Storage Phosphors for Medical Imaging
Abstract
:1. Introduction
1.1. Medical Radiography
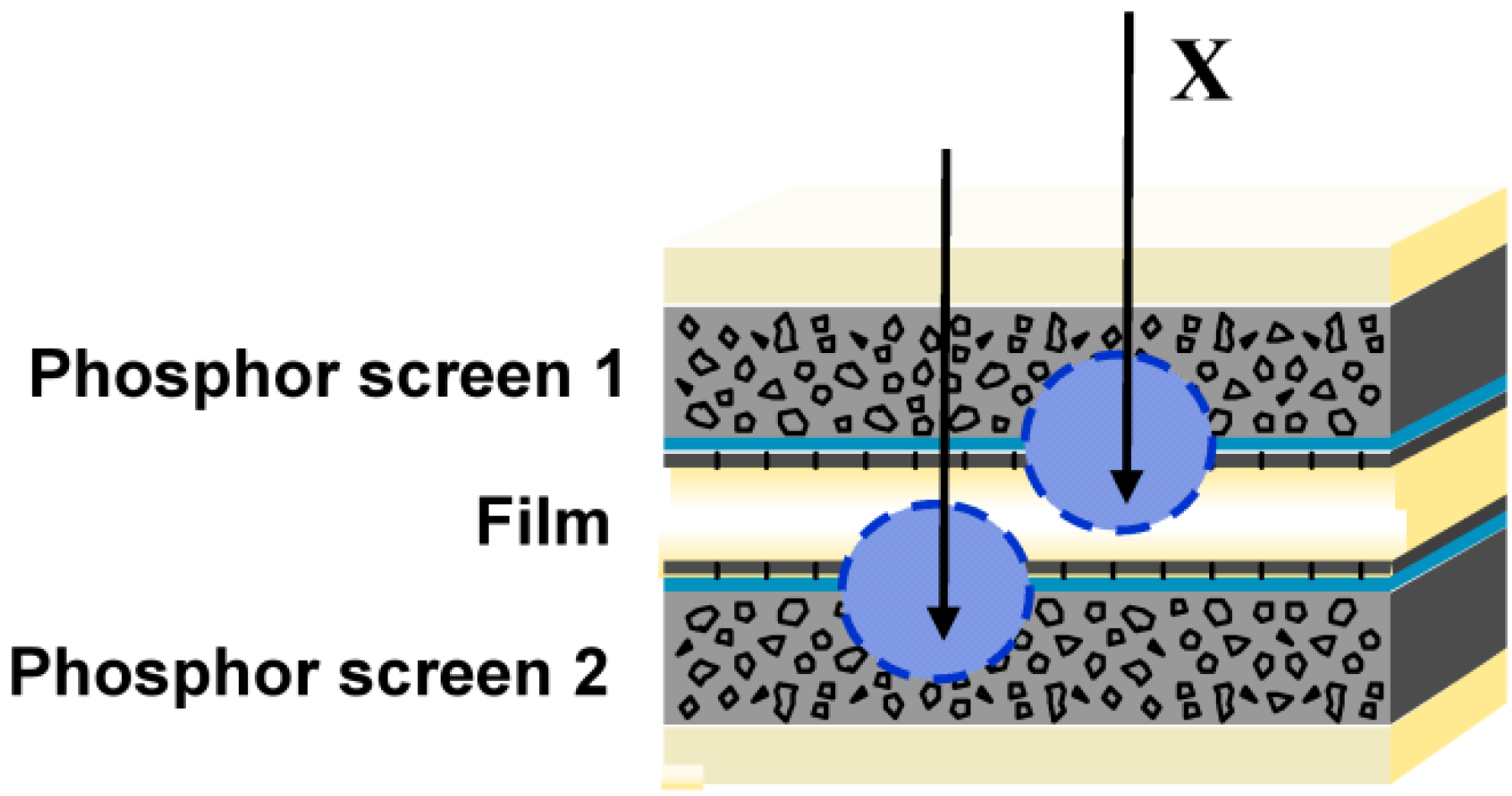
1.2. Screen/Film Radiography
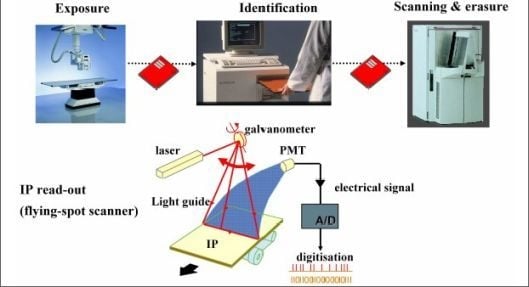
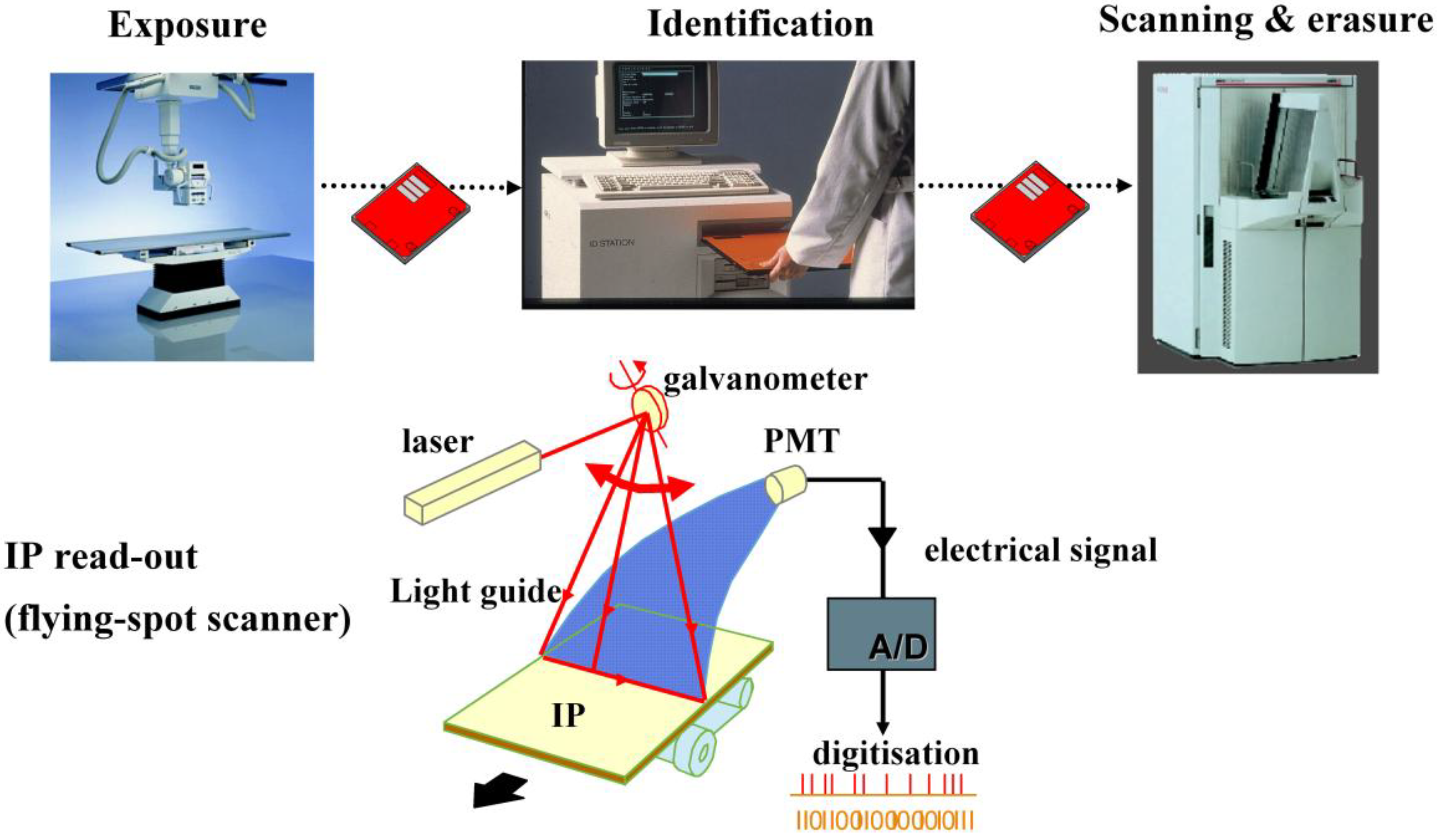
1.3. Computed Radiography (CR)


2. CR Phosphor Requirements
- It should have a high X-ray absorption for medical X-ray spectra, i.e., for energies ranging from 20 keV to 140 keV.
- It should have high conversion efficiency. The amount of energy stored per unit X-ray energy should be high. This implies that a large fraction of the mobile charge carriers created by the absorbed X-ray quanta is converted into trapped electrons and holes forming defect aggregates leading to PSL. The energy needed to create a trapped electron and hole in the storage phosphors in commercial use is estimated to be:
- 100 eV in BaFBr:Eu2+
- 67 eV in CsBr:Eu2+.
- The image information stored in the phosphor should have slow fading in dark at room temperature, i.e., electron- and hole traps should be stable. In practice, the so-called dark-decay of the stored image in a CR plate is between 10 and 25% in the first hour after X-ray exposure.
- The stimulation spectrum should be in the range covered by inexpensive solid state lasers. Currently laser diodes are commercially available with wavelengths ranging from 375 nm to 1800 nm, but red and IR lasers exhibit the best price/performance ratio. In addition, the stimulation light should not produce free charges that can give rise to PSL. This excludes lasers with wavelengths in the blue and UV parts of the spectrum. Finally, the stimulation light and the stimulated emission should be spectrally separable. This is necessary since the emitted light is between 105 and 109 times weaker than the stimulation light, depending on X-ray spectrum and dose used to make the image. Color filters are applied to block the stimulation wavelength and transmit the emission light. Since the slope of the filter curves is finite, a stimulated emission with a wavelength close to that of the laser will also be attenuated. Altogether, this means that efficient stimulation of the trapped charges should be possible in the wavelength range between 500 and 1,500 nm. However, it is unlikely that trapped charges that can be stimulated with 1,000 to 1,500 nm light are stable at room temperature in the dark.
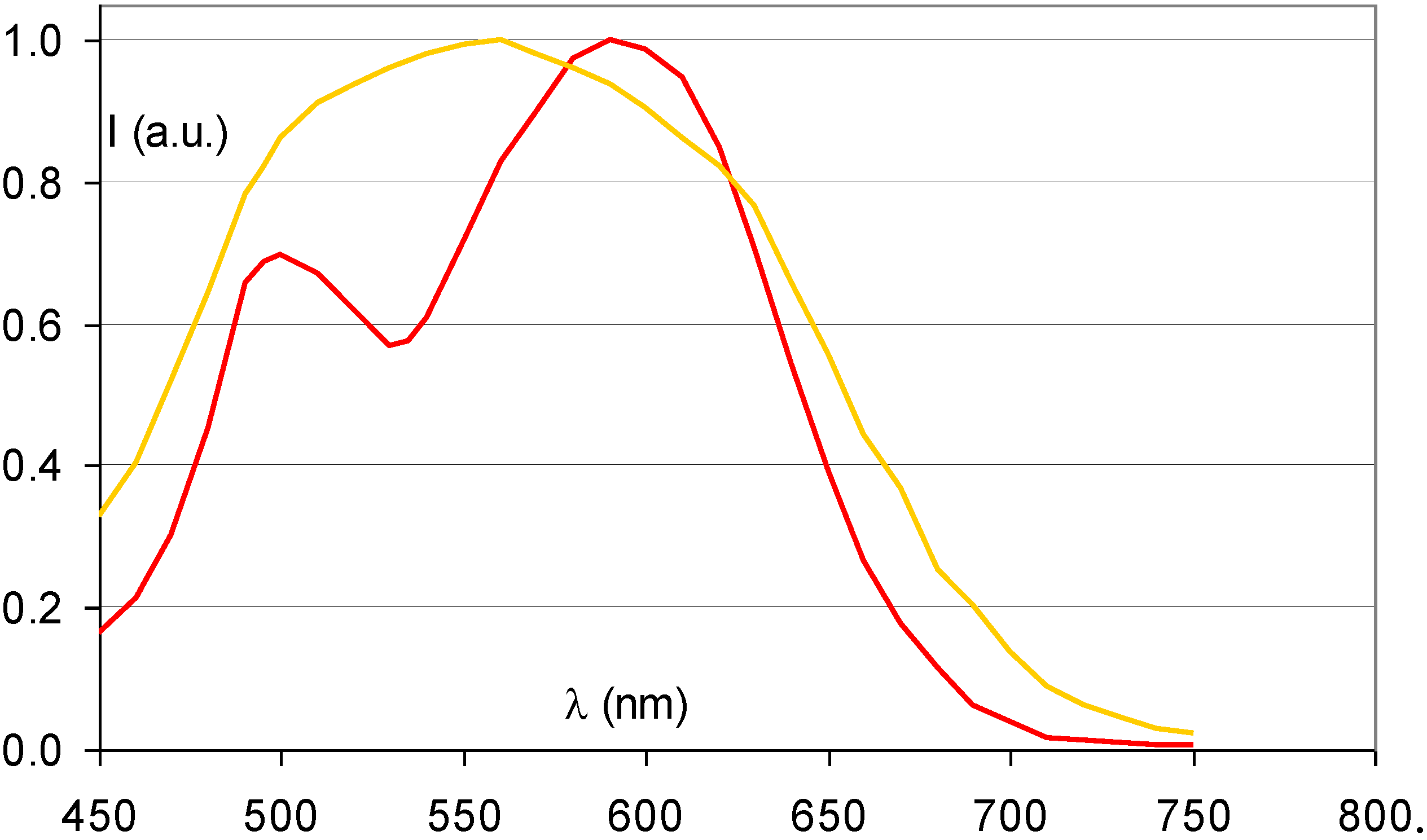
- The CR phosphor emission should match the sensitivity spectrum of the light detector (Figure 5). In flying-spot scanners with a PMT light detector, the emission is preferable below 500 nm and most preferably in the range between 350 and 450 nm.Figure 5. Quantum efficiency of a typical CR scanner PMT.
- The laser power required for CR plate stimulation should be low. This implies that both the oscillator strength and the recombination probability of the stimulated trap should be high. Actual CR scanner laser powers are between 5 and 30 mW.
- Since read-out of a complete image plate in less than one minute is desirable, the luminescence decay time should be short. In current flying-spot CR digitizers, the pixel read-out time does not exceed 2 µs. At the time the next pixel is stimulated, the light emission of the previous pixel should have decayed to at least 1/e of its initial value. Consequently, for use in flying-spot scanners the decay time should be maximum 2 µs.For line scanners phosphor decay time is much less critical. In these devices a complete line is stimulated and read-out at once and the line-time is typically in the millisecond range.
- The IP should be re-usable. Only roughly 50% of the stored energy (trapped charges) is released during scanning. Hence, the IP must be erased for re-use. For erasure the phosphor is illuminated with a strong light source. To allow efficient erasure, the amount of “persistent traps” that is formed upon X-ray radiation should be negligible.
- The phosphor should be stable under normal room conditions, i.e., its performance should not degrade when it is exposed to humidity and daylight.
- It should be possible to manufacture the phosphor on an industrial scale in a commercially viable way.
- Spatial and contrast resolution are important clinical requirements and are affected by phosphor quality as well. Smaller particles lead to higher resolution and to lower light output. The best compromise is obtained when the phosphor particles have a median size in the range between 1 and 10 μ.
