Horizontal Bilayer for Electrical and Optical Recordings
Abstract
:1. Introduction
- i
- Supported bilayers spread on the surface of various supporting substrates like glass, mica or Si/SiO2.
- ii
- Free-standing bilayer which is spread across a micro-aperture of 30–200 µm within a thin (≈12.5–50 µm) PTFE-septum or other types of suited polymer films.
2. Results and Discussion
2.1. Microchip Setup—Geometric Features, Materials of the Chip, Fabrication of the Aperture Hole & Chip Assembly
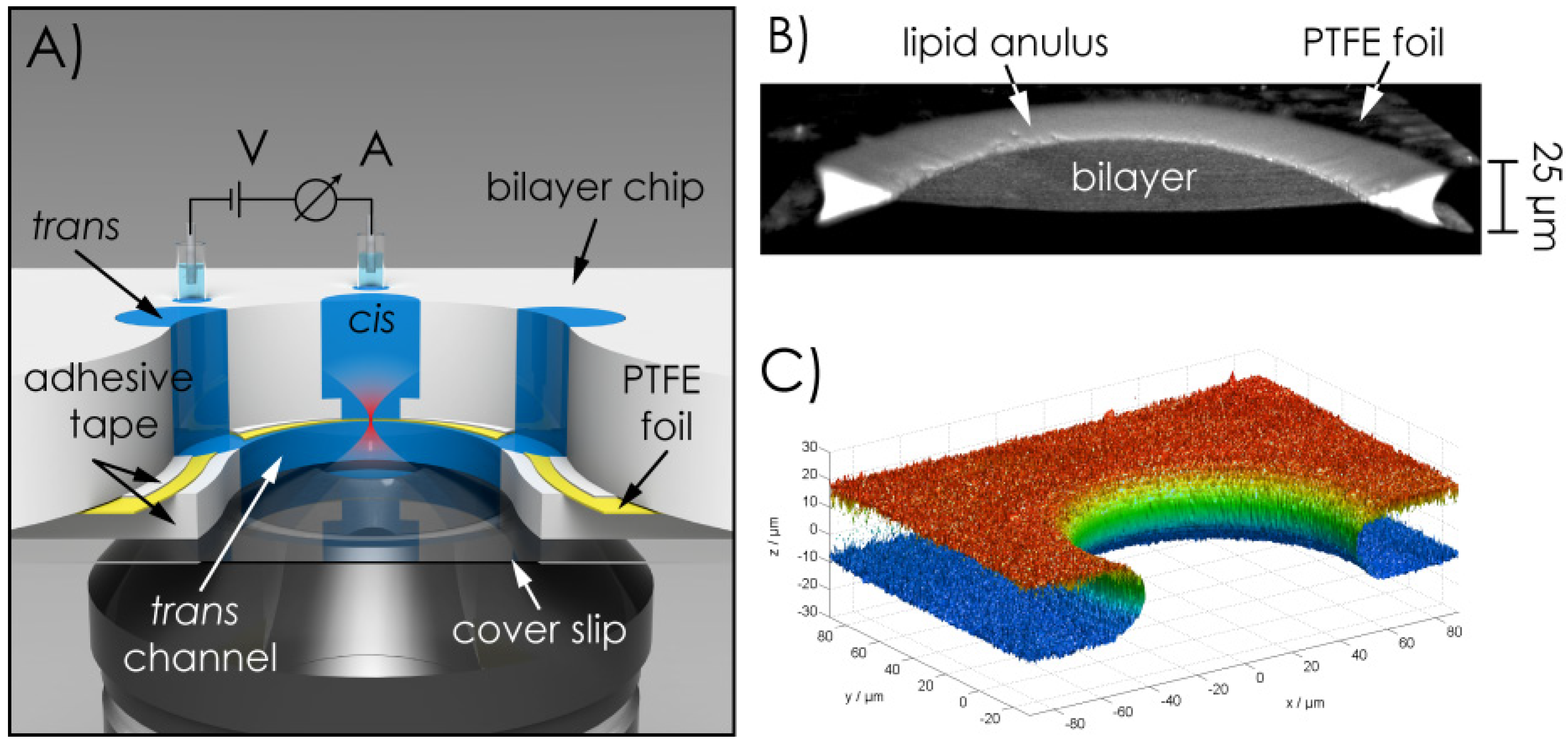
2.2. Bilayer Fabrication and Stability
2.2.1. Painting and Other Techniques
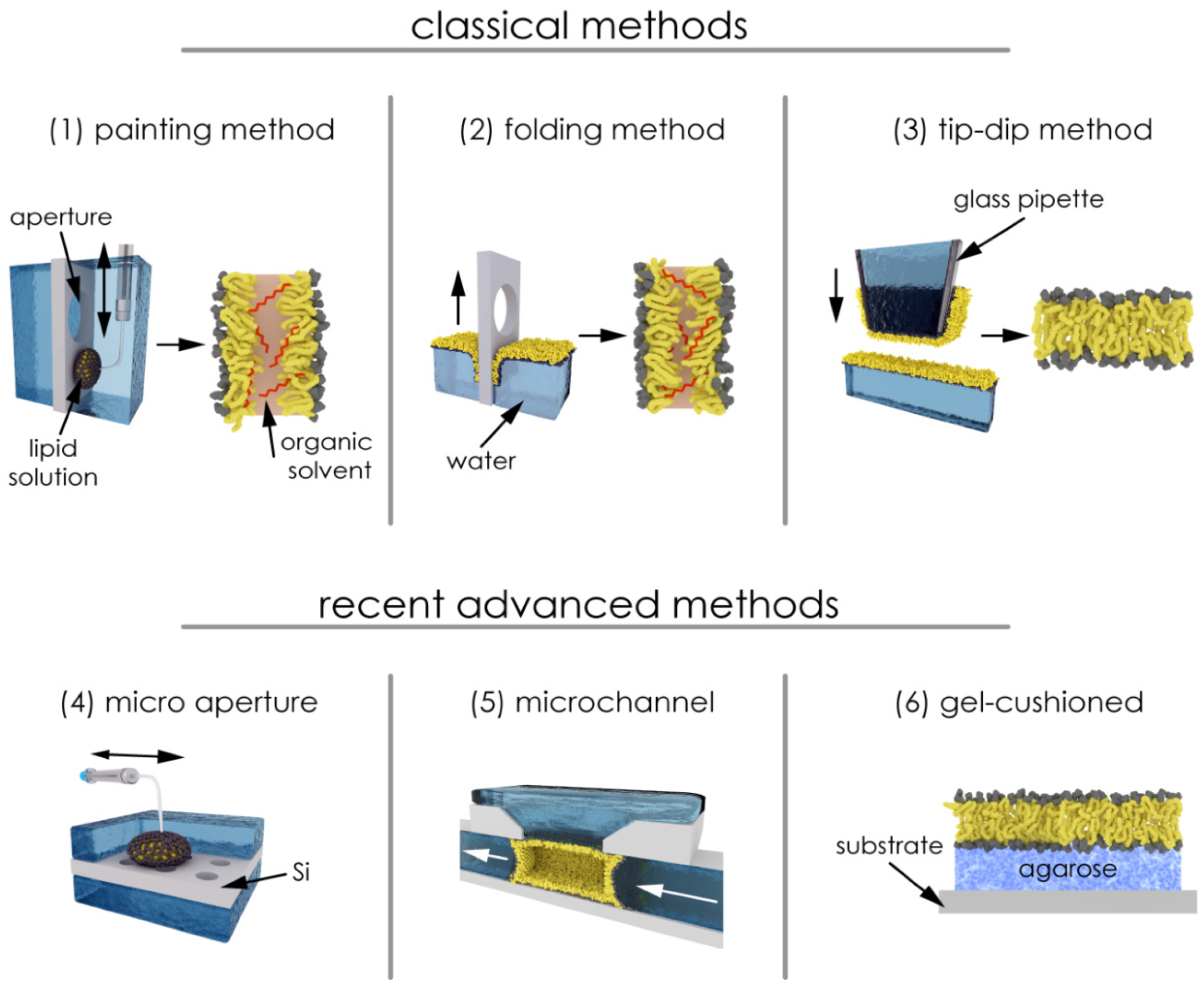
2.2.2. Solvent and Residual Solvent: Effects on the Bilayer Physical State
| Name | Composition | Type | References |
|---|---|---|---|
| Azolectin (Type IV-S, ≥30%) | PC (40%), PE (33%), PI (14%), lyso-PC (5%), CA (4%) | extract | [32] |
| E. coli Total Lipid Extract | PE (57,5%), unknown (17,6%), PG (15,1%), CA (9,8%) | extract | Avanti Polar Lipids, Inc. (Alabaster, AL, USA) |
| E. coli Polar Lipid Extract | PE (67%), PG (23,2%), CA (9,8%) | [14] | |
| DOPC:DOPE (70%:30%) | synthetic | [33] | |
| DOPC:DMPC:CO (1:3:1) | synthetic | ||
| DOPC:DSPC:CO (2–1:1:1) | synthetic | [28] | |
| Outer mitochondrial membrane | PC (51%), PE (35%), PI (9%), CA (4%), PS (1%) | synthetic | [34] |
| Inner mitochondrial membrane | PC (42%), PE (38%), CA (17%), PI (2%), PS (1%) | synthetic | [34,35] |
2.2.3. Contaminations Impeding Electrical and Optical Single-Molecule Measurements

2.2.4. Polymer-Supported Bilayer
2.3. Bilayer Optical Recording
2.3.1. Single-Molecule Fluorescence Recordings from Lipid Bilayer Membranes
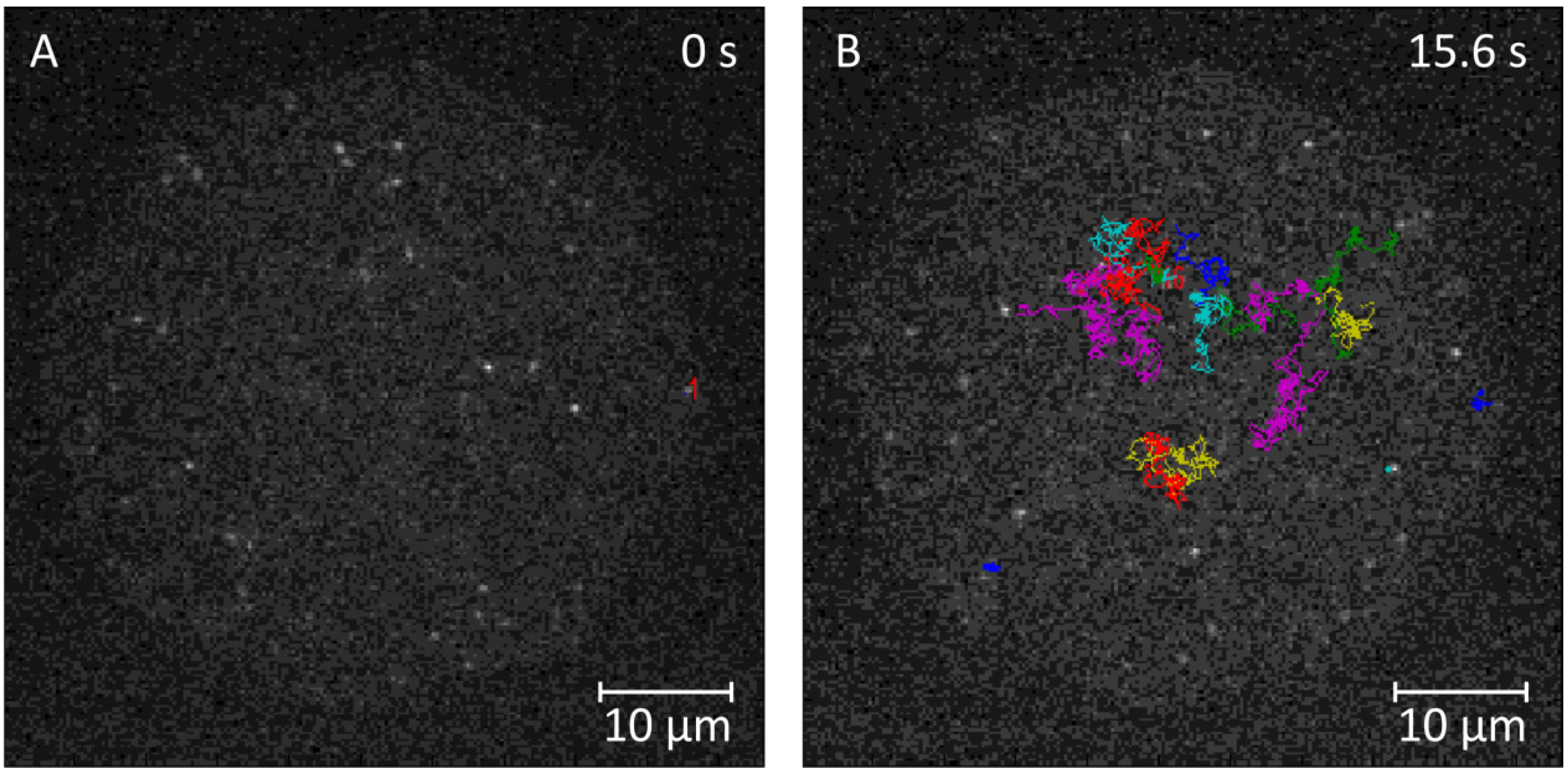
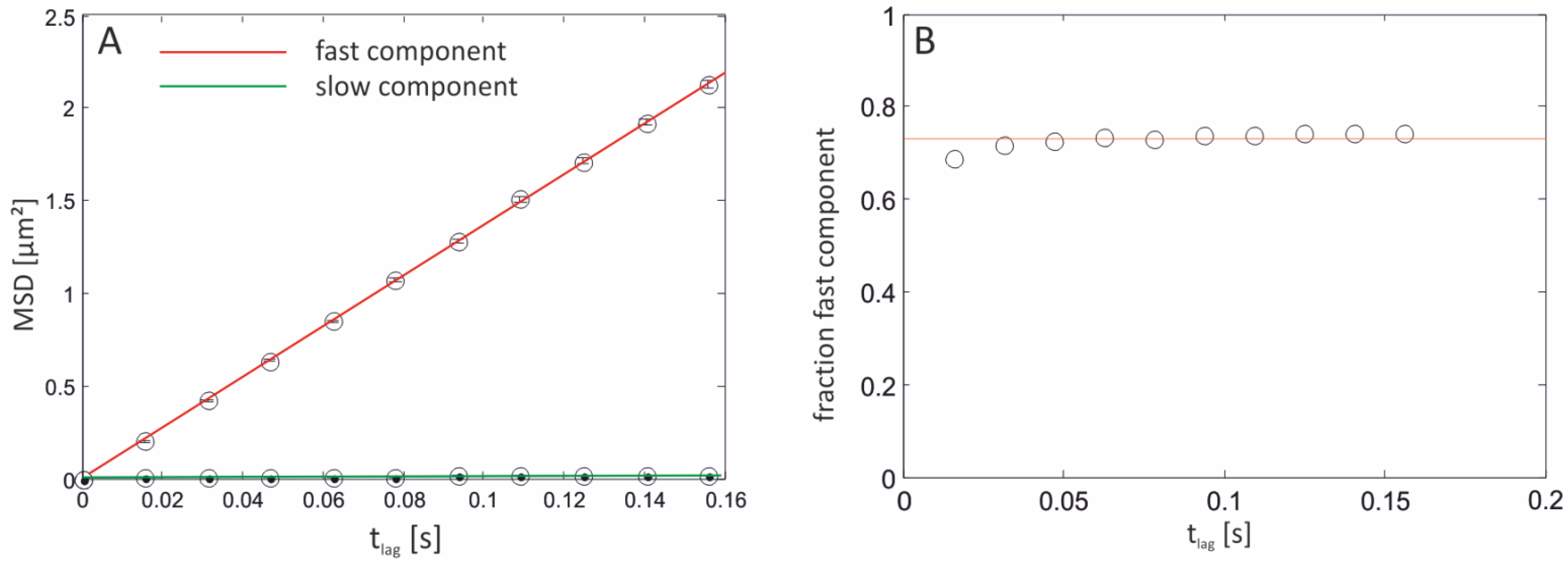
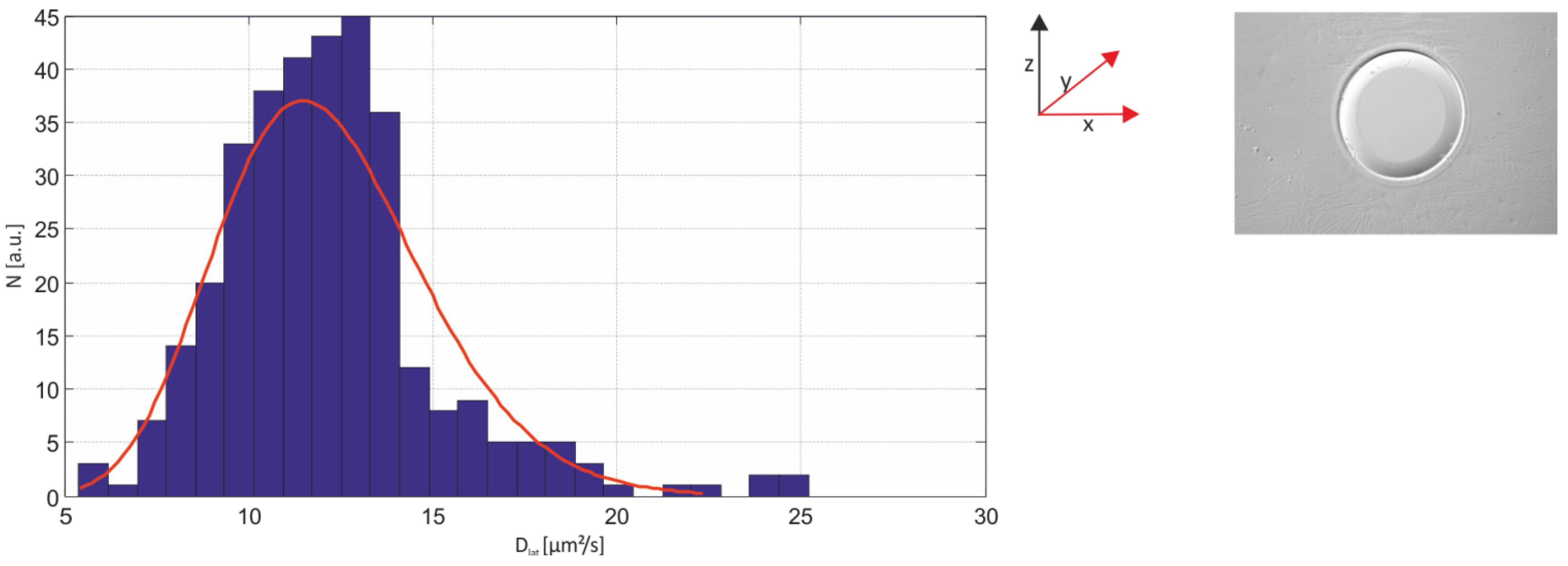
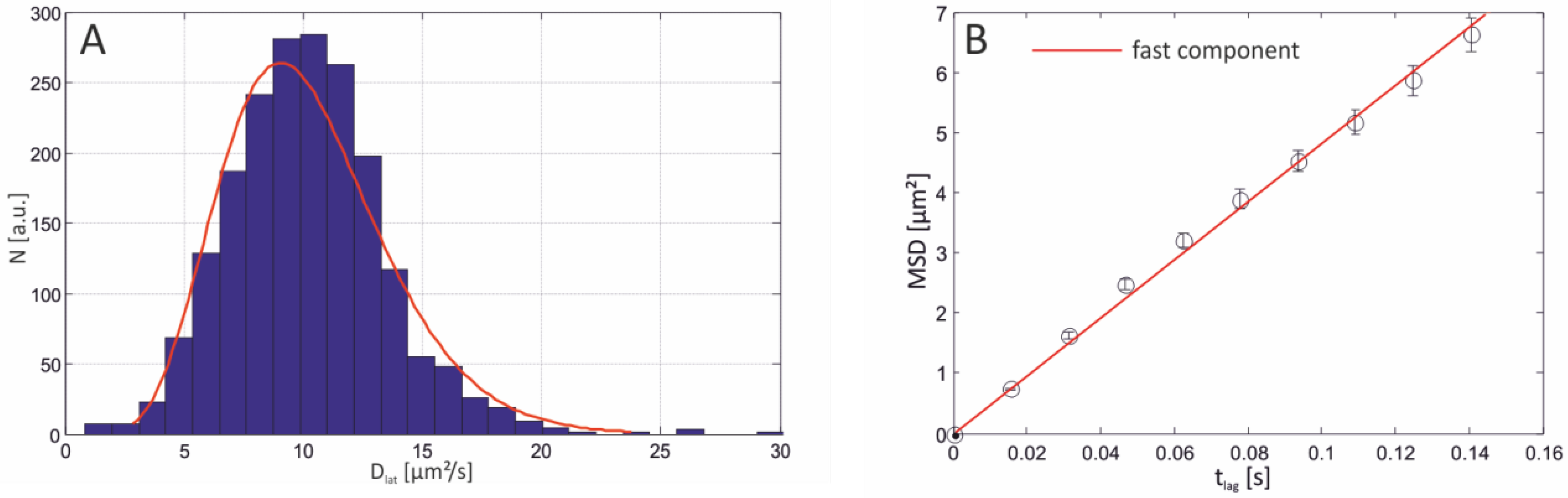
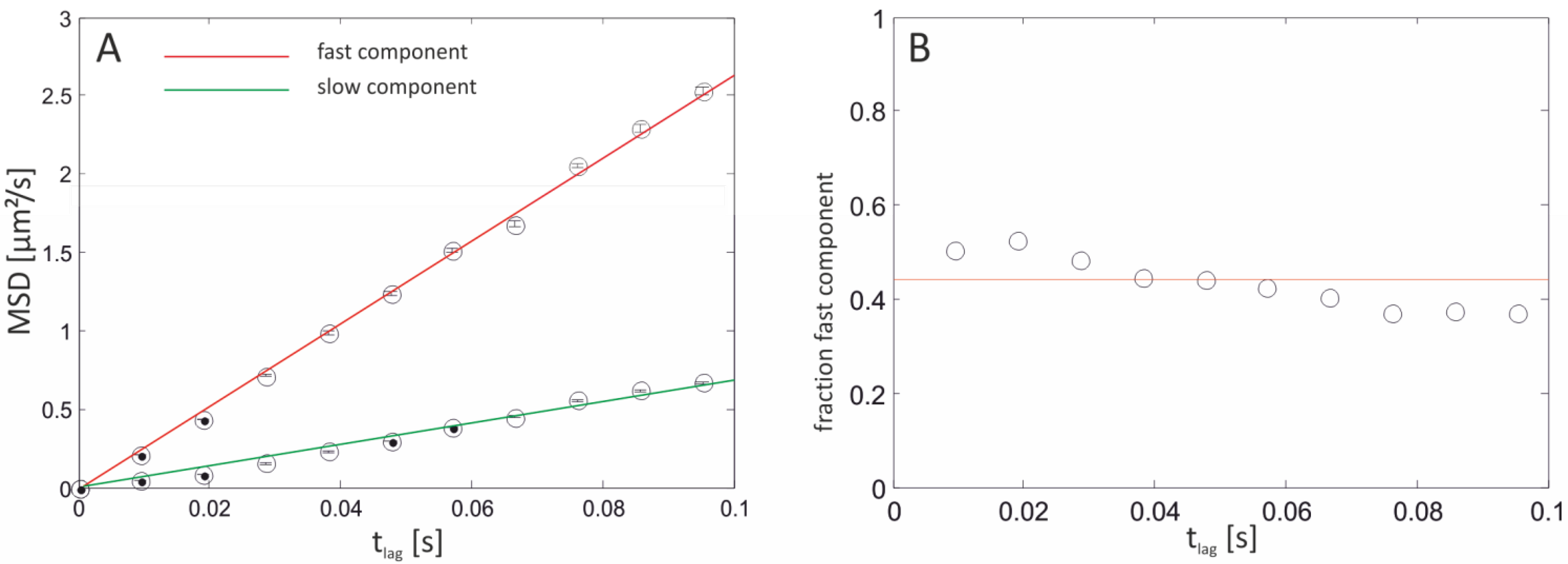
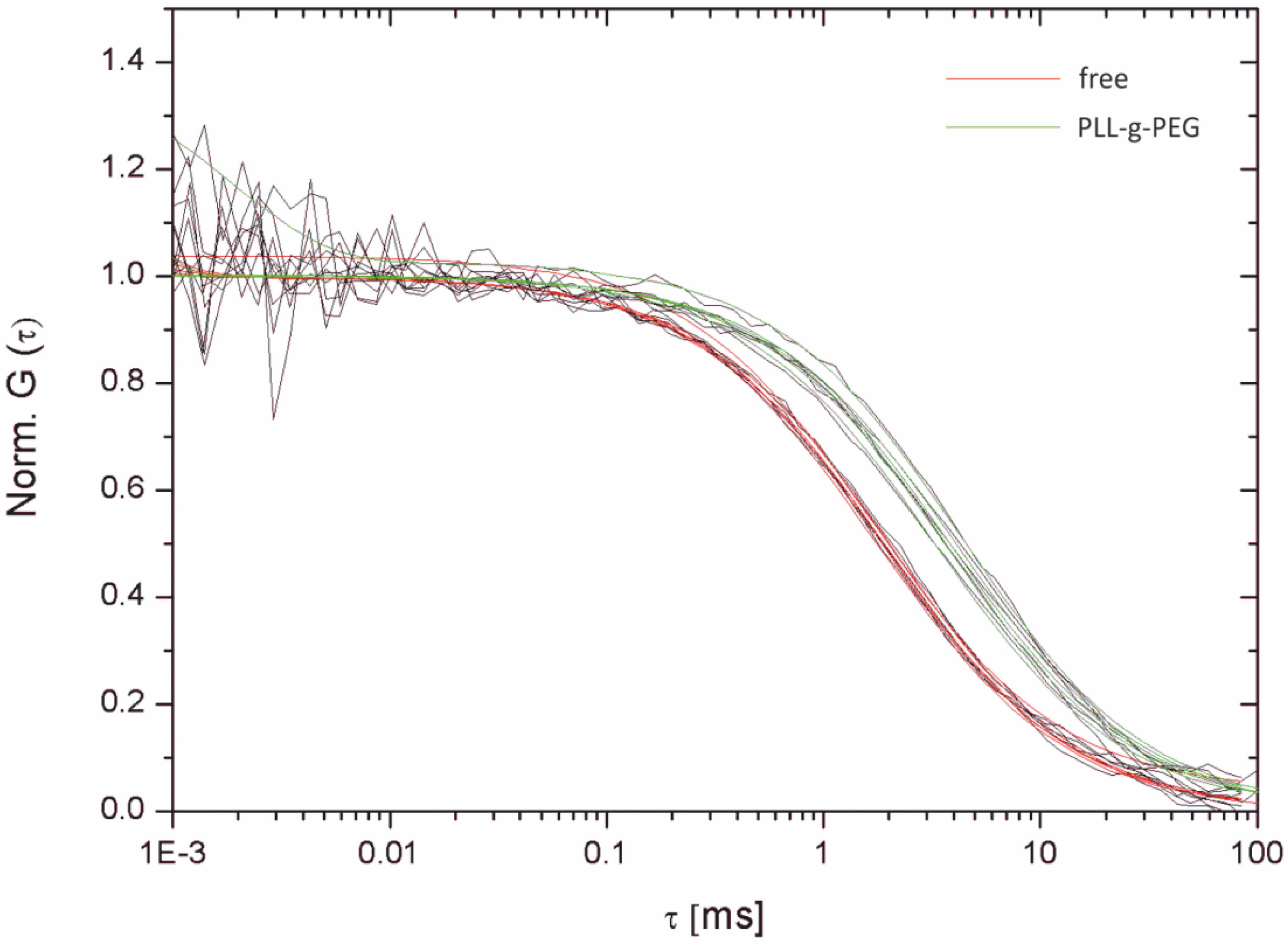
| Bilayer system | Dfast (µm2/s) | Dslow (µm2/s) | Fractionfast |
|---|---|---|---|
| Free-standing | 12.2 ± 2.07 | – | 1 |
| Free-Standing (FCS) | 13.51 ± 1.04 | ||
| Agarose-supported | 10.22 ± 1.86 | – | 1 |
| PLL-g-PEG-supported | 6.58 ± 0.129 | 1.718 ± 0.091 | 0.44 ± 0.08 |
