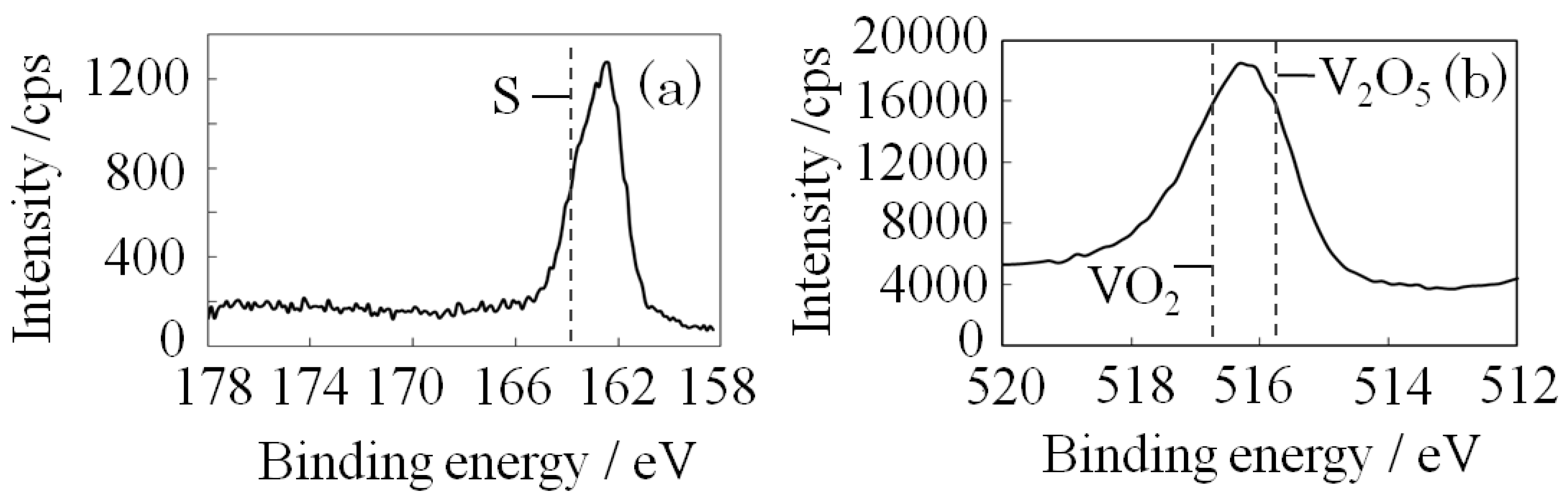
2. Results and Discussion
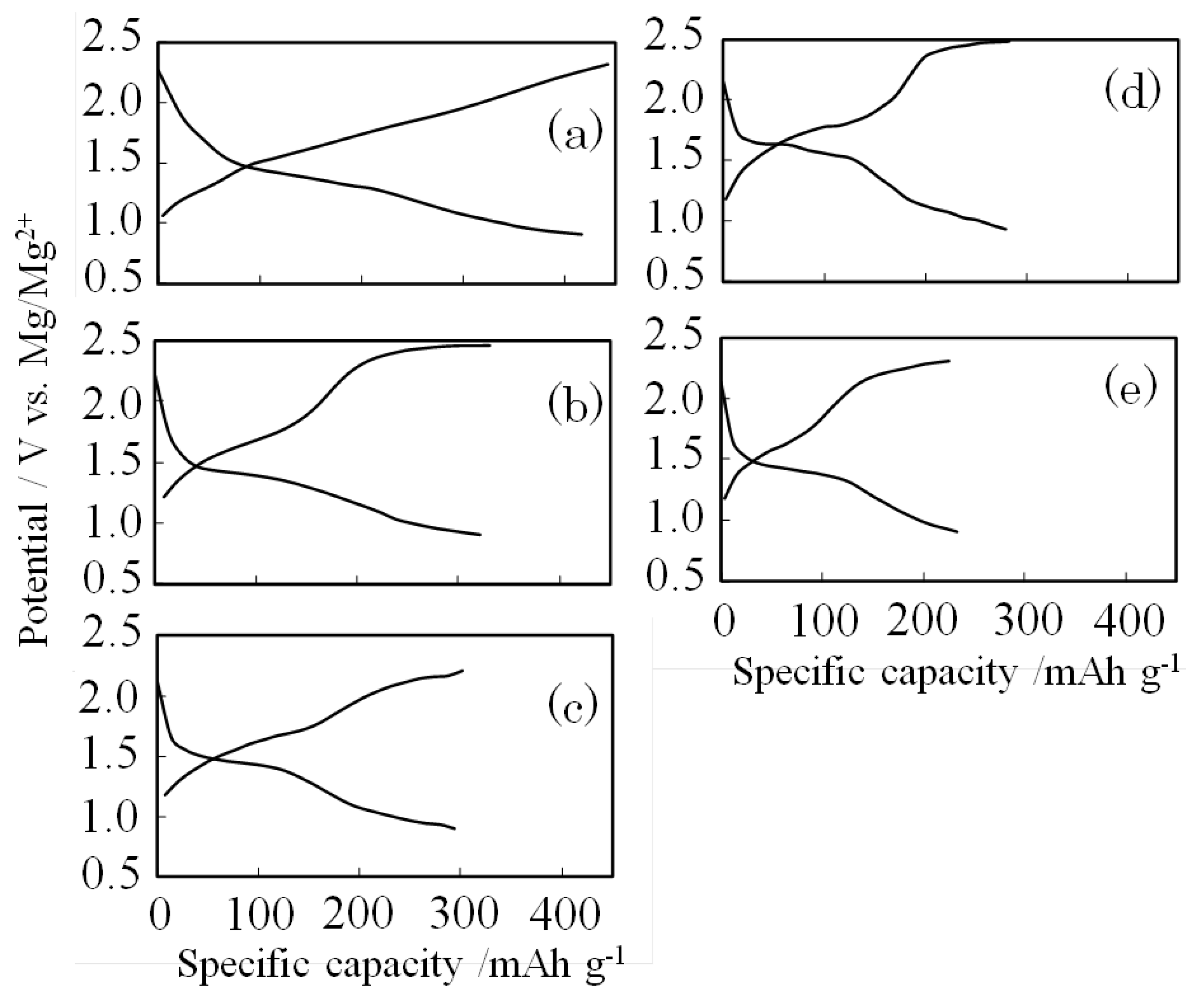
2.1. Electrochemical Characteristics
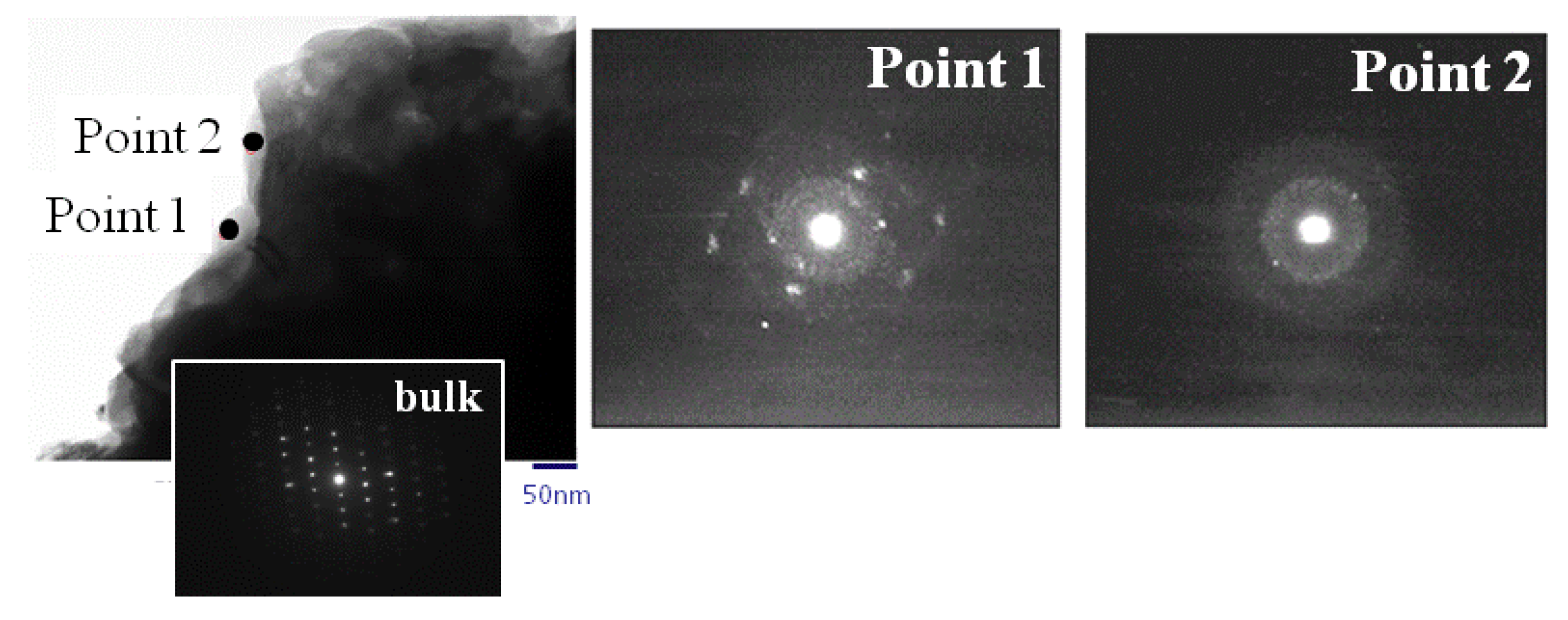
2.2. Structural Analysis
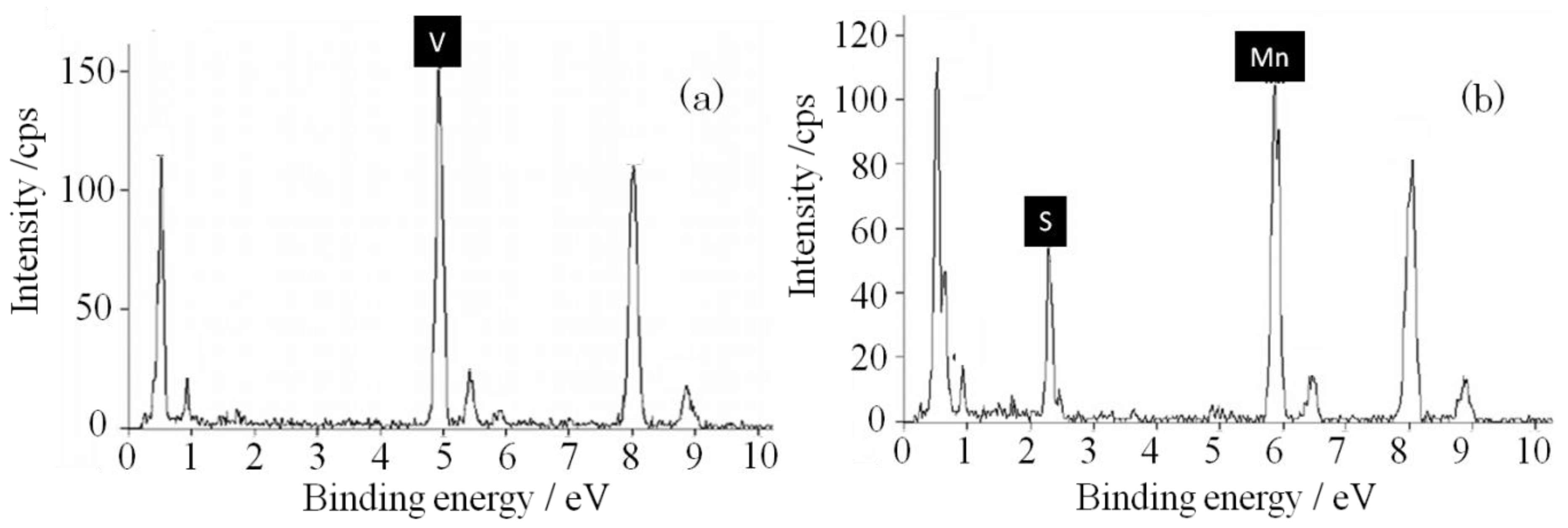

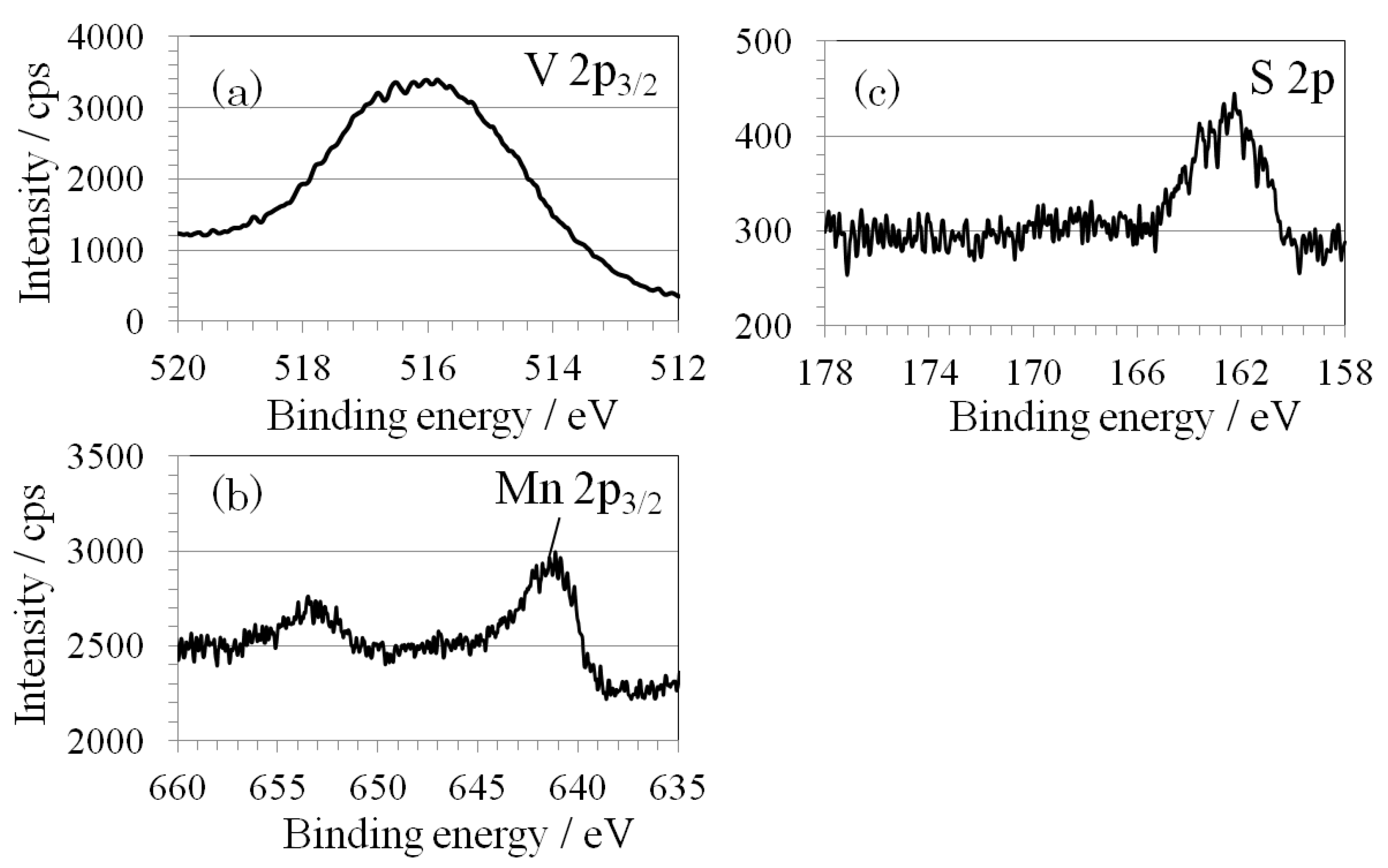
3. Experimental Section
3.1. Preparation of Cathode Material by CF-MWP
3.2. Electrochemical Characteristics
3.3. Structural Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Aurbach, D.; Lu, Z.; Schechter, A.; Gofer, Y.; Gizbar, H.; Turgeman, R.; Cohen, Y.; Moshkovich, M.; Levi, E. Prototype systems for rechargeable magnesium batteries. Nature 2000, 407, 724–727. [Google Scholar] [PubMed]
- Aurbach, D.; Cohen, Y.; Moshkovich, M. The study of reversible magnesium deposition by in situ scanning tunneling microscopy. Electrochem. Solid State Lett. 2001, 4, A113–A116. [Google Scholar] [CrossRef]