3. Powder Imaging Plates (PIP’s) for Medical CR
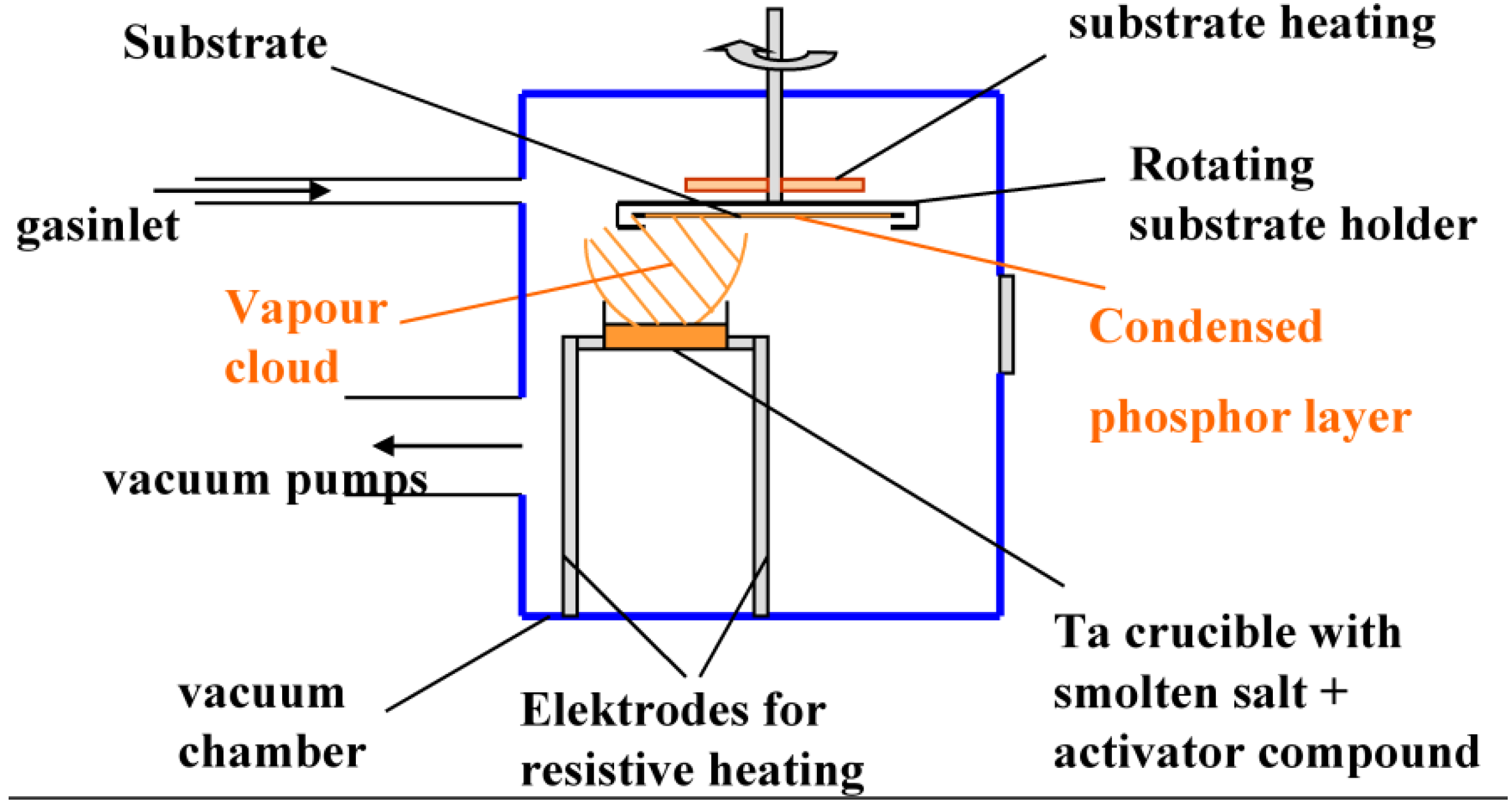
3.1. PIP Manufacturing

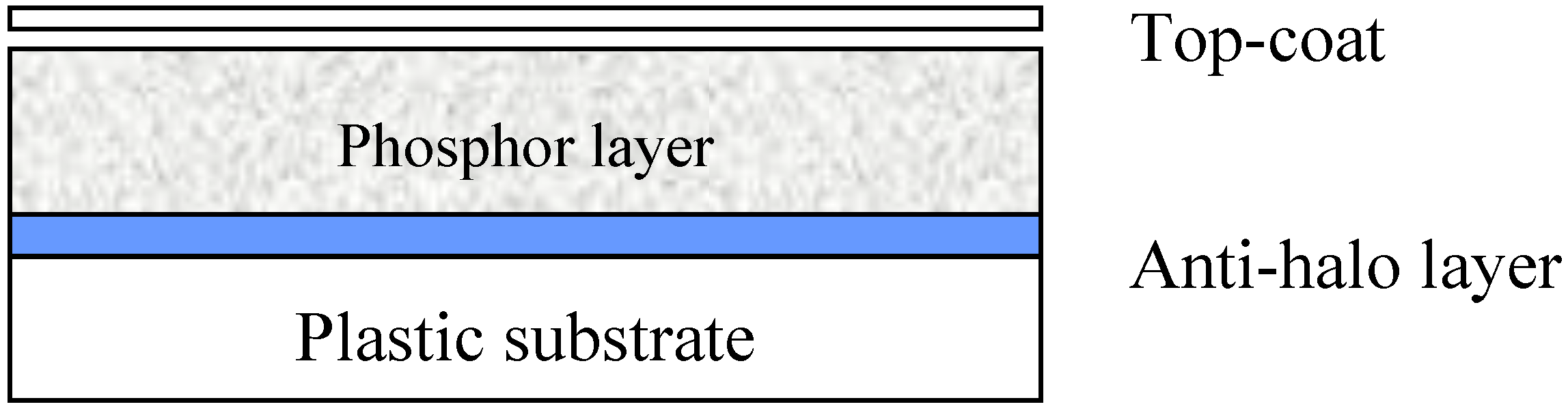
3.2. The BaFBr:Eu2+ Storage Phosphor

4. BaFBr:Eu2+ PSL Mechanism

4.1. Electron and Hole Center Production

4.2. Recombination of Trapped Electrons and Holes
4.3. Spatial Correlation of Defects
4.4. The Formation of Bromine Vacancies in the BaFBr Lattice

5. BaFBr:Eu2+ Engineering


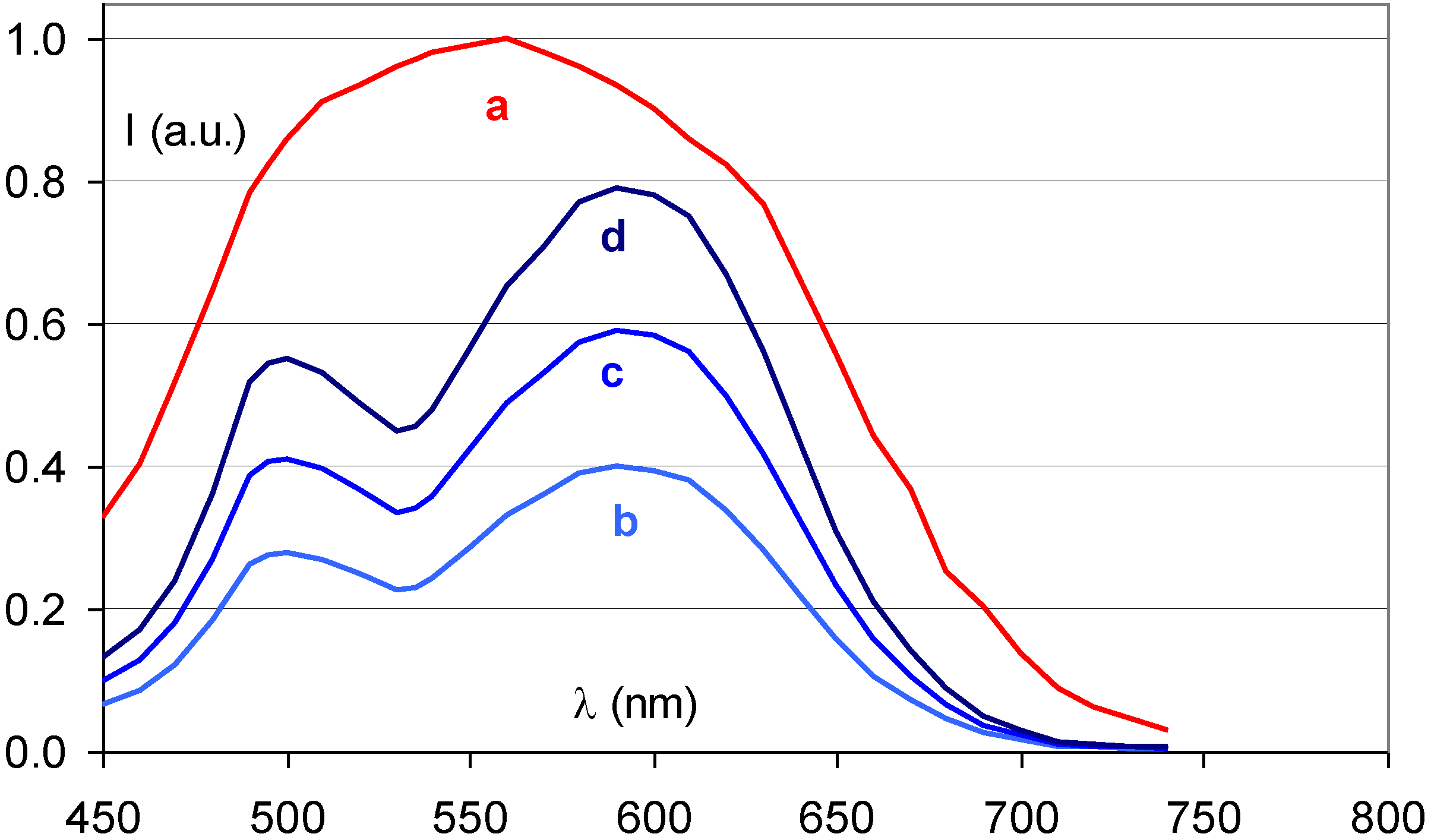


6. Alternative Storage Phosphors for Medical CR
- -
- the most promising candidates to replace BaFBr:Eu2+
- -
- storage phosphors having high X-ray absorption or useable for manufacturing thicker and, therefore, more absorbing phosphor screens.
- Conversion efficiency (CE): the total energy of stimulated light per unit area and per unit of X-ray dose produced by the phosphor in pJ/mm2/mR,
- Stimulation energy (SE): the laser energy per unit area required to release 63% of the stored energy in µJ/mm2.