| PLL-g-PEG-supported (FCS) | 6.96 ± 1.1 | – | 1 |
| Glass-supported | 3.42 ± 0.014 | 0.028 ± 0.004 | 0.73 ± 0.02 |
2.3.2. Fluorescence Anisotropy—Lipid Mixtures and Phases
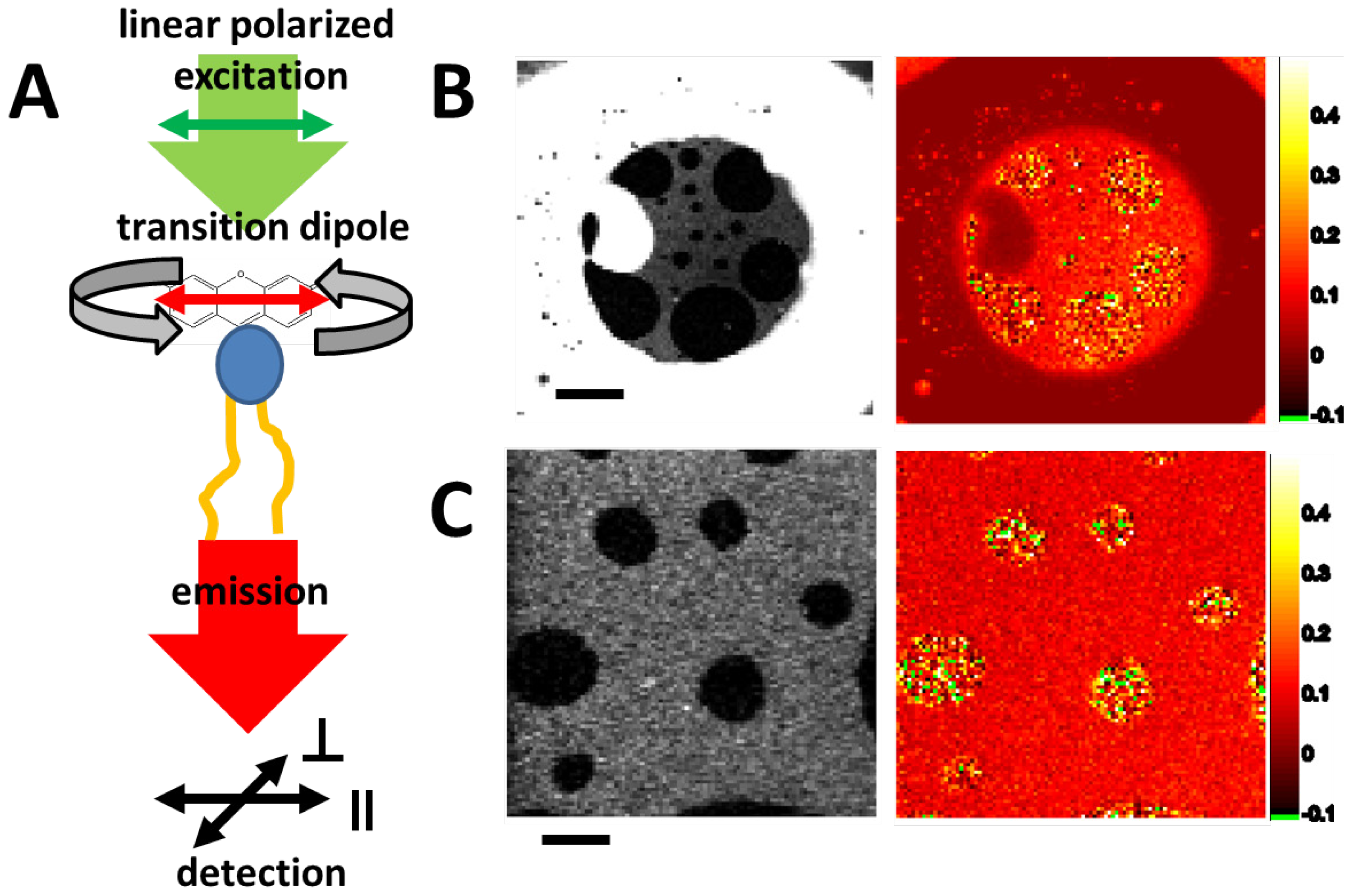
2.3.3. Combined Optical and Electrical Recording from a Horizontal Bilayer—Membrane-Integration of a Pore Forming Toxin

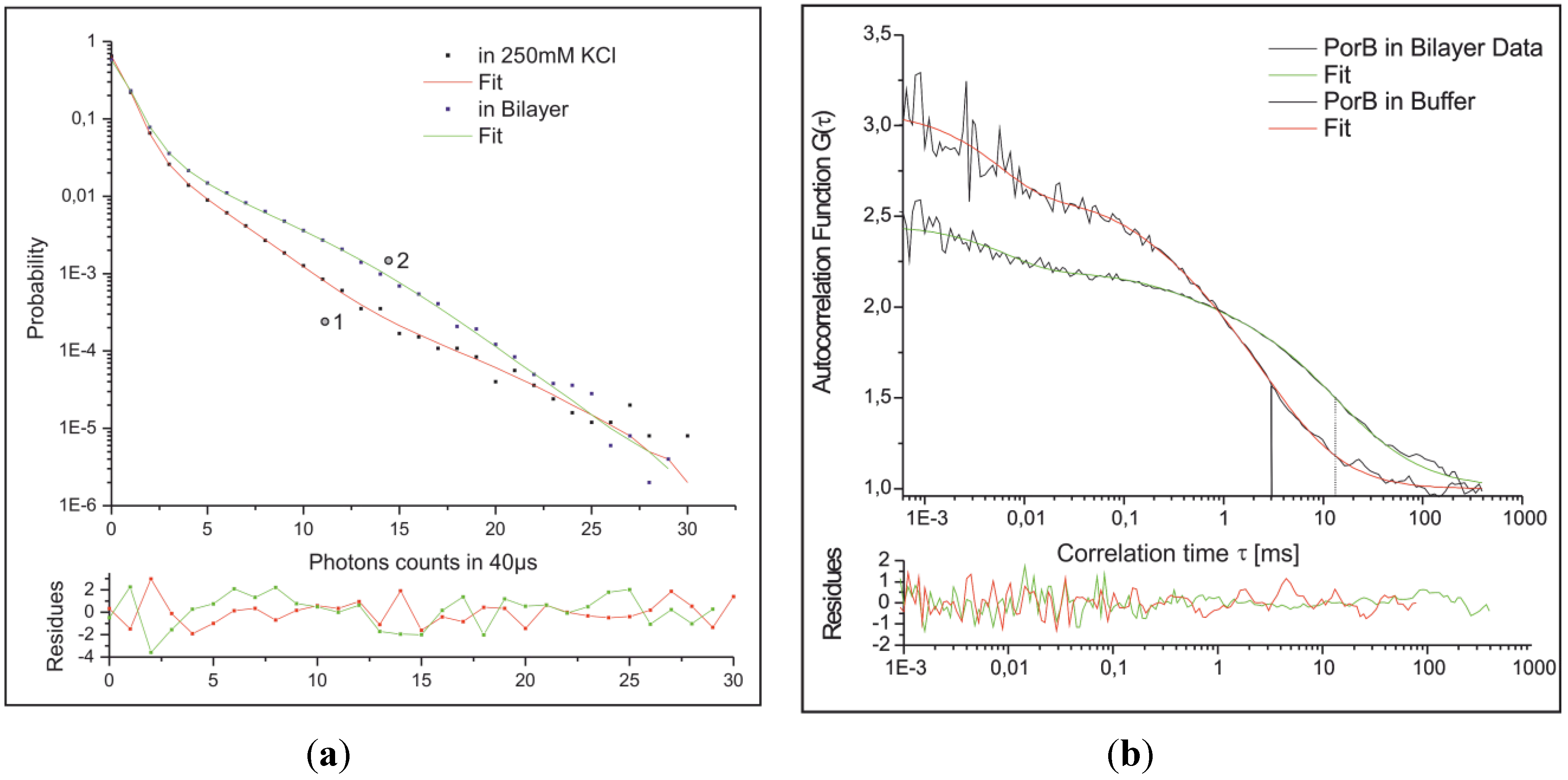
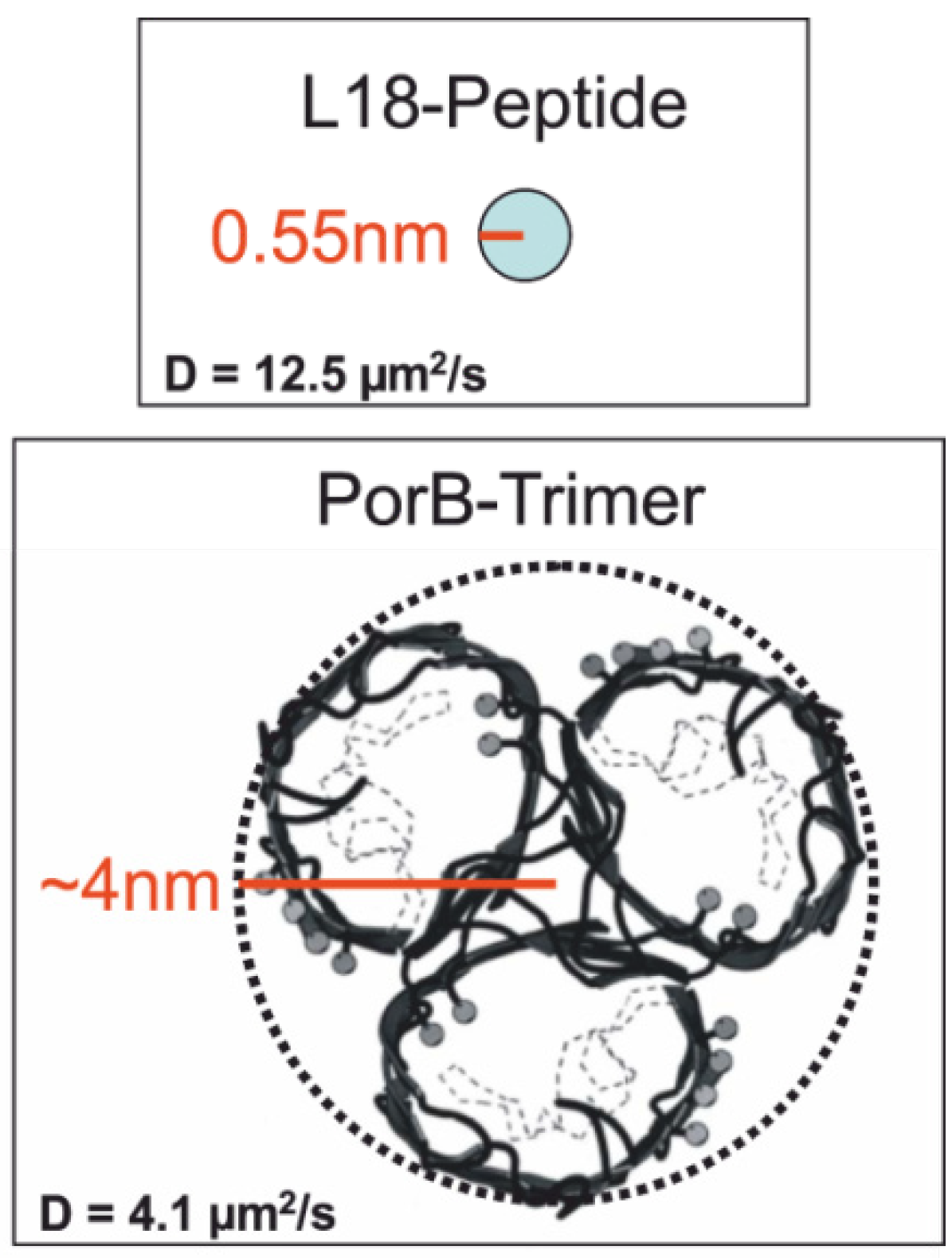
2.3.4. Electrical Recording from the PorB Containing Horizontal Bilayer

3. Experimental Section
3.1. Chemicals
3.2. Imaging
3.3. The Electro-Mechanical Micro-Hole Generator (EMMHG)
3.4. Bilayers
3.5. Fluorescence Labeling of PorB
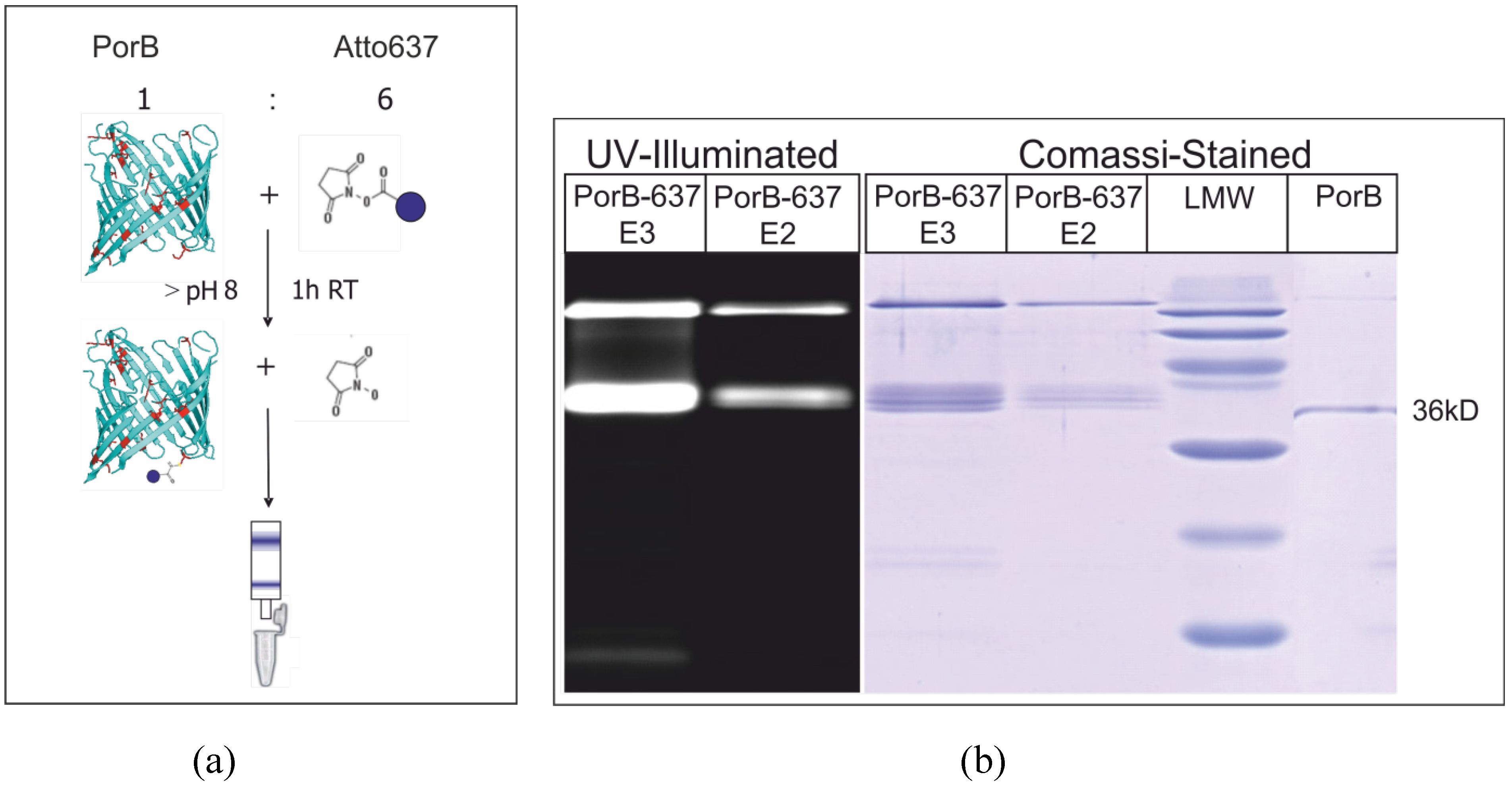
3.6. Electrical Recordings
4. Conclusions
Abbreviations
| FCS | Fluorescence Correlation Spectroscopy |
| FIDA | Fluorescence Intensity Distribution Analysis |
| FLT | Fluorescence Lifetime |
Acknowledgments
Appendix
References
- Zagnoni, M. Miniaturised technologies for the development of artificial lipid bilayer systems. Lab Chip 2012, 12, 1026–1039. [Google Scholar] [CrossRef] [PubMed]
- Trojanowicz, M.T. Miniaturized biochemical sensing devices based on planar bilayer lipid membranes. Fresenius J. Anal. Chem. 2001, 371, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Borisenko, V.; Lougheed, T.; Hesse, J.; Fureder-Kitzmuller, E.; Fertig, N.; Behrends, J.C.; Woolley, G.A.; Schutz, G.J. Simultaneous optical and electrical recording of single gramicidin channels. Biophys. J. 2003, 84, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Honigmann, A.; Pulagam, L.P.; Sippach, M.; Bartsch, P.; Steinhoff, H.J.; Wagner, R. A high resolution electro-optical approach for investigating transition of soluble proteins to integral membrane proteins probed by colicin A. Biochem. Biophys. Res. Commun. 2012, 427, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, M.; Iwamoto, M.; Kato, A.; Yoshikawa, K.; Oiki, S. Oriented reconstitution of a membrane protein in a giant unilamellar vesicle: experimental verification with the potassium channel KcsA. J. Am. Chem. Soc. 2011, 133, 11774–11779. [Google Scholar] [CrossRef] [PubMed]