- Novák, P.; Scheifele, W.; Haas, O. Magnesium insertion batteries—An alternative to lithium? J. Power Sources 1995, 54, 479–482. [Google Scholar] [CrossRef]
- Novák, P.; Scheifele, W.; Joho, F.; Haas, O. Electrochemical insertion of magnesium into hydrated vanadium bronzes. J. Electrochem. Soc. 1995, 142, 2544–2550. [Google Scholar] [CrossRef]
- Novák, P.; Desilvestro, J. Electrochemical insertion of magnesium in metal oxides and sulfides from aprotic electrolytes. J. Electrochem. Soc. 1993, 140, 140–144. [Google Scholar] [CrossRef]
- Feng, Z.; Yang, J.; NuLi, Y.; Wang, J. Sol–gel synthesis of Mg1.03Mn0.97SiO4 and its electrochemical intercalation behavior. J. Power Sources 2008, 184, 604–609. [Google Scholar] [CrossRef]
- Tao, Z.L.; Xu, L.N.; Gou, X.L.; Chen, J.; Yuan, H.T. TiS2 nanotubes as the cathode materials of Mg-ion batteries. Chem. Commun. 2004, 18, 2080–2081. [Google Scholar] [CrossRef]
- Mitelman, A.; Levi, M.D.; Lancry, E.; Levi, E.; Aurbach, D. New cathode materials for rechargeable Mg batteries: Fast Mg ion transport and reversible copper extrusion in CuMo6S8 compounds. Chem. Commun. 2007, 41, 4212–4214. [Google Scholar] [CrossRef]
- Inamoto, M.; Kurihara, H.; Yajima, T. Electrode performance of S-doped vanadium pentoxide as cathode active material for rechargeable magnesium battery. Hyomen Gijutsu 2011, 62, 516–520. [Google Scholar]
- Kurihara, H.; Yajima, T. Decomposition of toluene by atmospheric pressure microwave plasma generated using metal salt-impregnated carbon felt pieces. Chem. Lett. 2007, 36, 526–527. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, X. Electrochemical insertion of magnesium ions into V2O5 from aprotic electrolytes with varied water content. J. Colloid Interface Sci. 2004, 278, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Imamura, D.; Miyayama, M. Characterization of magnesium-intercalated V2O5/carbon composites. Solid State Ion. 2003, 161, 173–180. [Google Scholar] [CrossRef]
- Baddour-Hadjean, R.; Smirnov, M.B.; Smirnov, K.S.; Kazimirov, V.Y.; Gallardo-Amores, J.M.; Amador, U.; Arroyo-de Dompablo, M.E.; Pereira-Ramos, J.P. Lattice dynamics of β-V2O5: Raman spectroscopic insight into the atomistic structure of a high-pressure vanadium pentoxide polymorph. Inorg. Chem. 2012, 51, 3194–3201. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Inamoto, M.; Kurihara, H.; Yajima, T. Vanadium Pentoxide-Based Composite Synthesized Using Microwave Water Plasma for Cathode Material in Rechargeable Magnesium Batteries. Materials 2013, 6, 4514-4522. https://doi.org/10.3390/ma6104514
Inamoto M, Kurihara H, Yajima T. Vanadium Pentoxide-Based Composite Synthesized Using Microwave Water Plasma for Cathode Material in Rechargeable Magnesium Batteries. Materials. 2013; 6(10):4514-4522. https://doi.org/10.3390/ma6104514
Chicago/Turabian StyleInamoto, Masashi, Hideki Kurihara, and Tatsuhiko Yajima. 2013. "Vanadium Pentoxide-Based Composite Synthesized Using Microwave Water Plasma for Cathode Material in Rechargeable Magnesium Batteries" Materials 6, no. 10: 4514-4522. https://doi.org/10.3390/ma6104514