6.1. BaFBr:Eu2+ Alternatives
6.2. Higher Intrinsic Absorption
6.3. Elpasolite Storage Phosphor
6.4. Alkaline Earth Sulfide Storage Phosphors
6.5. Storage Phosphor Glass Ceramics
7. Needle Storage Phosphors for Medical CR
7.1. Needle Plate Manufacturing
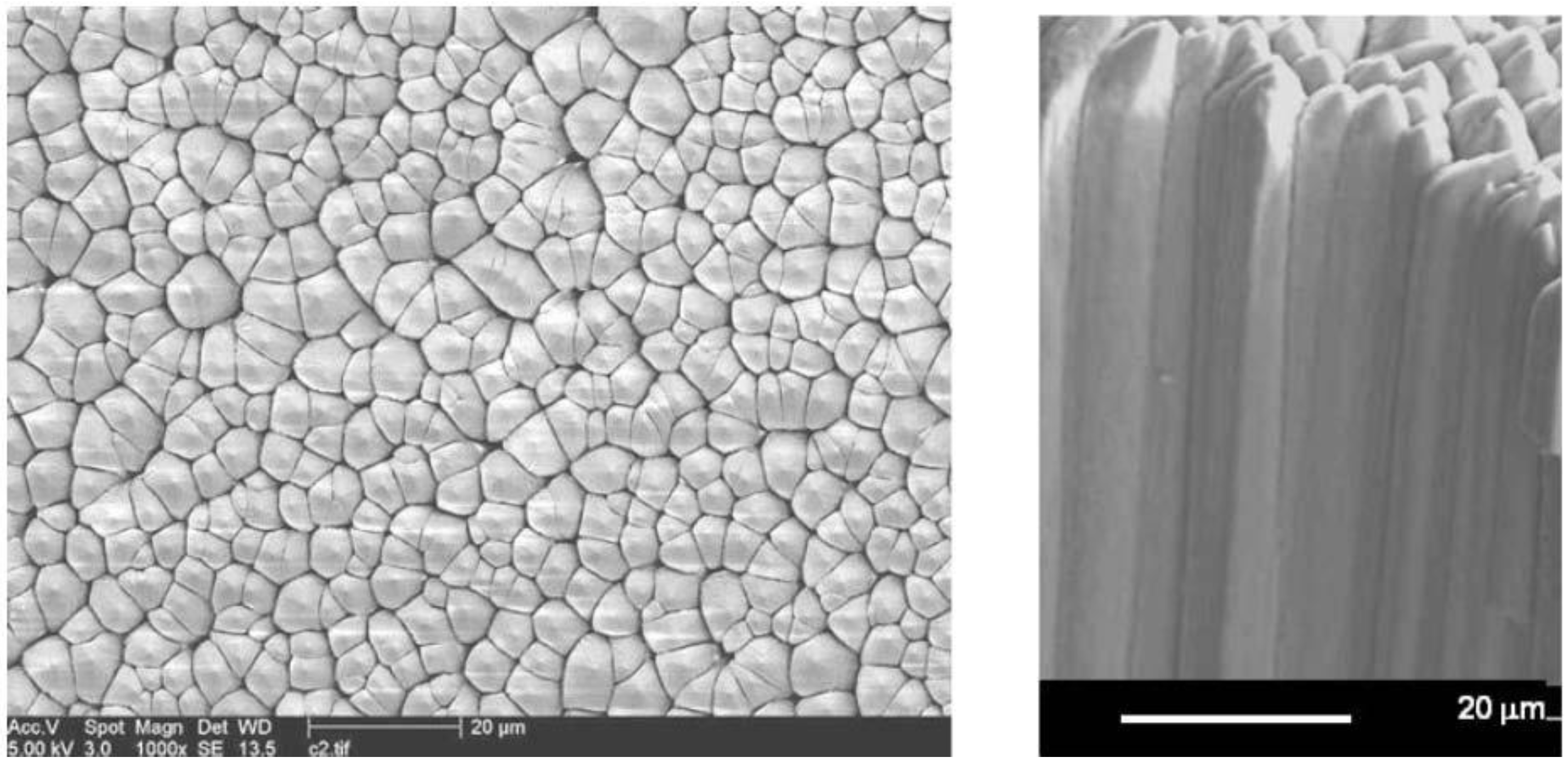
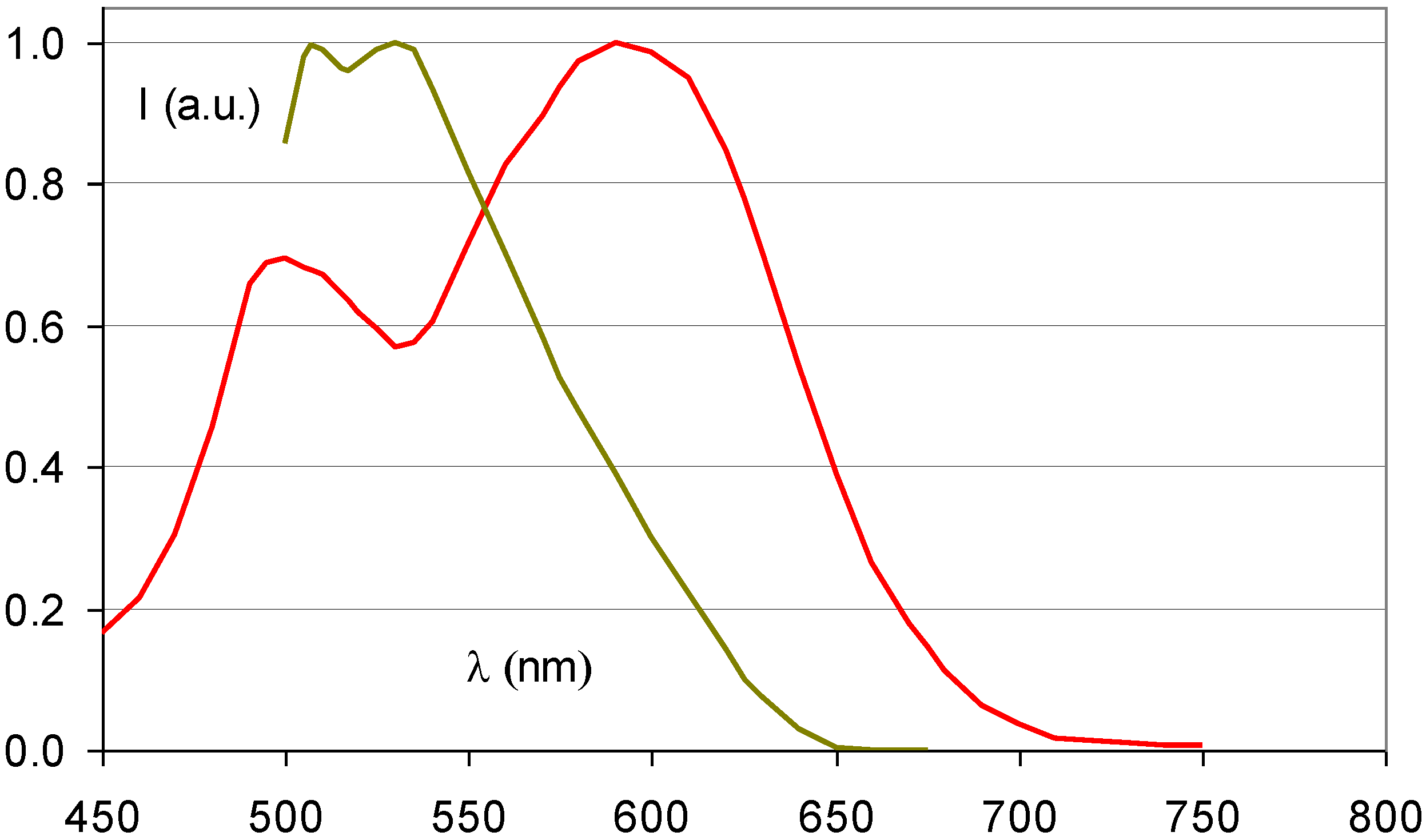
7.2. Needle Phosphors for Medical Imaging
7.3. CR Needle Phosphors

| Phosphor | Stimulating laser wavelengtd (nm) | Peak PSL wavelengtd (nm) | Peak stimulation wavelengtd (nm) | CE pJ/mm2/mR | SE μJ/mm2 |
| BaFBr:Eu2+ | 633 | 390 | 550 | 20 | 16 |
| BaFBr:Eu2+ | 680 | 390 | 550 | 14 | 28 |
| RbBr:In+ | 680 | 490 | 700 | 2 | 25 |
| RbBr:Ga+ | 680 | 550 | 705 | 6 | 4 |
| CsBr:In+ | 680 | 504 | 700 | 3 | 23 |
| CsBr:Ga+ | 680 | 515 | 685 | 6 | 4 |
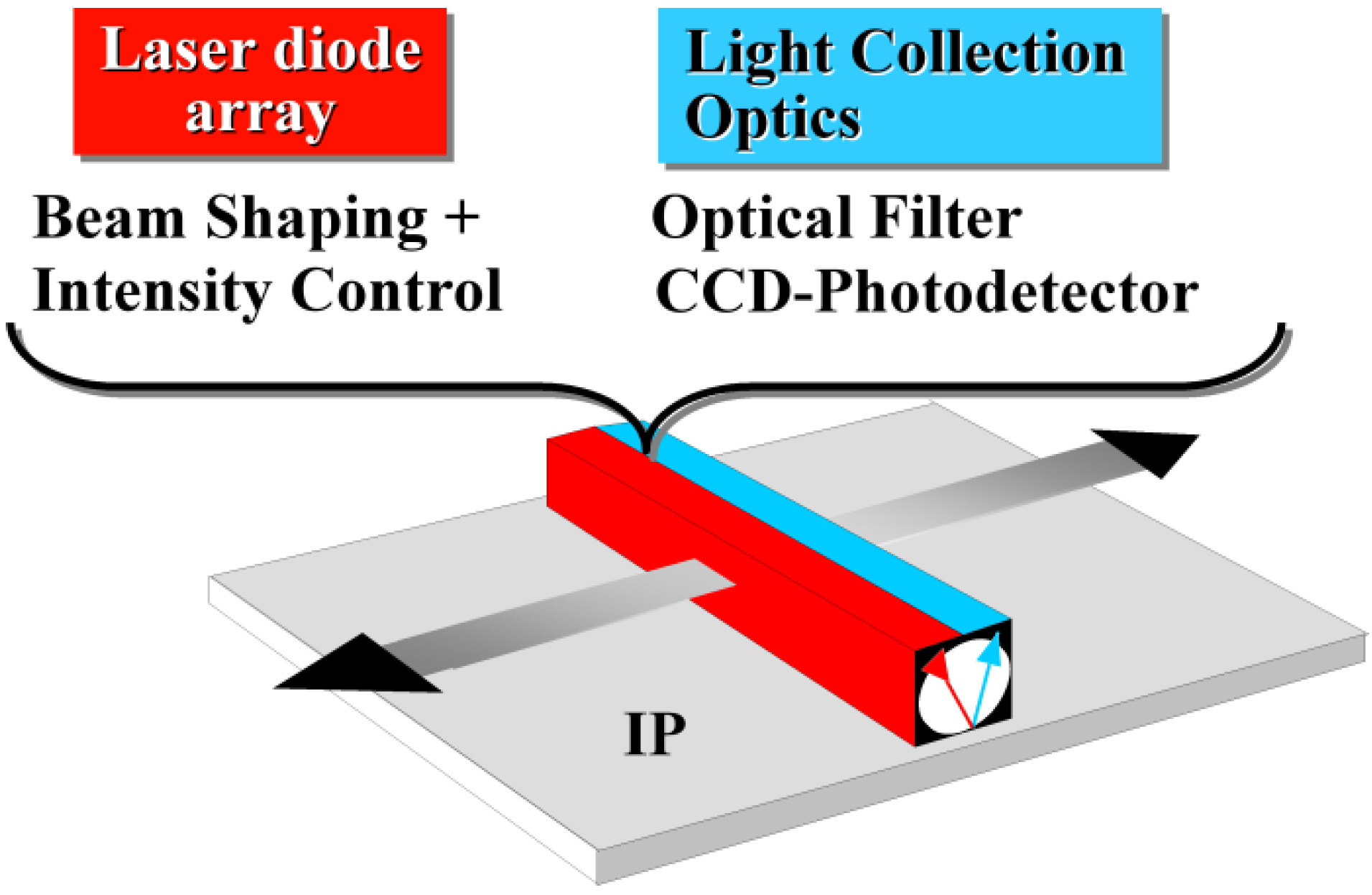
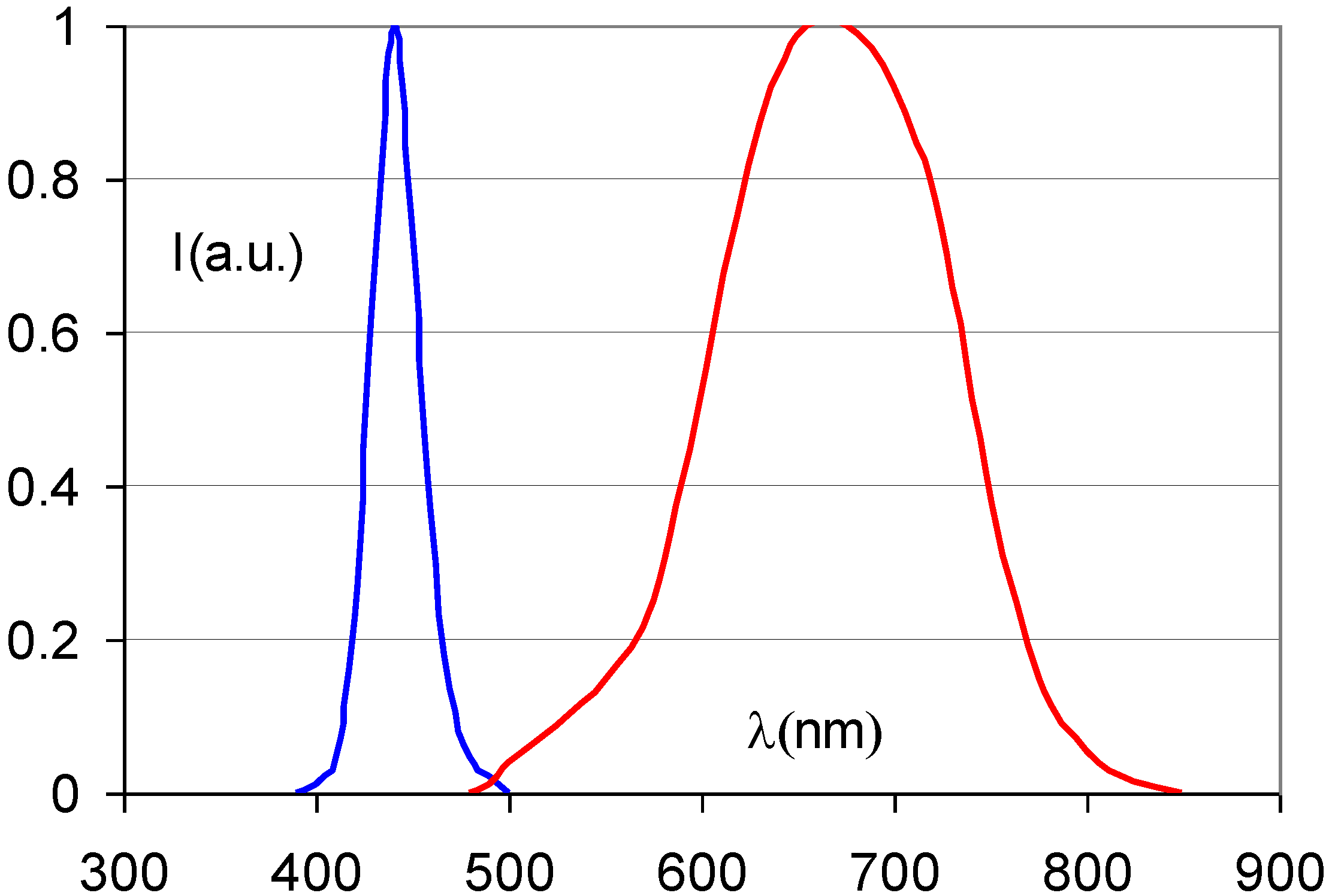
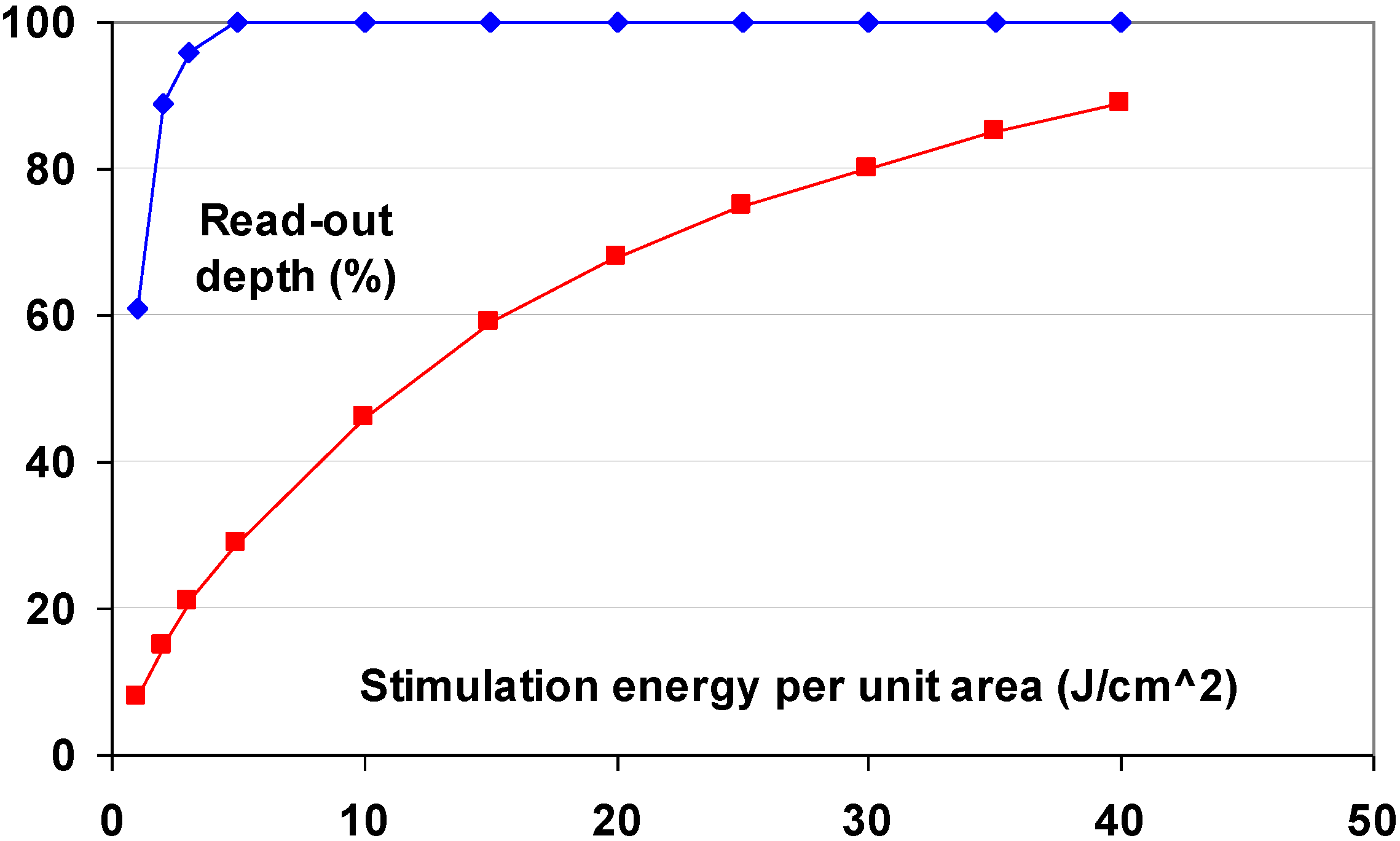
8. The CsBr:Eu2+ PSL Mechanism