- Doeven, M.K.; Folgering, J.H.; Krasnikov, V.; Geertsma, E.R.; van den Bogaart, G.; Poolman, B. Distribution, lateral mobility and function of membrane proteins incorporated into giant unilamellar vesicles. Biophys. J. 2005, 88, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Weiss, D.S. Site-specific fluorescence reveals distinct structural changes with GABA receptor activation and antagonism. Nat. Neurosci. 2002, 5, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.; Dempski, R.E. Examining the conformational dynamics of membrane proteins in situ with site-directed fluorescence labeling. J. Vis. Exp. 2011. [Google Scholar] [CrossRef]
- Demuro, A.; Parker, I. Imaging the activity and localization of single voltage-gated Ca2+ channels by total internal reflection fluorescence microscopy. Biophys. J. 2004, 86, 3250–3259. [Google Scholar] [CrossRef] [PubMed]
- Villar, G.; Heron, A.J.; Bayley, H. Formation of droplet networks that function in aqueous environments. Nat. Nano 2011, 6, 803–808. [Google Scholar] [CrossRef]
- Ide, T.; Yanagida, T. An artificial lipid bilayer formed on an agarose-coated glass for simultaneous electrical and optical measurement of single ion channels. Biochem. Biophys. Res. Commun. 1999, 265, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Ichikawa, T. A novel method for artificial lipid-bilayer formation. Biosens. Bioelectron. 2005, 21, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Schütz, G.J.; Baumgartner, W.; Gruber, H.J.; Schindler, H. Imaging of single molecule diffusion. Proc. Natl. Acad. Sci. USA 1996, 93, 2926–2929. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, R.; Krüger, V.; Meinecke, M.; Schulz, C.; Schmidt, B.; Grunau, S.D.; Guiard, B.; Wiedemann, N.; van der Laan, M.; Wagner, R.; Rehling, P.; Dudek, J. The channel-forming Sym1 protein is transported by the TIM23 complex in a presequence-independent manner. Mol. Cell. Biol. 2012, 32, 5009–5021. [Google Scholar] [CrossRef] [PubMed]
- Mueller, P.; Rudin, D.O.; Tien, H.T.; Wescott, W.C. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature 1962, 194, 979–980. [Google Scholar] [CrossRef] [PubMed]
- Wonderlin, W.F.; French, R.J.; Arispe, N.J. Recording and analysis of currents from single ion channels. In Neurophysiological Techniques; Humana Press: Totowa, NJ, USA, 1990; Volume 14, pp. 35–142. [Google Scholar]
- Salafsky, J.; Groves, J.T.; Boxer, S.G. Architecture and function of membrane proteins in planar supported bilayers: A study with photosynthetic reaction centers. Biochemistry 1996, 35, 14773–14781. [Google Scholar] [CrossRef] [PubMed]
- Harms, G.S.; Sonnleitner, M.; Schütz, G.J.; Gruber, H.J.; Schmidt, T. Single-molecule anisotropy imaging. Biophys. J. 1999, 77, 2864–2870. [Google Scholar] [CrossRef] [PubMed]
- Harsman, A.; Bartsch, P.; Hemmis, B.; Kruger, V.; Wagner, R. Exploring protein import pores of cellular organelles at the single molecule level using the planar lipid bilayer technique. Eur. J. Cell Biol. 2011, 90, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Harsman, A.; Krüger, V.; Bartsch, P.; Honigmann, A.; Schmidt, O.; Meisinger, C.; Wagner, R. Protein conducting nanopores. J. Phys. Condens. Matter 2010, 22, 454102–454122. [Google Scholar] [CrossRef] [PubMed]
- White, S.H. Analysis of the torus surrounding planar lipid bilayer membranes. Biophys. J. 1972, 12, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Mueller, P.; Rudin, D.O.; Tien, R.T.; Westcott, W.C. Reconstitution of excitable cell membrane structure in vitro. Circ. Res. 1962, 26, 1167–1171. [Google Scholar] [CrossRef]
- Montal, M.; Mueller, P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. USA 1972, 69, 3561–3566. [Google Scholar] [CrossRef] [PubMed]
- Manoli, I.; Alesci, S.; Blackman, M.R.; Su, Y.A.; Rennert, O.M.; Chrousos, G.P. Mitochondria as key components of the stress response. Trends Endocrinol. Metab. 2007, 18, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Mancusso, R.; Gregorio, G.G.; Liu, Q.; Wang, D.-N. Structure and mechanism of a bacterial sodium-dependent dicarboxylate transporter. Nature 2012, 491, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Hirano-Iwata, A.; Oshima, A.; Kimura, Y.; Niwano, M. Chapter three—Stable and reproducible bilayer lipid membranes based on silicon microfabrication techniques. In Advances in Planar Lipid Bilayers and Liposomes; Aleš, I., Ed.; Academic Press: New York, NY, USA, 2010; Volume 11, pp. 71–86. [Google Scholar]