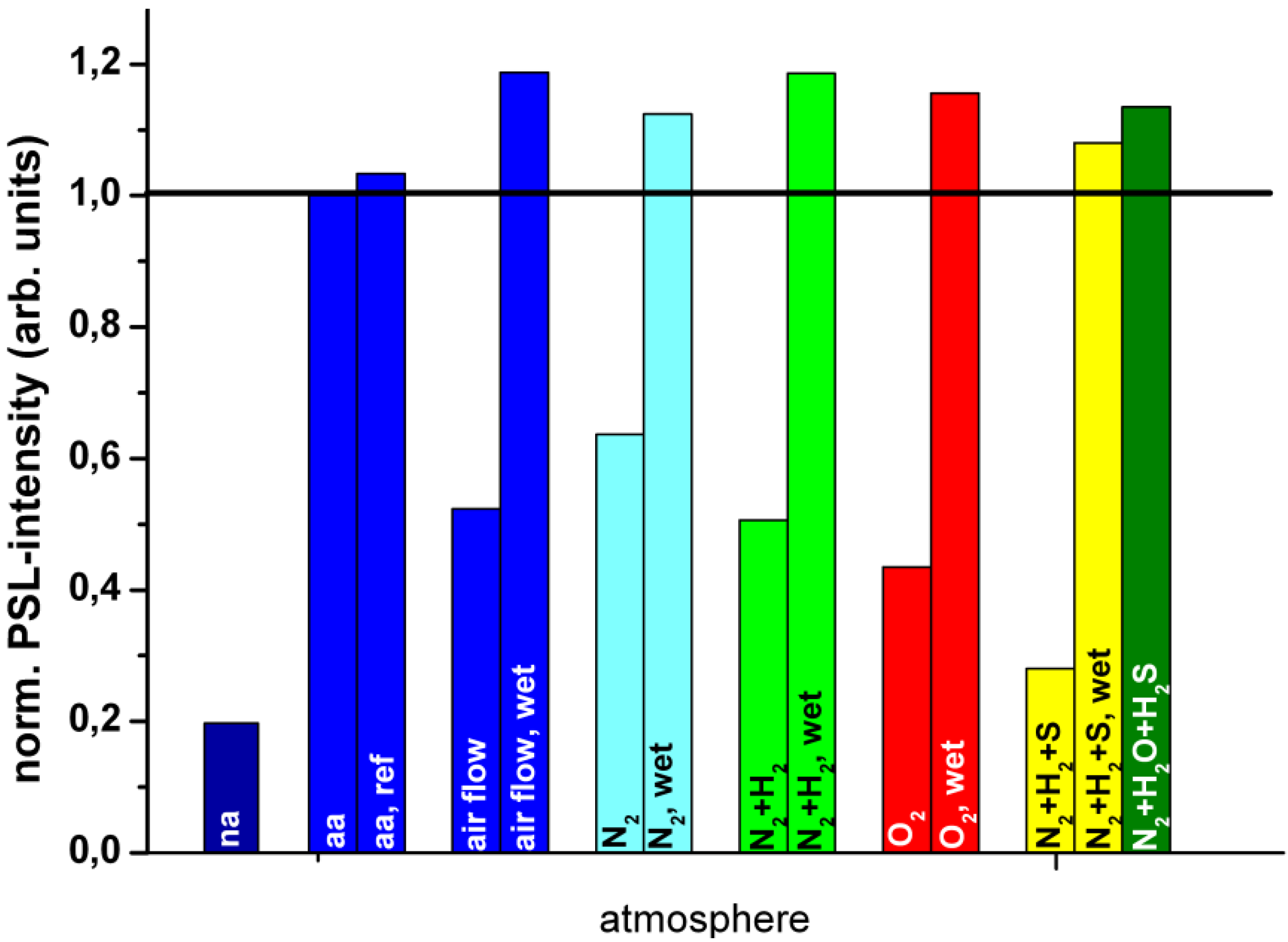

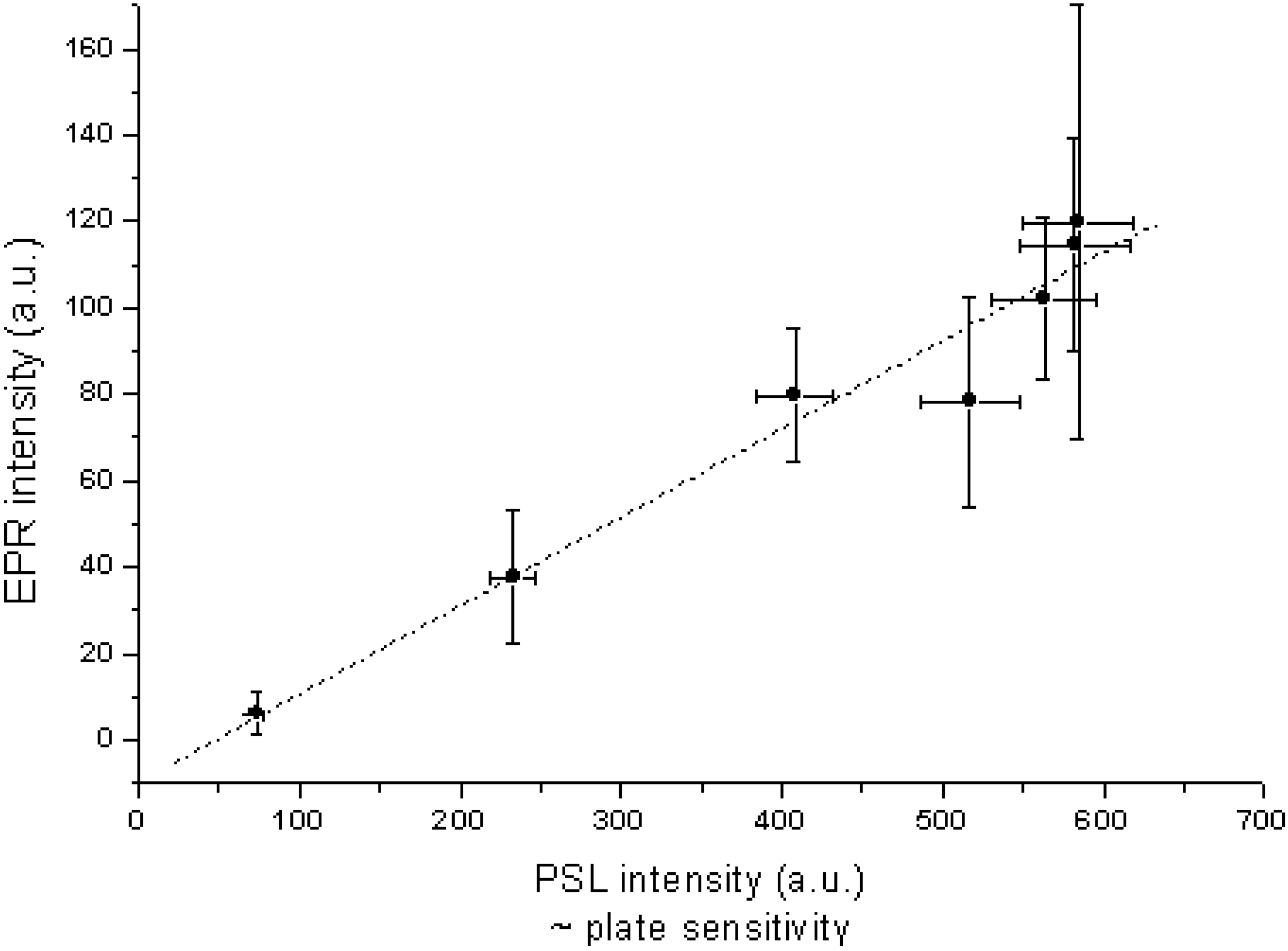
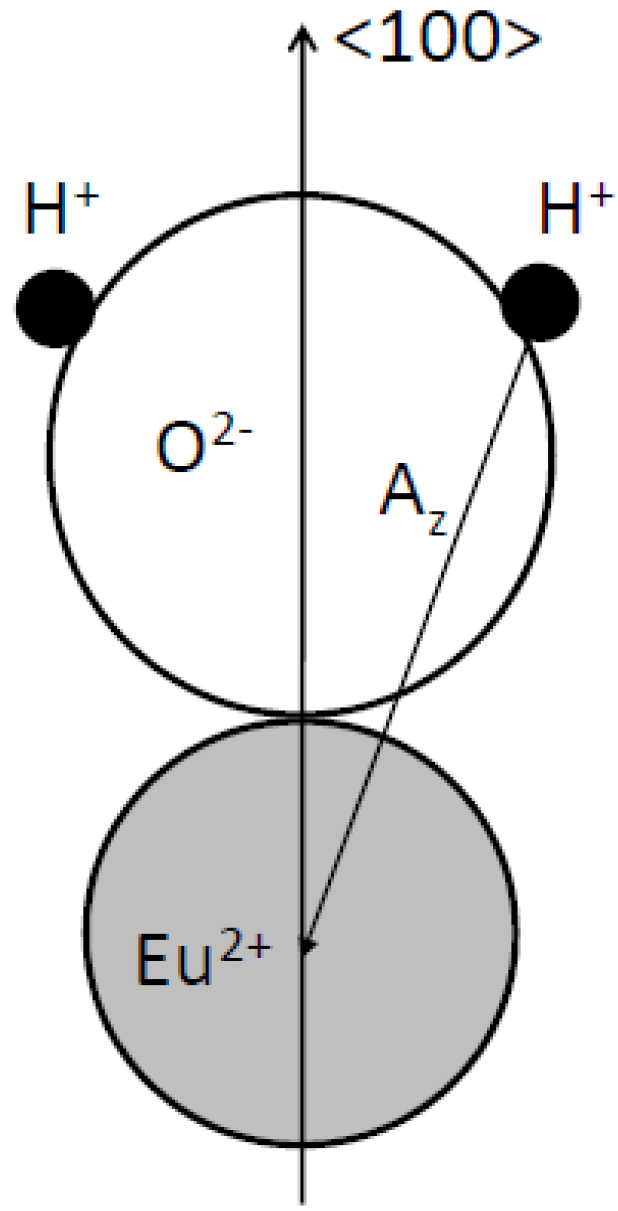
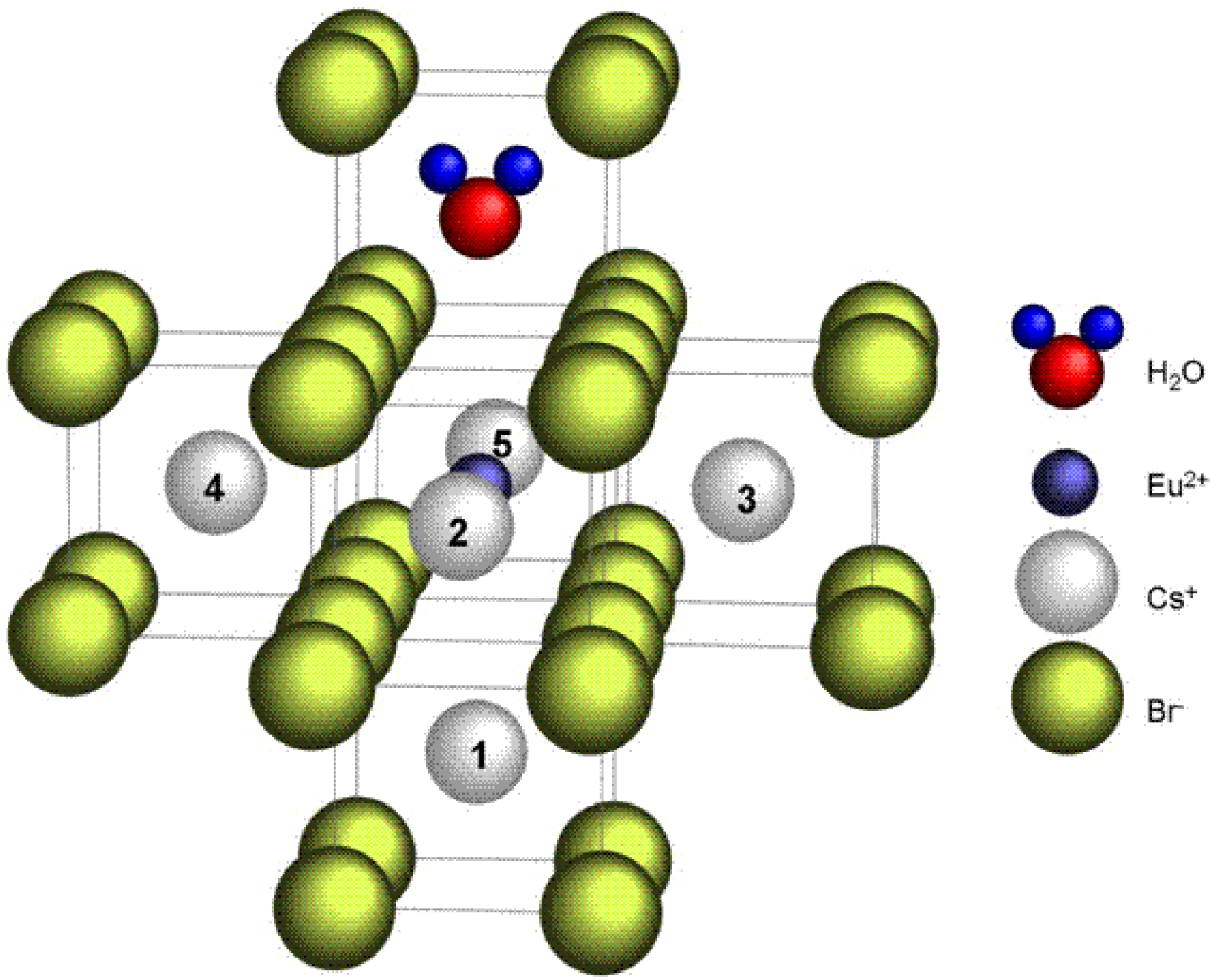

9. Image Quality of CR Systems
9.1. Definition of Medical Radiography System’s Image Quality
9.1.1. Signal-to-Noise Ratio (SNR)
9.1.2. Detective Quantum Efficiency (DQE)
9.1.3. Noise Sources
9.1.4. Noise as a Function of Spatial Frequency
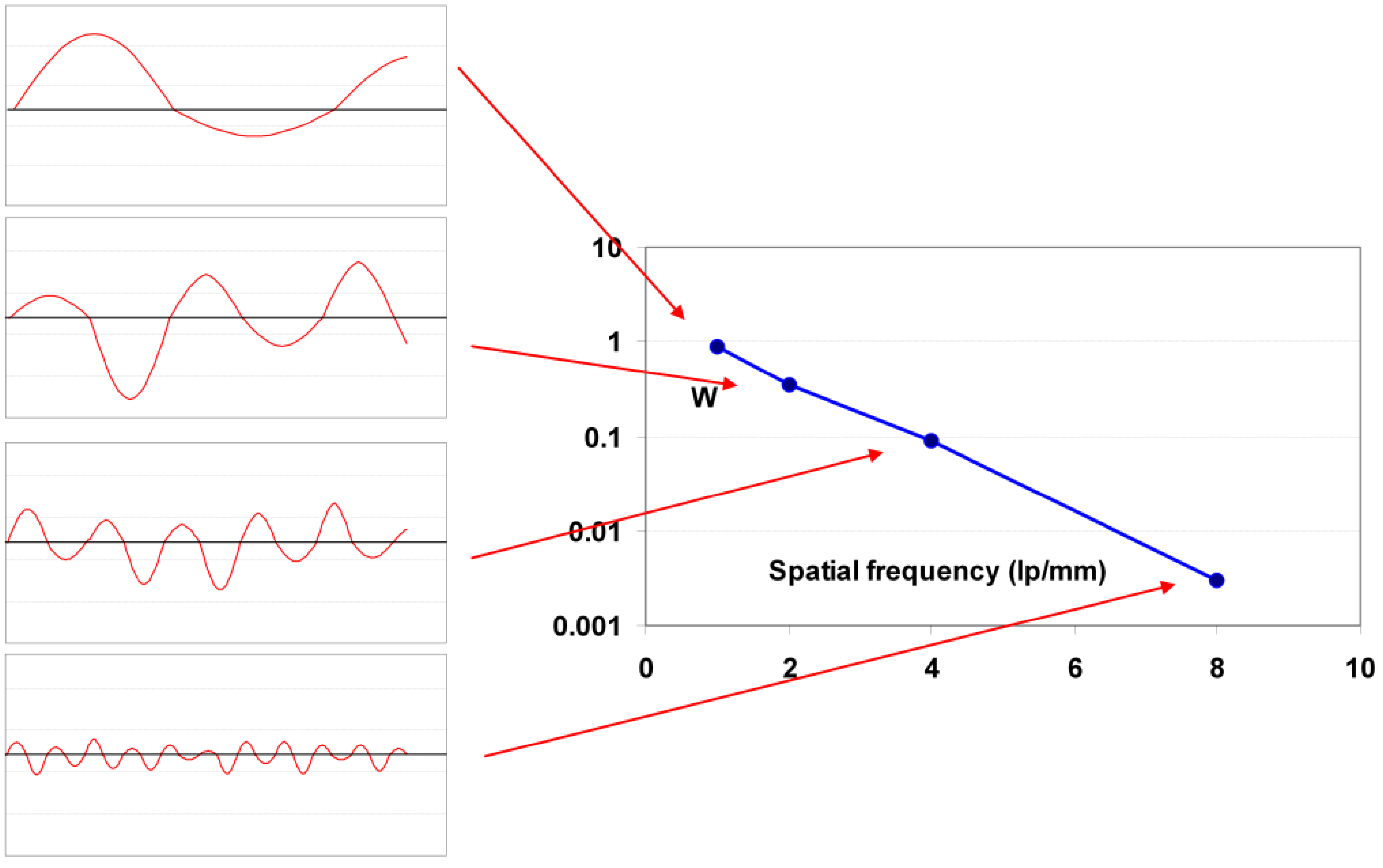
9.1.5. Resolution and Modulation Transfer Function (MTF)
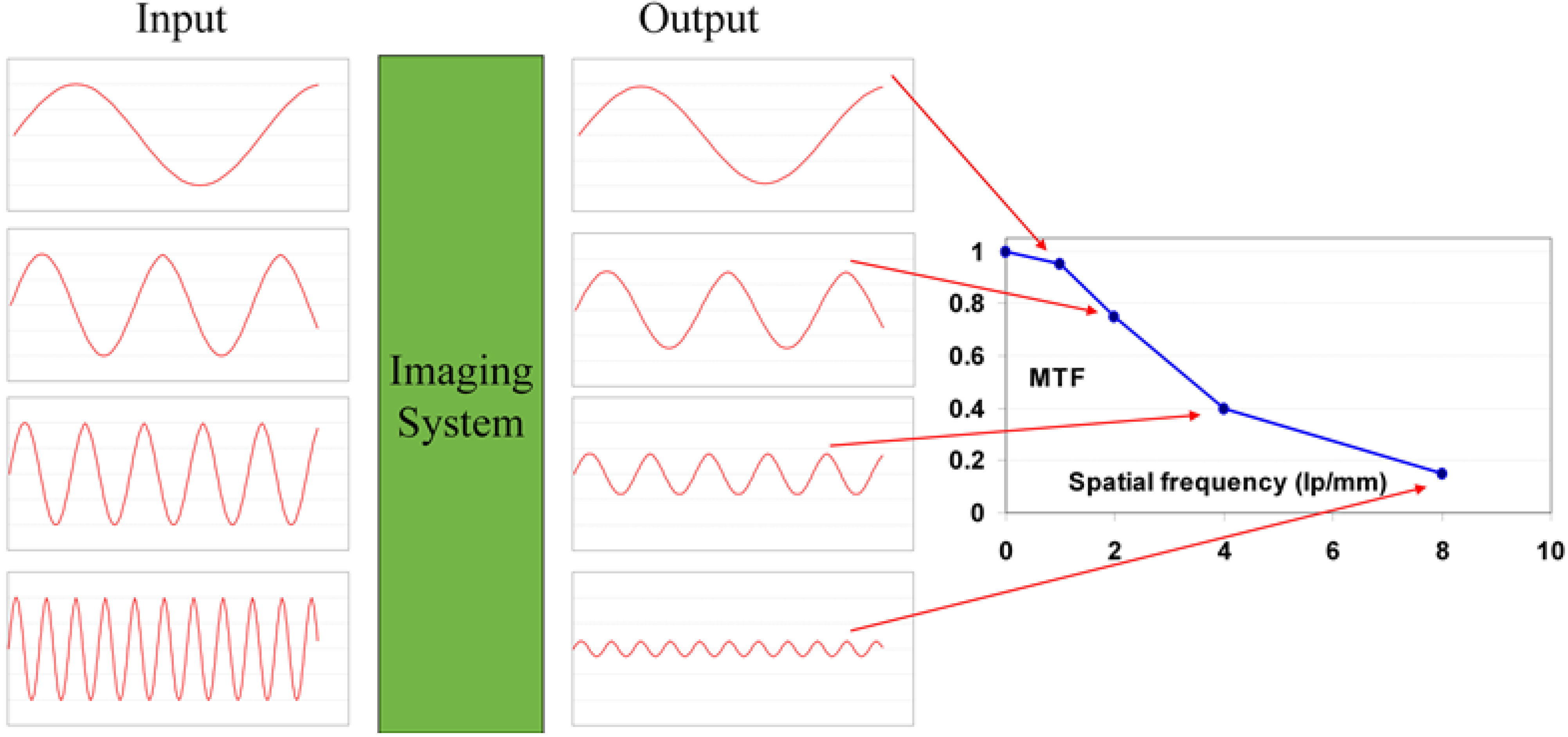
9.1.6. DQE as a Function of Spatial Frequency
9.2. Measurement of Image Quality
9.2.1. Measurement of MTF
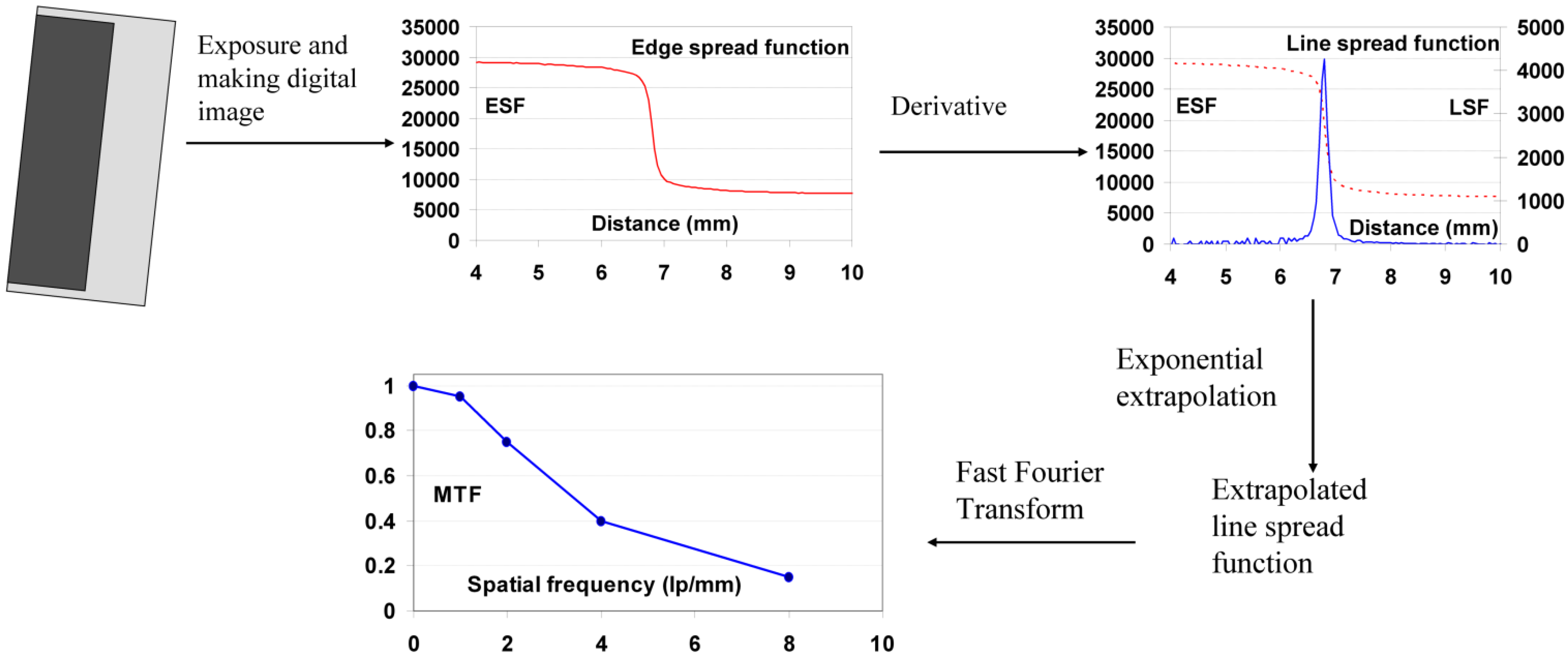
9.2.2. Measurement of W
9.2.3. Measurement of Air Kerma and Translation to Number of Quanta
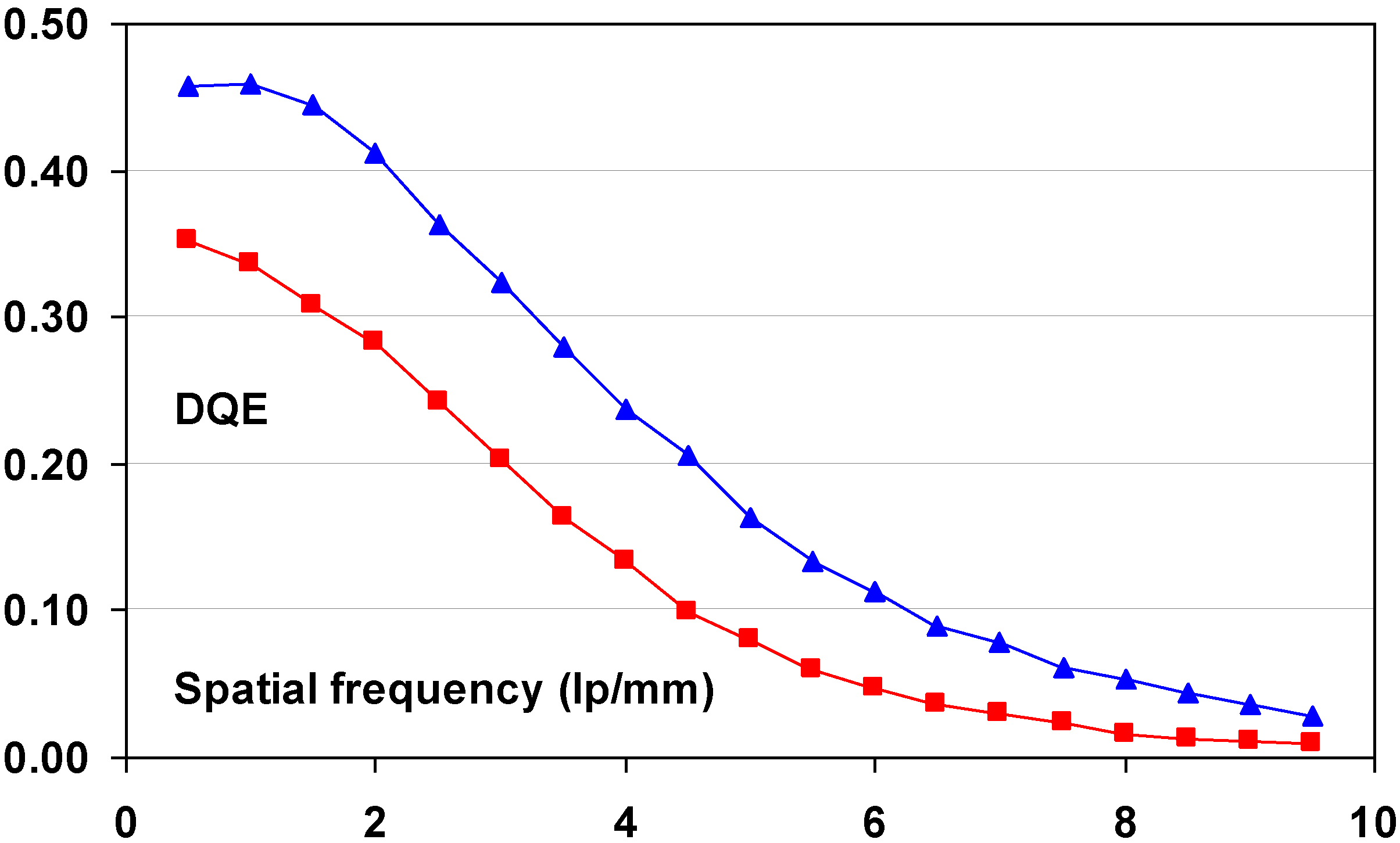
9.3. Relation between Imaging Plate Characteristics and Image Quality (DQE)
9.3.1. Simple DQE Model
9.3.2. Relation between DQE and IP Characteristics
- The diameter of the laser beam halo increases with the depth in the layer. Hence, spatial resolution and MTF in CR decrease with increasing phosphor layer thickness.
- Another factor of influence is the color of the substrate on which the phosphor layer is coated. The more the substrate absorbs the stimulation light, the higher MTF will be, because reflected stimulation light will diffuse further in the lateral direction, thereby increasing the size of the stimulating halo.
- Resolution can be improved by mixing anti-halo dye or pigment in the phosphor layer or top-coat. This, obviously, limits lateral light diffusion.
- The shorter the distance between two light scattering events, i.e., the shorter the mean free path of the photons in the phosphor layer, the shorter will be the distance over which light can travel before being absorbed. This explains why the resolution offered by PIP improves with decreasing phosphor particle size and in NIP with decreasing needle diameter.