- White, S.H.; Petersen, D.C.; Simon, S.; Yafuso, M. Formation of planar bilayer membranes from lipid monolayers. A critique. Biophys. J. 1976, 16, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Honigmann, A.; Walter, C.; Erdmann, F.; Eggeling, C.; Wagner, R. Characterization of horizontal lipid bilayers as a model system to study lipid phase separation. Biophys. J. 2010, 98, 2886–2894. [Google Scholar] [CrossRef] [PubMed]
- White, S.H. Thickness changes in lipid bilayer membranes. Biochim. Biophys. Acta 1970, 196, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Fahey, P.F.; Koppel, D.E.; Barak, L.S.; Wolf, D.E.; Elson, E.L.; Webb, W.W. Lateral diffusion in planar lipid bilayers. Science 1977, 195, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Gennis, R.B. Biomembranes Moleculare Structure and Function; Springer Verlag: New York, NY, USA, 1989. [Google Scholar]
- Miller, C.; Racker, E. Fusion of phospholipid vesicles reconstituted with cytochrome c oxidase and mitochondrial hydrophobic protein. J. Membr. Biol. 1976, 26, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Meinecke, M.; Cizmowski, C.; Schliebs, W.; Kruger, V.; Beck, S.; Wagner, R.; Erdmann, R. The peroxisomal importomer constitutes a large and highly dynamic pore. Nat. Cell Biol. 2010, 12, 273–277. [Google Scholar] [PubMed]
- Voelker, D. Lipid synthesis and transport in mitochondrial biogenesis. In Mitochondrial Function and Biogenesis; Springer: Heidelberg, Berlin, 2004; Volume 8, pp. 267–291. [Google Scholar]
- Krueger, V.; Deckers, M.; Hildenbeutel, M.; van der Laan, M.; Hellmers, M.; Drekers, C.; Preuss, M.; Herrmann, J.M.; Rehling, P.; Wagner, R.; Meinecke, M. The mitochondrial oxidase-assembly-protein1 (Oxa1) insertase forms a membrane pore in lipid bilayers. J. Biol. Chem. 2012, 32, 5009–5021. [Google Scholar]