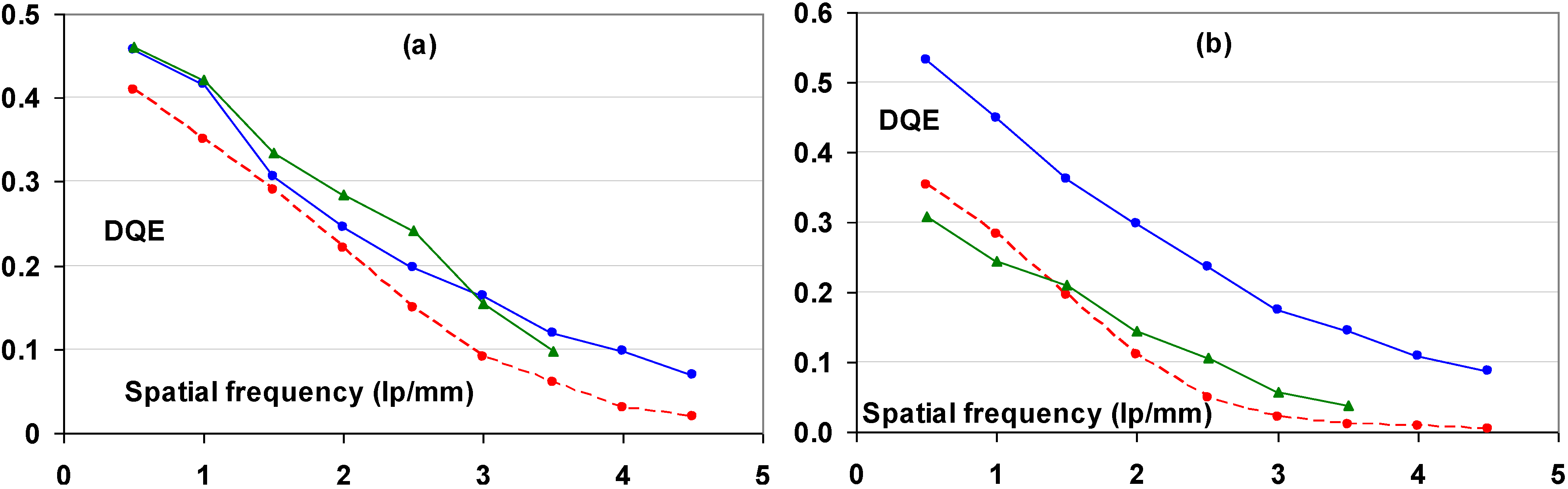
9.4. Technical and Clinical Image Quality of CR Systems with PIP and NIP
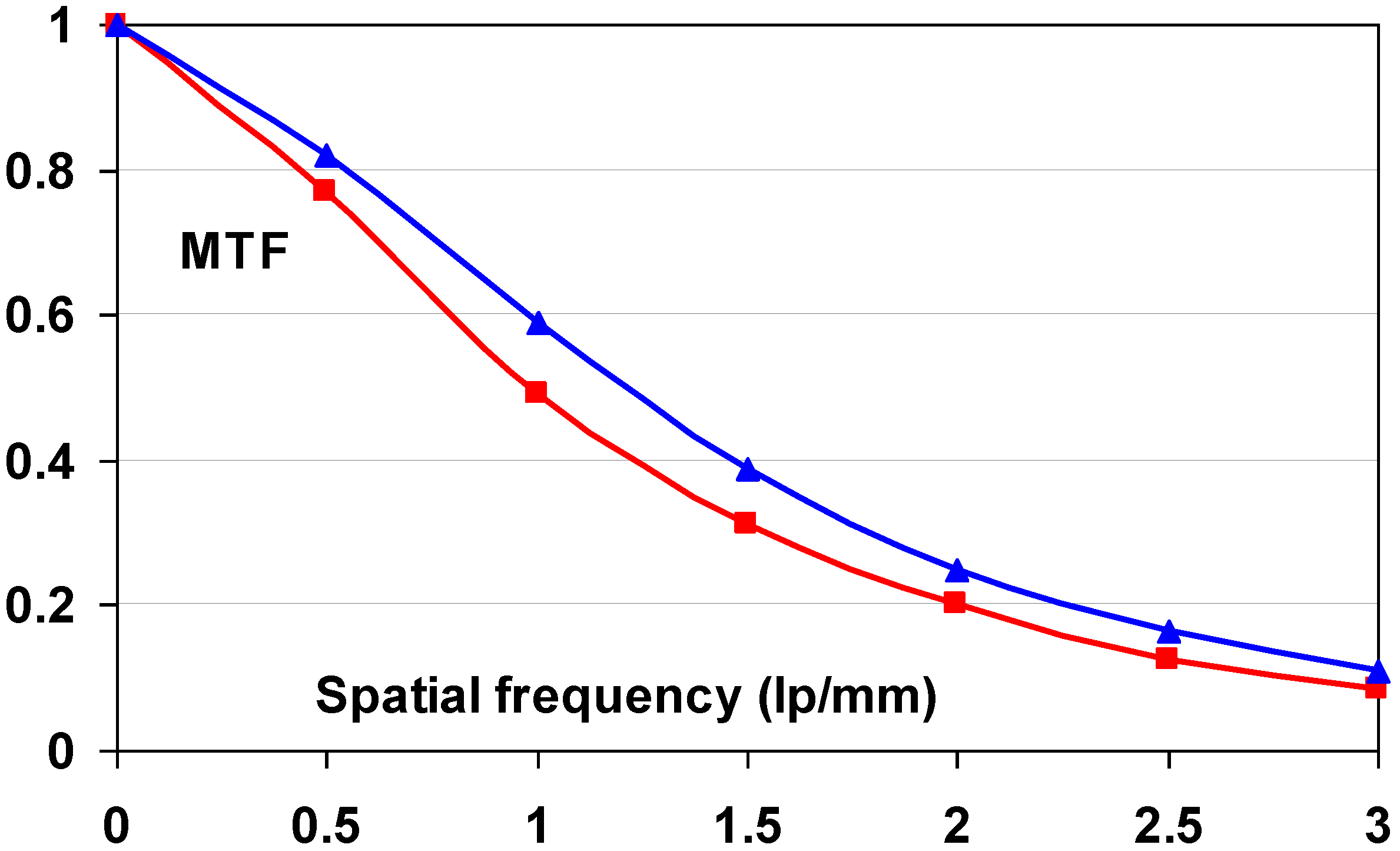
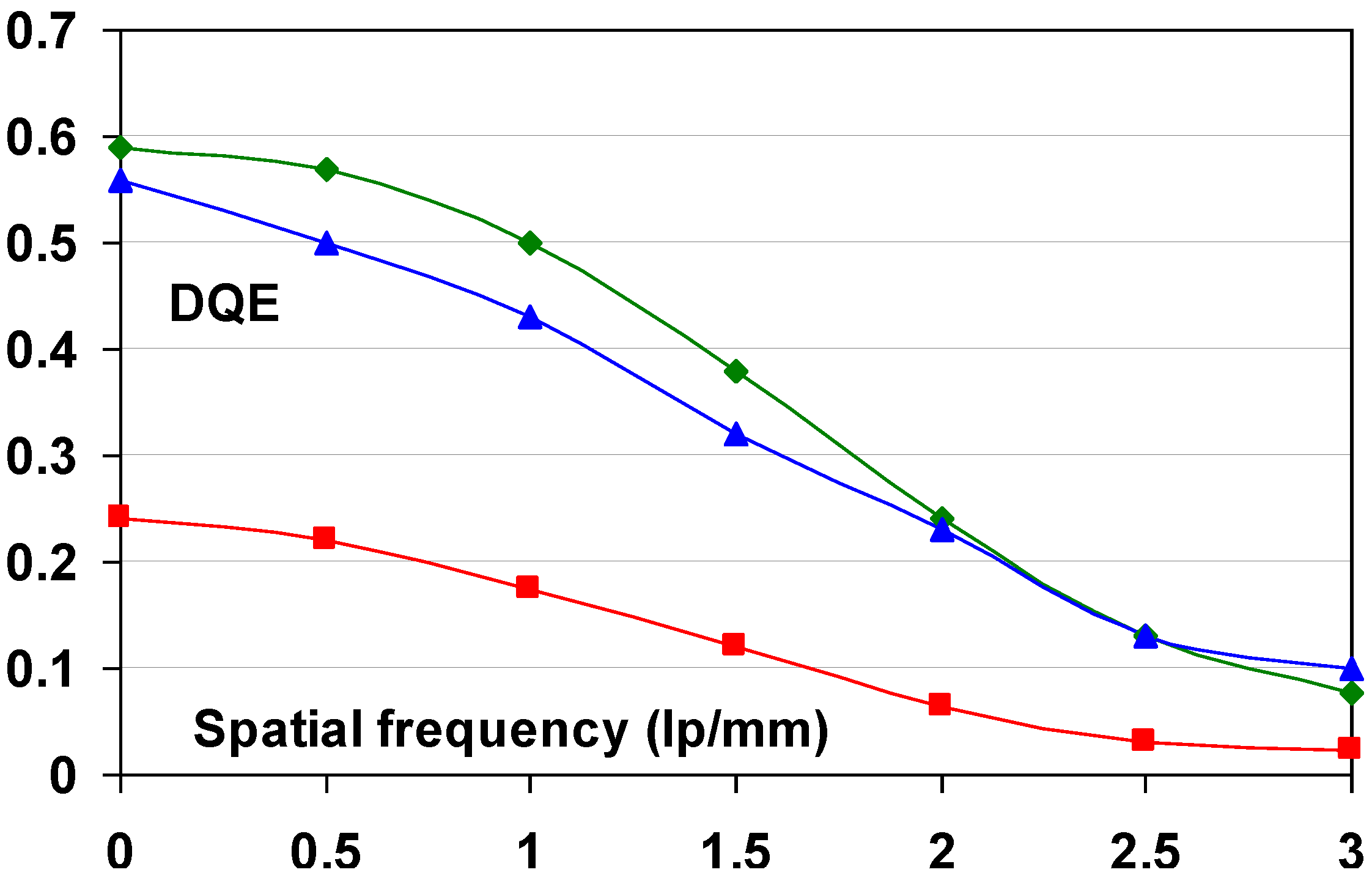
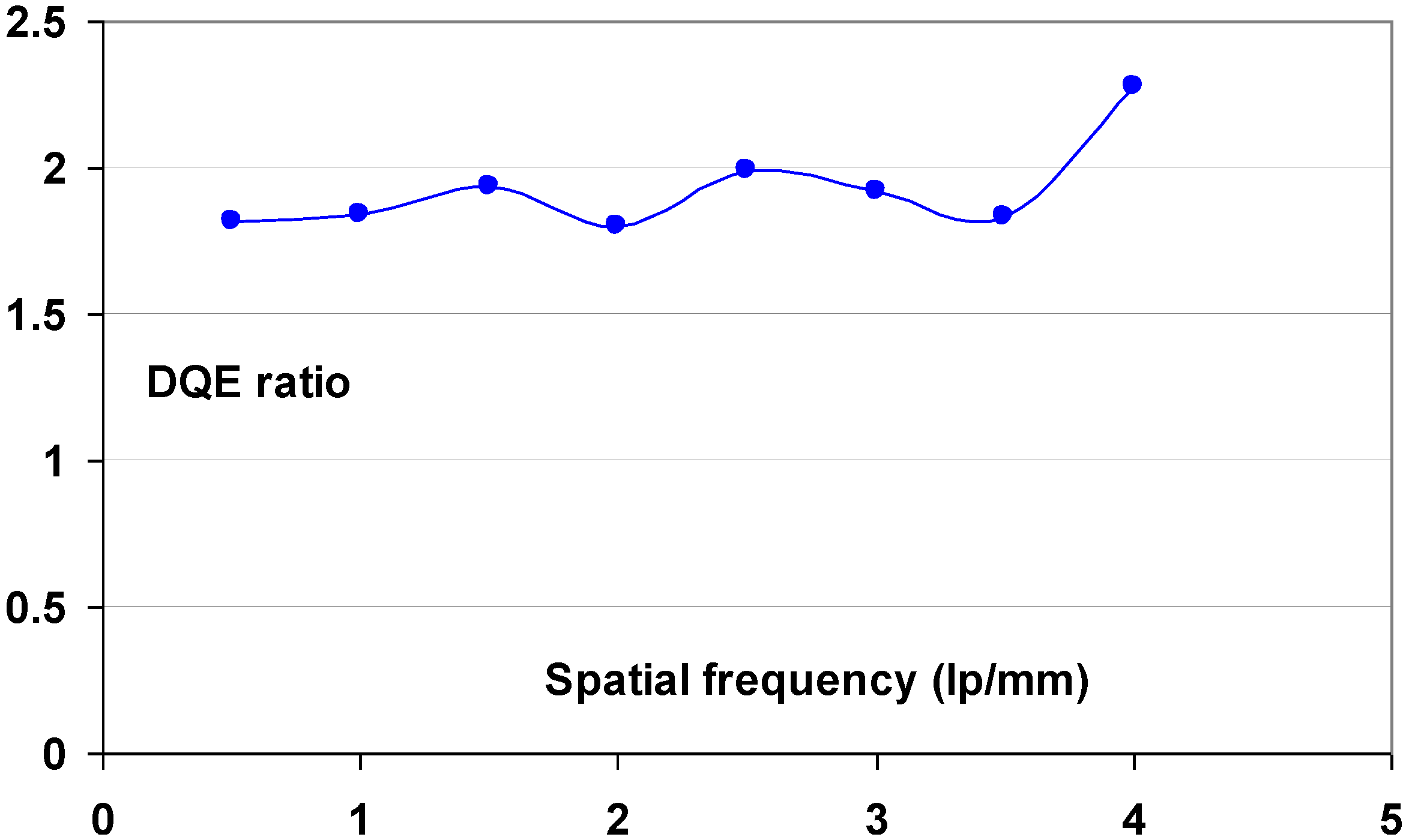
- (1) The Institute for Clinical Radiology in Munich evaluated image quality and anatomical detail depiction [112]. Dose-reduced radiographs were made with NIP and compared to full dose PIP images. 24 Supine chest radiographs were made with PIP at standard dose and compared to follow-up studies with NIP in which the dose was reduced by 50%. In a blind study, 6 independent readers rated the PIP and dose-reduced NIP images equally. The same group also evaluated the low-contrast performance of DX-S® compared to that of a PIP CR system [113]. A total of 36 images of a CDRAD phantom were made using nine different exposure conditions. A blind study of the images by five radiologists and five physicists lead to the conclusion that for all but two of the exposure settings NIP allowed visualization of significantly lower contrast levels. The remaining two settings also showed a trend toward better low contrast depiction with NIP.
- (2) A comparison of imaging performance with different doses in skeletal radiography was made for a PIP based CR system, DX-S® and a DR system [114]. The DR system was a Siemens flat-panel detector based on a CsI:Tl+ scintillator layer and an amorphous silicon sensor layer. Five independent blinded radiologists evaluated 72 plain radiographs of the feet of six human cadavers obtained with four surface entrance doses. The conclusion was that the radiation dose can be reduced by 75% in clinical skeletal imaging of peripheral extremities when NIP is used instead of PIP in CR. The DR system allowed a dose reduction of 50%.
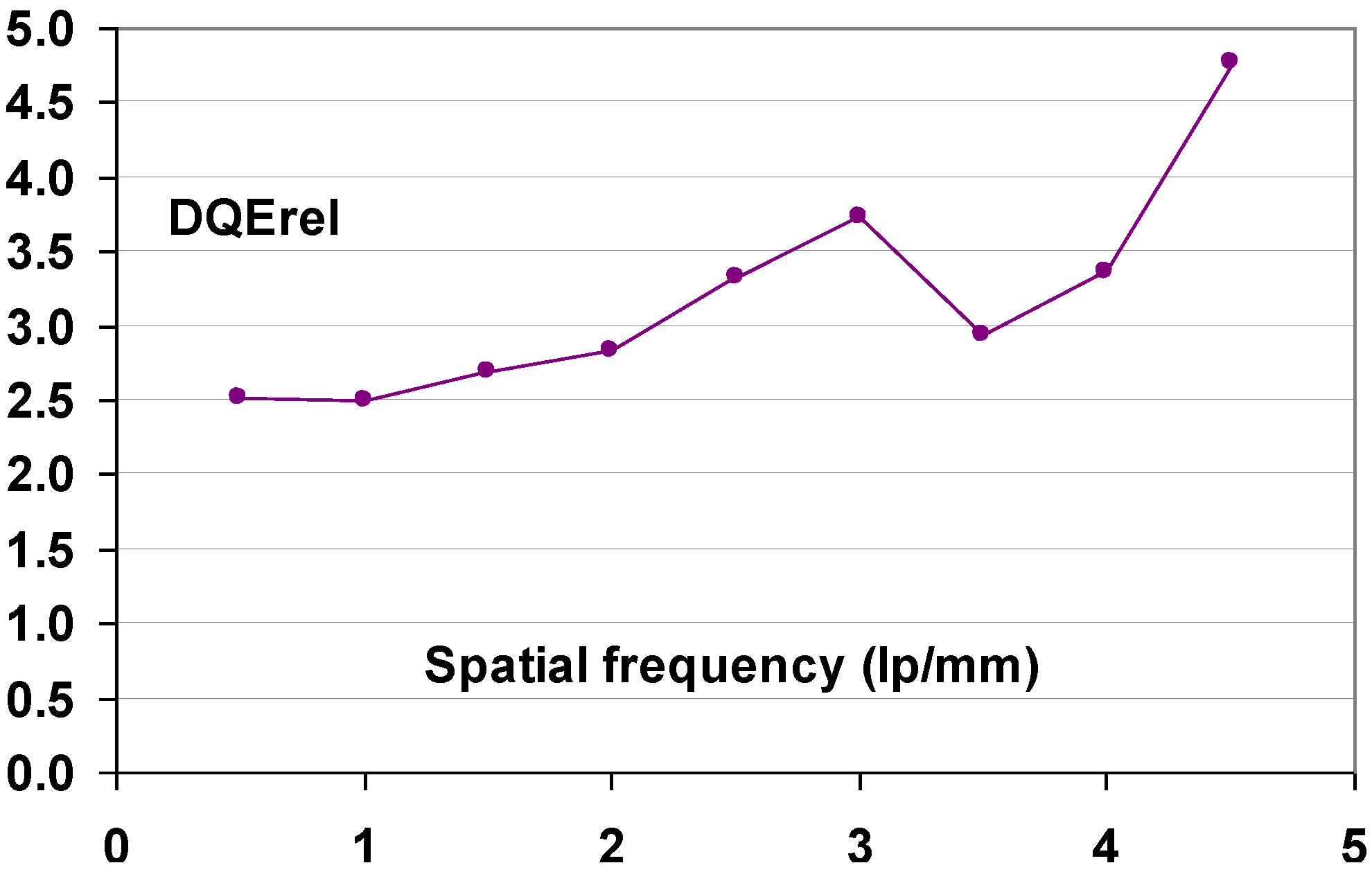
- (3) Physical image quality was compared for two CR and two flat-panel DR systems [115]. The CR systems were DX-S® and the PIP based Agfa ADC Compact Plus®. The first DR system, DR1, was a General Electric Revolution XQ/I® with an a-Si panel and a CsI:Tl+ scintillator; the second, DR2, was a Philips Diagnost®, based on the same technology. Image quality was assessed with a contrast-detail object and acrylic material to simulate clinical conditions. Important image quality differences were observed. DX-S® and DR2 showed similar image quality and were superior compared to DR1 and ADC Compact Plus®. It was further concluded that DX-S® provides better low-contrast detectability and a potential for dose reduction and that for doses over 0.2 mGy it provides even better image quality than DR1 and DR2.
- (4) Smans et al. tested CR image quality in neonatal imaging [116]. For typical acquisition parameters of neonatal chest X-ray examinations, the threshold-contrast detectability in simulated and acquired images of a contrast-detail phantom was compared. This was done for the Agfa ADC Compact Plus® with PIP and for DX-S®. Good agreement was found between the threshold-contrast curves of the simulated and experimentally acquired images and the superiority of NIP for neonatal imaging was confirmed.
- (5) Ludewig et al. simulated the conditions in neonatal radiological practice by making thoracic radiographs of cats. They compared DX-S® to ADC Compact Plus®. The conclusion was that a dose reduction of 50% seems possible without relevant deterioration of image quality [117].

10. Outlook
11. Conclutions
Acknowledgements
References
- Andriole, K.P. Computed Radiography Technology Overview. In RSNA Categorical Course in Physics: Technology Update and Quality Improvement of Diagnostic X-ray Imaging Equipment; Gould, R.G., Boone, J.M., Eds.; Radiological Society of North America Publication: Oak Brook, IL, USA, 1996; pp. 141–152. [Google Scholar]
- Luckey, G.W. Apparatus and method for producing images corresponding to patterns of high energy radiation. US Patent 3,859,527, 1975. [Google Scholar]
- Lakshmanan, A.R. Radiation induced defects and photostimulated luminescence process in BaFBr:Eu2+. Phys. Stat. Sol. (a) 1996, 153, 3–27. [Google Scholar] [CrossRef]
- Yen, W.M.; Weber, M.J. Inorganic Phosphors, Compositions, Preparation and Optical Properties; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Stevels, A.L.N.; Pingault, F. BaFCl:Eu2+, a new phosphor for X-ray intensifying screens. Philips Res. Repts. 1975, 30, 277–290. [Google Scholar]
- Nicklaus, E.; Fischer, F. F-centres of two types in BaFCl crystals. Phys. Stat. Sol. (b) 1972, 52, 453–460. [Google Scholar] [CrossRef]
- Kotera, N.; Eguchi, S.; Miyahara, J.; Matsumoto, H.; Kato, H. Method and apparatus for recording and reproducing a radiation image. US Patent 4,239,968, 1980. [Google Scholar]
- Sonoda, M.; Takano, M.; Miyahara, J.; Kato, H. Computed radiography utilizing scanning laser stimulated luminescence. Radiology 1983, 148, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Miyahara, J.; Shibahara, Y. Photostimulated luminescence (PSL) and color centers in BaFX:Eu2+ (X=Cl,Br,I) phosphors. J. Electrochem. Soc. 1985, 132, 1492–1494. [Google Scholar] [CrossRef]
- Hangleiter, Th.; Koschnick, F.-K.; Spaeth, J.-M.; Eachus, R.S. Photo-stimulated emission of X-irradiated BaFBr:Eu2+. Radiat. Eff. Defects Solids 1991, 615, 119–121. [Google Scholar]
- Koschnick, F.K. Magnetische Resonanzuntersuchungen am Röntgenspeicherleuchtstoff BaFBr:Eu. PhD Thesis, Universität Paderborn, Paderborn, Germany, 1991. [Google Scholar]
- von Seggern, H. Photostimulable X-ray storage phosphors: A review of present understanding. Braz. J. Phys. 1999, 29, 255–268. [Google Scholar]
- Ye, B.; Lin, J.-H.; Su, M.-Z. Two types of f-centers in BaFBr. J. Luminesc. 1988, 40-41, 323–324. [Google Scholar] [CrossRef]
- Takahashi, K.; Kohda, K.; Miyahara, J. Mechanism of photostimulated luminescence in BaFX: Eu2+ (X = Cl, Br) phosphors. J. Luminesc. 1984, 31-32, 266–268. [Google Scholar] [CrossRef]
- Itoh, N. Creation of lattice defects by electronic excitation in alkali halides. Adv. Phys. 1982, 31, 491–551. [Google Scholar] [CrossRef]
- Hangleiter, Th.; Koschnick, F.K.; Spaeth, J.-M.; Nuttall, R.H.D.; Eachus, R.S. Temperature dependence of the photostimulated luminescence of X-irradiated BaFBr:Eu2+. J. Phys. Condens. Matter 1990, 2, 6837–6846. [Google Scholar] [CrossRef]
- Eachus, R.S.; Koschnick, F.K.; Spaeth, J.-M.; Nuttall, R.H.D. The roles of oxygen impurities and defect aggregation in the performance of the storage phosphor BaFBr:Eu2+. In Proceedings of International Conference on Defects in Insulating Materials, Schloss Nordkirchen, Germany, 16–22 August 1992; Kanert, O., Spaeth, J.-M., Eds.; World Scientific: Singapore, 1992; pp. 267–269. [Google Scholar]
- Eachus, R.S.; Nuttall, R.H.D.; McDugle, W.G.; Koschnick, F.K.; Spaeth, J.M. The structure and mechanism of formation of OF−, OCl− and (O2−)Cl centers in X and UV-irradiated BaFCl. In Proceedings of International Conference on Defects in Insulating Materials, Nordkirchen, Germany, 16–22 August 1992; Kanert, O., Spaeth, J.M., Eds.; World Scientific: Singapore, 1992; pp. 1172–1174. [Google Scholar]
- Koschnick, F.K.; Spaeth, J.M.; Eachus, R.S.; McDugle, W.G.; Nuttall, R.H.D. Experimental evidence for the aggregation of photostimulable centers in BaFBr:Eu2+ single crystals by cross relaxation spectroscopy. Phys. Rev. Lett. 1991, 67, 3571–3574. [Google Scholar] [CrossRef] [PubMed]
- Eachus, R.S.; McDugle, W.G.; Nuttall, R.H.D.; Ohm, M.T.; Koschnik, F.K.; Hangleiter, T.; Spaeth, J.M. Radiation-produced electron and hole centres in oxygen-containing BaFBr: I EPR and ODEPR studies. J. Phys. Condens. Matter 1991, 3, 9327–9338. [Google Scholar] [CrossRef]
- Thoms, M.; von Seggern, H.; Winnacker, A. Spatial correlation and photostimulability of defect centers in the X-ray storage phosphor BaFBr:Eu2+. Phys. Rev. B Condens. Matter 1991, 44, 9240–9247. [Google Scholar] [CrossRef] [PubMed]
- von Seggern, H.; Voigt, T.; Knüpfer, W.; Lange, G. Physical model of the photostimulated luminescence of X-ray irradiated BaFBr:Eu2+. J. Appl. Phys. 1988, 64, 1405–1412. [Google Scholar] [CrossRef]
- von Seggern, H. X-ray storage phosphors: Physical mechanisms and applications. Cryst. Latt. Def. Amorph. Mat. 1989, 18, 399–417. [Google Scholar]
- de Leeuw, D.M.; Kovats, T.; Herko, S.P. Kinetics of Photostimulated Luminescence in BaFBr:Eu. J. Electrochem. Soc. 1987, 134, 491–493. [Google Scholar] [CrossRef]
- Koschnick, F.K.; Spaeth, J.M.; Eachus, R.S. Study of spatial correlations between radiation-induced defects and the activator Eu2+ in the X-ray storage phosphor BaFBr:Eu2+ with optical detection of electron paramagnetic resonance. J. Phys. Condens. Matter 1992, 4, 8919–8932. [Google Scholar] [CrossRef]
- Ivey, H.F. Spectral location of the absorption due to color centers in Alkali Halide crystals. Phys. Rev. 1947, 72, 341–343. [Google Scholar] [CrossRef]
- Nanto, H.; Araki, T.; Daimon, M.; Kusano, E.; Kinbara, A.; Kawabata, K.; Nakano, Y. Optically Stimulated Luminescence in an Imaging Plate Using BaFI:Eu. Radiat Prot Dosimetry 2002, 100, 385–388. [Google Scholar] [CrossRef]
- Nakano, Y.; Gido, T.; Honda, S.; Maezawa, A.; Wakamatsu, H.; Yanagita, T. Improved computed radiography image quality from a BaFI:Eu photostimulable phosphor plate. Med. Phys. 2002, 29, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Terrell, D.R.; Adriaensens, A.D.; Neyens, L.M.; Tecotzky, M.K.J.; Benjamin, S.D. Photostimulable phosphor for use in radiography. US Patent 5,514,298, 1995. [Google Scholar]
- Klee, R.J. An x-ray diffraction study of (Ba,Sr)F1+xBr1−x;Eu2+, a new x-ray image receptor material. J. Phys. D Appl. Phys. 1995, 28, 2529–2533. [Google Scholar] [CrossRef]
- Dietze, C.; Hangleiter, T.; Willems, P.; Leblans, P.J.R.; Struye, L.; Spaeth, J.M. Photostimulation redshift for nonstoichiometric Ba1−x Srx FBr:Eu2+. J. Appl. Phys. 1996, 80, 1074–1078. [Google Scholar] [CrossRef]
- Schweizer, S.; Spaeth, J.M.; Bastow, T.I. Generation of F centres and hole centres in the non-stoichiometric X-ray storage phosphor BaFBr. J. Phys. Condens. Matter 1998, 10, 9111–9122. [Google Scholar] [CrossRef]
- Schweizer, S. Physics and current understanding of X-ray storage phosphors. Phys. Stat. Sol. (A) 2001, 2, 335–393. [Google Scholar]
- Meijerink, A. Photostimulated luminescence and thermally stimulated luminescence of (Ba,Sr)F1+xBr1−x:Eu2+. Mater. Chem. Phys. 1996, 44, 170–177. [Google Scholar]
- Batentschuk, M.; Hackenschmied, P.; Winnacker, A. Optimization of mixed storage phosphors of the type (Ba,Sr)F1+xBr1−x:Eu for digital radiography. Mat. Res. Soc. Symp. Proc. 1999, 560, 27–32. [Google Scholar] [CrossRef]
- Hackenschmied, P.; Li, H.; Epelbaum, E.; Fasbender, R.; Batentschuk, M.; Winnacker, A. Energy transfer in Ba1−xSrxFBr:Eu storage phosphors as a function of Sr and Eu concentration. Radiat. Meas. 2001, 33, 669–674. [Google Scholar] [CrossRef]
- Dietze, C.; Hangleiter, T.; Willems, P.; Leblans, P.; Struye, L.; Spaeth, J.M. Photostimulation redshift for nonstoichiometric Ba1−xSrxFBr:Eu2+. J. Appl. Phys. 1996, 80, 1074–1078. [Google Scholar] [CrossRef]
- Schweizer, S.; Willems, P.; Leblans, P.J.R.; Struye, L. Electron traps in Ca2+- or Sr2+-doped BaFBr:Eu2+ X-ray storage phosphors. J. Appl. Phys. 1996, 79, 4157–4165. [Google Scholar] [CrossRef]
- Schlapp, M.; Bulur, E.; von Seggern, H. Photo-stimulated luminescence of calcium co-doped BaFBr:Eu2+ x-ray storage phosphors. J. Phys. D Appl. Phys. 2003, 36, 103–108. [Google Scholar] [CrossRef]
- Hosoi, Y.; Saito, H.; Kojima, Y.; Arakawa, S.; Takahashi, K. Mammographic imaging properties of an experimental SrFBr:Eu2+ imaging plate. Med. Imaging VI Instrum. 1992, 1651, 236–245. [Google Scholar]
- Meijerink, A.; Blasse, G.; Struye, L. A new photostimulable phosphor: Eu2+ activated bariumbromosilicate (Ba5SiO4Br6). Mater. Chem. Phys. 1989, 21, 261–270. [Google Scholar] [CrossRef]
- Meijerink, A. Luminescence of new X-ray storage phosphors. PhD Thesis, University of Utrecht, Utrecht, The Netherlands, 1990; pp. 35–43. [Google Scholar]
- Struye, L.; Leblans, P. Method for recording and viewing or reproducing a radiation image by means of an infrared-stimulable phosphor. European Patent 0559258, 1993. [Google Scholar]
- Meijerink, A.; Blasse, G. Photoluminescence and thermoluminescence properties of Ca2PO4Cl:Eu2+. J. Phys. Condens. Matter 1990, 2, 3619–3628. [Google Scholar] [CrossRef]
- Schipper, W.J.; Hamelink, J.J.; Langeveld, E.M.; Blasse, G. Trapping of electrons by H+ in the X-ray storage phosphor Ba3(PO4)2:Eu2+,La3+. J. Phys. D. Appl. Phys. 1993, 26, 1487–1492. [Google Scholar] [CrossRef]
- Zych, E.; Trojan-Pieggza, J.; Hreniak, D.; Strek, W. Properties of Tb-doped vacuum sintered Lu2O3 storage phosphor. J. Appl. Phys. 2003, 94, 1318–1324. [Google Scholar] [CrossRef]
- Pawlik, T.; Spaeth, J.M. Optical and magnetooptical investigation of the X-ray storage phosphor Cs2NaYF6:Ce3+. In Proceedings of International Conference on Inorganic Scintillators and Their Applications SCINT95; Dorenbos, P., Van Eijk, C.W.E., Eds.; Delft University Press: Delft, The Netherlands, 1996; pp. 392–395. [Google Scholar]
- Leblans, P.; Willems, P.; Struye, L.; Spaeth, J.M.; Pawlik, T. Class of high energy detecting phosphors. . European Patent 1994. [Google Scholar]
- Chakrabarti, K.; Mathur, V.K.; Thomas, L.A.; Abbundi, R.J. Charge trapping and mechanism of stimulated luminescence in CaS:Ce,Sm. J. Appl. Phys. 1989, 65, 2021–2023. [Google Scholar] [CrossRef]
- Chakrabarti, K.; Mathur, V.K.; Rhodes, J.F.; Abbundi, R.J. Stimulated luminescence in rare-earth doped MgS. J. Appl. Phys. 1989, 64, 1363–1366. [Google Scholar] [CrossRef]
- Nanto, H.; Hirai, Y.; Ikeda, M.; Kadota, M.; Nishishita, J.; Nasu, S.; Douguchi, Y. A novel image storage sensor using photostimulated luminescence in SrS:Eu,Sm phosphor for electromagnetic waves such as X-rays, UV-rays and visible light. Sens. Actuators A 1996, 53, 223–226. [Google Scholar] [CrossRef]
- Soltani, P. System for reading a phosphor screen used in filmless radiography. World Patent 1998/02780, 1996. [Google Scholar]
- Dorenbos, P. The 5d level positions of the trivalent lanthanides in inorganic compounds. J. Lumin. 2000, 91, 155–176. [Google Scholar] [CrossRef]
- Secu, M.; Schweitzer, S.; Spaeth, J.M.; Edgar, A.; Williams, G.V.M.; Rieser, U. Photostimulated luminescence from a fluorobromozirconate glass-ceramic and the effect of crystallite size and phase. J. Phys. Condens. Matter 2003, 15, 1097–1108. [Google Scholar] [CrossRef]
- Thornton, J.A. Influence of substrate temperature and deposition rate on structure of thick sputtered Cu coatings. J. Vac. Sci. Technol. 1975, 12, 830–835. [Google Scholar] [CrossRef]
- Thornton, J.A. High rate thick film growth. Annu. Rev. Mater. Sci. 1977, 7, 239–260. [Google Scholar] [CrossRef]
- Schmitt, B.; Fuchs, M.; Hell, E.; Knüpfer, W.; Hackenschmiedt, P.; Winnacker, A. Structured alkali halides for medical applications. Nucl. Instrum. Methods Phys. Res. Sect. B 2002, 191, 800–804. [Google Scholar] [CrossRef]
- Stevels, A.L.N.; Schrama-de Pauw, A.D.M. Vapour-deposited CsI:Na layers, II, screens for application in X-ray imaging devices. Philips Res. Rep. 1974, 29, 353–367. [Google Scholar]
- Brinkman, P. CsI(Na) scintillation crystals. Phys. Lett. 1965, 15, 305. [Google Scholar] [CrossRef]
- Bates, C.W. Scintillation in Thin Films of CsI(Na) and CsI(Tl) due to Low Energy X-rays, Electrons and Protons. Adv. Electron. Electron Phys. 1969, 28, 451–459. [Google Scholar]
- Wang, J.; Blackburn, T.F. X-ray Image Intensifiers for Fluoroscopy. Radiographics 2000, 20, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Stevels, A.L.N. New phosphors for X-ray screens. Med. Mundi 1975, 20, 12–22. [Google Scholar]
- Kano, T.; Takahashi, T.; Okajima, K.; Umetani, K.; Ataka, S.; Yokouchi, H.; Suzuki, R. Laser-stimulable transparant CsI:Na film for a high quality x-ray imaging sensor. Appl. Phys. Lett. 1986, 49, 1117–1118. [Google Scholar] [CrossRef]
- Perez-Mendez, V. Digital Radiography: Present Detectors and Future Developments Lawrence Berkeley Laboratory Reports 29441. 1990.