- Becker, W.; Bergmann, A.; Haustein, E.; Petrasek, Z.; Schwille, P.; Biskup, C.; Kelbauskas, L.; Benndorf, K.; Klocker, N.; Anhut, T.; Riemann, I.; Konig, K. Fluorescence lifetime images and correlation spectra obtained by multidimensional time-correlated single photon counting. Microsc. Res. Tech. 2006, 69, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Jeon, T.J.; Poulos, J.L.; Schmidt, J.J. Long-term storable and shippable lipid bilayer membrane platform. Lab Chip 2008, 8, 1742–1744. [Google Scholar] [CrossRef] [PubMed]
- Schutz, G.J.; Schindler, H.; Schmidt, T. Single-molecule microscopy on model membranes reveals anomalous diffusion. Biophys. J. 1997, 73, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Saxton, M.J. Single-particle tracking: the distribution of diffusion coefficients. Biophys. J. 1997, 72, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Sheetz, M.P.; Elson, E.L. Single particle tracking. Analysis of diffusion and flow in two-dimensional systems. Biophys. J. 1991, 60, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Tamm, L.K.; McConnell, H.M. Supported phospholipid bilayers. Biophys. J. 1985, 47, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Kalb, E.; Frey, S.; Tamm, L.K. Formation of supported planar bilayers by fusion of vesicles to supported phospholipid monolayers. Biochim. Biophys. Acta 1992, 1103, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Ladha, S.; Mackie, A.R.; Harvey, L.J.; Clark, D.C.; Lea, E.J.; Brullemans, M.; Duclohier, H. Lateral diffusion in planar lipid bilayers: A fluorescence recovery after photobleaching investigation of its modulation by lipid composition, cholesterol, or alamethicin content and divalent cations. Biophys. J. 1996, 71, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Bayley, H.; Cronin, B.; Heron, A.; Holden, M.A.; Hwang, W.L.; Syeda, R.; Thompson, J.; Wallace, M. Droplet interface bilayers. Mol. BioSyst. 2008, 4, 1191–1208. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, J.; Xiong, J.-Y.; Liu, X.-Y. Determination of agarose gel pore size: Absorbance measurements vis a vis other techniques. J. Phys. Conf. Ser. 2006, 28, 83–86. [Google Scholar] [CrossRef]
- Lee, S.; Müller, M.; Ratoi-Salagean, M.; Vörös, J.; Pasche, S.; de Paul, S.M.; Spikes, H.A.; Textor, M.; Spencer, N.D. Boundary lubrication of oxide surfaces by poly(l-lysine)-g-poly(ethylene glycol) (PLL-g-PEG) in aqueous media. Tribol. Lett. 2003, 15, 231–239. [Google Scholar] [CrossRef]