- Rowlands, J.A.; Yorkston, J. Flat Panel Detectors for Digital Radiography. In Handbook of Medical Imaging, vol. 1 Physics and Psychophysics; Beutel, J., Kunel, H.L., Van Metter, R.L., Eds.; SPIE Publications: Bellingham, WA, USA, 2000; pp. 223–328. [Google Scholar]
- Kobayashi, H.; Tanaka, S.; Iwase, N.; Yoshiyama, H. Photostimulated luminescence in BaFBr:Eu2+ evaporated films. J. Lumin. 1988, 40/41, 819–820. [Google Scholar] [CrossRef]
- Amitani, K.; Kano, A.; Tsuchino, H.; Shimada, F. X-ray Imaging System Utilizing New Photostimulable Phosphor. In Proceedings of SPIE’s Conference and Exhibition: Electric Imaging, 26th Fall Symposium, San Diego, CA, USA, October 1986; Printing of Paper Summaries. pp. 180–183.
- Amitani, K.; Kano, A.; Tsuchino, H.; Shimada, F. A new photostimulable phosphor detector for digital radiography. Konica Tech. Rep. 1988, 1, 120–125. [Google Scholar]
- von Seggern, H.; Voigt, T.; Schwarzmichel, K. Physical mechanisms of photostimulation in medical X-ray storage phosphors. Siemens Forsch. Entwickl. 1988, 17, 125–130. [Google Scholar]
- von Seggern, H.; Meijerink, A.; Voigt, T.; Winnacker, A. Photostimulated mechanism of x-ray-irradiated RbBr:Tl. J. Appl. Phys. 1989, 66, 4418–4424. [Google Scholar] [CrossRef]
- Sato, A.; Hara, H.; Kano, A. Development of x-ray direct digitizer Regius Model 530 and Regius Model 330M. Konica Tech. Rep. 1999, 12, 117–120. [Google Scholar]
- Rogulis, U.; Schweizer, S.; Assmann, S.; Spaeth, J.M. Ga2+ hole centers and photostimulated luminescence in the x-ray storage phosphor RbBr:Ga+. J. Appl. Phys. 1998, 84, 4537–4542. [Google Scholar] [CrossRef]
- Rogulis, U.; Schweizer, S.; Assmann, S.; Spaeth, J.M. Photostimulated luminescence process in the x-ray storage phosphor CsBr:Ga+. J. Appl. Phys. 2000, 8, 207–211. [Google Scholar] [CrossRef]
- Hangleiter, T.; Rogulis, U.; Dietze, C.; Spaeth, J.M.; Willems, P.; Leblans, P.J.R. The X-ray Storage Phosphors RbI:Tl+ and KBr:In+ and other In+ and Ga+ Doped Alkali Halides. In Proceedings of International Conference on Inorganic Scintillators and Their Applications, SCINT95, Delft, The Netherlands, 28 August–1 September 1995; Delft University Press: Delft, The Netherland, 1996; pp. 452–455. [Google Scholar]
- Leblans, P.; Struye, L.; Gebele, H. A new detector for digital radiography: The best of two worlds. Phys. Med. 1999, XV, 301–304. [Google Scholar]
- Niilisk, A.; Soovik, T.; Tatarly, V. Luminescence Decay Time for Ga+ Centres in Alkali Halides. Phys. Stat. Sol. (B) 1974, 64, 135–138. [Google Scholar] [CrossRef]
- Frankenberger, J.; Mair, S.; Herrmann, C.; Lamotte, J.; Fasbender, R. Reflective and Transmissive CR Scanhead Technology on Needle Image Plates. Proc. SPIE 2005, 5745, 499–510. [Google Scholar]
- Leblans, P.; Struye, L.; Willems, P. New needle-crystalline CR detector. J. Digital Imaging 2000, 13, 117–120. [Google Scholar] [CrossRef]
- Leblans, P.J.R.; Struye, L.; Willems, P. New needle-crystalline CR detector. Proc. SPIE 2001, 4320, 59–67. [Google Scholar]
- Vandenbroucke, D.A.N.; Leblans, P.J.R. CR mammography: Image quality measurement and model calculation for needle vs. powder imaging plate. Lect. Notes Comput. Sci. 2010, 6136, 219–226. [Google Scholar]
- Leblans, P.; Struye, S. A cesium bromide storage phosphor with narrow emission spectrum upon UV-excitation. European Patent 1217633, 2001. [Google Scholar]
- Hackenschmied, P.; Schierning, G.; Batentschuk, M.; Winnacker, A. Precipitation-induced photostimulated luminescence in CsBr:Eu2+. J. Appl. Phys. 2003, 93, 5109–5112. [Google Scholar] [CrossRef]
- Weidner, M.; Osvet, A.; Meister, F.; Batentschuk, M.; Winnacker, A.; Tahon, J.-P.; Leblans, P. Luminescence spectroscopy of Eu2+ in CsBr:Eu needle image plates (NIPs). Radiat. Meas. 2007, 42, 661–664. [Google Scholar] [CrossRef]
- Weidner, M. Einfluss der Temperbedigungen auf Lumineszenz- und Speicherzentren in CsBr:Eu2+ Nadelbildplatten. PhD Thesis, Universität Erlangen-Nürnberg, Nürnberg, Germany, 2009. [Google Scholar]
- Appleby, G.A.; Zimmerman, J.; Hesse, S.; Karg, O.; von Seggern, H. Sensitization of the photostimulated X-ray storage-phosphor CsBr:Eu2+ following room-temperature hydration. J. Appl. Phys. 2009, 20, 54–58. [Google Scholar]
- Schweizer, S.; Rogulis, U.; Assmann, S.; Spaeth, J.M. RbBr and CsBr doped with Eu2+ as new competitive X-ray storage phosphors. Radiat. Meas. 2001, 33, 486–486. [Google Scholar]
- Fowler, W.B. Physics of Color Centres; Academic Press: San Diego, CA, USA, 1968. [Google Scholar]
- Hackenschmied, P.; Zeitler, G.; Batentschuk, M.; Winnacker, A.; Schmitt, B.; Fuchs, M.; Hell, E.; Knüpfer, W. Storage performance of X-ray irradiated doped CsBr. Nucl. Instrum. Methods Phys. Res. Sect. B 2002, 191, 163–167. [Google Scholar] [CrossRef]
- Hackenschmied, P. Funktion und Herstellung von Speicherleuchtstoffen für die digitale Röntgendiagnostik. PhD Thesis, Universität Erlangen-Nürnberg, Nürnberg, Germany, 2002. [Google Scholar]
- Schierning, G. Untersuchung von Nanophasen und deren Einfluss auf den Speichermechanismus von CsBr:Eu. PhD Thesis, Diss, Universität Erlangen-Nürnberg, Nürnberg, Germany, 2005. [Google Scholar]
- Schierning, G.; Batentschuk, M.; Winnacker, A.; Struye, L.; Tahon, J.-P.; Leblans, P. Correlation of the dielectric constant and the PSL-sensitivity in CsBr:Eu image plates. In Proceedings of LUMDETR 2006: European Conference on Luminescent Detectors and Transformers of Ionizing Radiation No. 6, Lviv, Ukraine, 19–23 June 2007; Volume 42, pp. 657–660.
- Tahon, J.P.; Project Manager Phosphors. Personal communication, Agfa HealthCare NV: Mortsel, België, 2011.
- Hesse, S.; Zimmerman, J.; von Seggern, H.; Ehrenberg, H.; Fuess, H. The role of segregations and oxygen doping in the photostimulation mechanism of CsBr:Eu2+. Radiat. Meas. 2007, 42, 638–643. [Google Scholar] [CrossRef]
- Hesse, S.; Zimmerman, J.; von Seggern, H.; Ehrenberg, H.; Fuess, H.; Fasel, C.; Riedel, R. CsEuBr3: Crystal structure and its role in the photostimulation of CsBr:Eu2+. J. Appl. Phys. 2006, 100, 083506:1–083506:5. [Google Scholar] [CrossRef]
- Loncke, F.; Vrielinck, H.; Matthys, P.; Callens, F.; Tahon, J.-P.; Leblans, P. Paramagnetic Eu2+ center as a probe for the sensitivity of CsBr x-ray needle image plates. Appl. Phys. Lett. 2008, 92, 204102:1–204102:3. [Google Scholar] [CrossRef]
- Loncke, F.; Vrielinck, H.; Matthys, P.; Callens, F.; Tahon, J.-P.; Leblans, P. Electron paramagnetic resonance study of a Eu2+ related defect in CsBr:Eu needle imaging plates for computed radiography. Spectrochmica Acta Part A 2008, 69, 1322–1326. [Google Scholar] [CrossRef]
- Loncke, F.; Vrielinck, H.; Matthys, P.; Callens, F.; Tahon, J.P.; Leblans, P. Temperature dependence of Eu-related EPR spectra in CsBr:Eu needle image plates. Phys. Rev. B 2009, 79, 174102:1–174102:9. [Google Scholar] [CrossRef]
- Loncke, F. Magnetic Resonance Study of Dopant Related Defects in X-ray Storage Phosphors. PhD Thesis, Faculteit Wetenschappen, Vakgroep Vastestofwetenschappen, Universiteit Gent, Gent, Belgium, 2009. [Google Scholar]
- Vrielinck, H.; Loncke, F.; Tahon, J.P.; Leblans, P.; Matthys, P.; Callens, F. Electron nuclear double resonance study of photostimulated luminescence active centers in CsBr:Eu2+ medical imaging plates. Phys. Rev. B 2011, 83, 054102:1–054102:9. [Google Scholar] [CrossRef]
- Lidiard, A.B. Handbuch der Physik; Flügge, S., Ed.; Springer: Berlin, Germany, 1957; Volume 20, p. 246. [Google Scholar]
- Granfors, P.R. Medical Electrical Equipment—Characteristics of Digital X-ray Imaging Devices—Determination of the Detective Quantum Efficiency; IEC standard: IEC62220-1; IEC: Geneva, Switzerland, 2003. [Google Scholar]
- Samei, E.; Flynn, M.J.; Reimann, D.A. A method for measuring the presampled MTF of digital radiographic systems using an edge test device. Med. Phys. 1998, 25, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.B.; Mangiafico, P.A.; Simoni, P.U. Noise power spectra of images from digital mammography detectors. Med. Phys. 1999, 26, 1279–1293. [Google Scholar] [CrossRef] [PubMed]
- Aufrichtig, R.; Su, Y.; Cheng, Y.; Granfors, P.R. Measurement of the noise power spectrum in digital x-ray detectors. Proc. Spie 2001, 4320, 363–372. [Google Scholar]
- Shaw, R.; Van Metter, R.L. An analysis of the fundamental limitations of screen-film systems for x-ray detection: I. General theory. Proc. SPIE 1984, 454, 133–141. [Google Scholar]
- Dick, C.E.; Motz, J.W. Image information transfer properties of X-ray fluorescent screens. Med. Phys. 1981, 11, 37–46. [Google Scholar]
- Hillen, W.; Schiebel, U.; Zaengel, T. Imaging performance of a digital storage phosphor system. Med. Phys. 1987, 14, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Schaetzing, R.; Fasbender, R.; Kersten, P. New high-speed scanning technique for computed radiography. Proc. SPIE 2002, 4682, 511–522. [Google Scholar]
- Shimada, K.; Yasuda, H.; Arakawa, S.; Kuwabara, T.; Takasu, A.; Iwabuchi, Y.; Katou, M. Comparison in image quality and noise component of columnar phosphor plate and powder phosphor plate. Proc. SPIE 2007, 6510, 651044. [Google Scholar]
- Willems, P.; Leblans, P.J.R.; De Winter, B.M.F. Needle vs. Powder Imaging Plate for Computer Radiography: Image quality Measurement and Model Calculation. In Proceedings of NIP21: International Conference on Digital Printing Technologies, Baltimore, MD, USA, September 2005; Volume 21, pp. 657–660.
- Mackenzie, A.; Honey, I.D. Characterization of noise sources for two generations of computed radiography systems using powder and crystalline photostimulable phosphors. Med. Phys. 2007, 34, 3345–3357. [Google Scholar] [CrossRef] [PubMed]
- Körner, M.; Wirth, S.; Treitl, M.; Reiser, M.; Pfeifer, K.J. Initial Clinical Results with a New Needle Screen Storage Phosphor System in Chest Radiograms. Fortschr. Röntgenstr. 2005, 177, 1491–1496. [Google Scholar] [CrossRef]
- Körner, M.; Treitl, M.; Schaetzing, R.; Pfeifer, K.J.; Reiser, M.; Wirth, S. Depiction of low-contrast detail in digital radiography; comparison of powder- and needle-structured storage phosphor systems. Invest. Radiol. 2006, 41, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Wirth, S.; Treitl, M.; Reiser, M.F.; Körner, M. Imaging performance with different doses in skeletal radiography: Comparison of a needle-structured and a conventional storage phosphor system with a flat-panel detector. Radiology 2009, 250, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.M.; Ordiales, J.M.; Guibelalde, E.; Prieto, C.; Vano, E. Physical image quality comparison of four types of digital detector for chest radiology. Radiat. Prot. Dosim. 2008, 129, 140–143. [Google Scholar] [CrossRef]
- Smans, K.; Vandenbroucke, D.; Pauwels, H.; Struelens, L.; Vanhavere, F.; Bosmans, H. Validation of an image simulation technique for two computed radiography systems: An application to neonatal imaging. Med. Phys. 2010, 37, 2092–2100. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, E.; Hirsch, W.; Bosch, B.; Gäbler, K.; Sorge, I.; Succow, D.; Werrmann, A.; Gosch, D. Untersuchungen zur Qualität von Thoraxaufnahmen bei Katzen mit einem auf einer Nadelstruktur basierenden Speicherfoliensystem—Modelluntersuchungen zur Bewertung der Bildqualität bei Nuegeborenen. Fortschr. Röntgenstr. 2010, 182, 122–132. [Google Scholar] [CrossRef]
- Herrmann, C.; Frankenberger, J.; Reiser, G.; Lamotte, J. Optimization of a CR system comprising line-scanning and needle image plate technology with respect to examinations of extremities. Proc. SPIE 2007, 6510, 1–9. [Google Scholar]
- Peloschek, P.; Langs, G.; Semturs, F. First experiences with a new, needle-based CR mammography system: Evaluation of image quality and dose levels and comparison to other currently available CR and DR systems and to a highly sensitive screen-film system. In Presented at the European Congress of Radiology in Vienna, Vienna, Austria, 4–8 March 2011; 2011. Poster C-1156. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Leblans, P.; Vandenbroucke, D.; Willems, P. Storage Phosphors for Medical Imaging. Materials 2011, 4, 1034-1086. https://doi.org/10.3390/ma4061034
Leblans P, Vandenbroucke D, Willems P. Storage Phosphors for Medical Imaging. Materials. 2011; 4(6):1034-1086. https://doi.org/10.3390/ma4061034
Chicago/Turabian StyleLeblans, Paul, Dirk Vandenbroucke, and Peter Willems. 2011. "Storage Phosphors for Medical Imaging" Materials 4, no. 6: 1034-1086. https://doi.org/10.3390/ma4061034
APA StyleLeblans, P., Vandenbroucke, D., & Willems, P. (2011). Storage Phosphors for Medical Imaging. Materials, 4(6), 1034-1086. https://doi.org/10.3390/ma4061034