- Sonnleitner, A.; Schütz, G.J.; Schmidt, T. Free Brownian motion of individual lipid molecules in biomembranes. Biophys. J. 1999, 77, 2638–2642. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.; Sackmann, E. Translational and rotational drag coefficients for a disk moving in a liquid membrane associated with a rigid substrate. J. Fluid Mech. 1988, 194, 553–561. [Google Scholar] [CrossRef]
- Allender, D.W.; Schick, M. Phase separation in bilayer lipid membranes: effects on the inner leaf due to coupling to the outer leaf. Biophys. J. 2006, 91, 2928–2935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Granick, S. Lipid diffusion compared in outer and inner leaflets of planar supported bilayers. J. Chem. Phys. 2005, 123, 211104:1–211104:4. [Google Scholar]
- Gidwani, A.; Holowka, D.; Baird, B. Fluorescence anisotropy measurements of lipid order in plasma membranes and lipid rafts from RBL-2H3 mast cells. Biochemistry 2001, 40, 12422–12429. [Google Scholar] [CrossRef] [PubMed]
- Kozjak-Pavlovic, V.; Dian-Lothrop, E.A.; Meinecke, M.; Kepp, O.; Ross, K.; Rajalingam, K.; Harsman, A.; Hauf, E.; Brinkmann, V.; Gunther, D.; Herrmann, I.; Hurwitz, R.; Rassow, J.; Wagner, R.; Rudel, T. Bacterial porin disrupts mitochondrial membrane potential and sensitizes host cells to apoptosis. PLoS Pathog. 2009, 5, e1000629:1–e1000629:15. [Google Scholar] [CrossRef]
- Zeth, K.; Kozjak-Pavlovic, V.; Faulstich, M.; Fraunholz, M.; Hurwitz, R.; Kepp, O.; Rudel, T. Structure and function of the PorB porin from disseminating N. gonorrhoeae. Biochem. J. 2012. In Press. [Google Scholar]
- Fluka 53394 Atto 637 NHS. Available online: http://www.sigmaaldrich.com/etc/medialib/docs/Sigma/Datasheet/6/53394dat.Par.0001.File.tmp/53394dat.pdf (accessed on 6 December 2012).
- Meseth, U.; Wohland, T.; Rigler, R.; Vogel, H. Resolution of fluorescence correlation measurements. Biophys. J. 1999, 76, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Gambin, Y.; Lopez-Esparza, R.; Reffay, M.; Sierecki, E.; Gov, N.S.; Genest, M.; Hodges, R.S.; Urbach, W. Lateral mobility of proteins in liquid membranes revisited. Proc. Natl. Acad. Sci. USA 2006, 103, 2098–2102. [Google Scholar] [CrossRef] [PubMed]
- Achtman, M.; Neibert, M.; Crowe, B.A.; Strittmatter, W.; Kusecek, B.; Weyse, E.; Walsh, M.J.; Slawig, B.; Morelli, G.; Moll, A.; et al. Purification and characterization of eight class 5 outer membrane protein variants from a clone of Neisseria meningitidis serogroup A. J. Exp. Med. 1988, 168, 507–525. [Google Scholar] [CrossRef] [PubMed]
- ATTO-TEC Atto-Dyes Recommended Procedures for Labeling. Available online: http://www.atto-tec.com/fileadmin/user_upload/Katalog_Flyer_Support/Procedures.pdf (accessed on 6 December 2012).
- Hinnah, S.C.; Wagner, R.; Sveshnikova, N.; Harrer, R.; Soll, J. The chloroplast protein import channel Toc75: Pore properties and interaction with transit peptides. Biophys. J. 2002, 83, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Dertinger, T.; Pacheco, V.; von der Hocht, I.; Hartmann, R.; Gregor, I.; Enderlein, J. Two-focus fluorescence correlation spectroscopy: A new tool for accurate and absolute diffusion measurements. Chemphyschem 2007, 8, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Schwille, P.; Meyer-Almes, F.J.; Rigler, R. Dual-color fluorescence cross-correlation spectroscopy for multicomponent diffusional analysis in solution. Biophys. J. 1997, 72, 1878–1886. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bartsch, P.; Walter, C.; Selenschik, P.; Honigmann, A.; Wagner, R. Horizontal Bilayer for Electrical and Optical Recordings. Materials 2012, 5, 2705-2730. https://doi.org/10.3390/ma5122705
Bartsch P, Walter C, Selenschik P, Honigmann A, Wagner R. Horizontal Bilayer for Electrical and Optical Recordings. Materials. 2012; 5(12):2705-2730. https://doi.org/10.3390/ma5122705
Chicago/Turabian StyleBartsch, Philipp, Claudius Walter, Philipp Selenschik, Alf Honigmann, and Richard Wagner. 2012. "Horizontal Bilayer for Electrical and Optical Recordings" Materials 5, no. 12: 2705-2730. https://doi.org/10.3390/ma5122705
APA StyleBartsch, P., Walter, C., Selenschik, P., Honigmann, A., & Wagner, R. (2012). Horizontal Bilayer for Electrical and Optical Recordings. Materials, 5(12), 2705-2730. https://doi.org/10.3390/ma5122705




