Thermodynamic Origin of the Vitreous Transition
Abstract
:1. Introduction
2. Model
2.1. New Equations Governing the Crystal Nucleation



2.2. The Ideal Glass Transition Temperature T0 and The Energy Saving Associated with Crystal Formation



 are respected when the double solution corresponds to a minimum value of εlps0 larger than 1. It is given by (7,8) and occurs when 9 − 4 × (2 − εlps0) × εlps0/θ20lps = 0:
are respected when the double solution corresponds to a minimum value of εlps0 larger than 1. It is given by (7,8) and occurs when 9 − 4 × (2 − εlps0) × εlps0/θ20lps = 0:


3. Review of Experimental Results and Discussion
3.1. Presentation of Table 1 and Figure 1
| Glass | Tm | Tg | θg | T01 | T02 | T0g | εls0 | εlgs0 | T0m | References | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | As2S3 | 585 | 481 | −0.178 | 270 | 319 | 1.822 | 1.732 | 363 | [32,33] | |
| 2 | Propylene glycol | 214 | 167 | −0.220 | 117 | 108 | 1.780 | 1.671 | 125 | [26,34,35] | |
| 3 | SiO2 | 1,993 | 1,473 | −0.261 | 300 | 1.36 | [2,36] | ||||
| 4 | Propylene carbonate | 217 | 160 | −0.263 | 130 | 102 | 1.737 | 1.606 | 119 | [35] | |
| 5 | polystyrene | 513 | 375 | −0.269 | 323 | 239 | 1.731 | 1.596 | 280 | [26,37] | |
| 6 | Pd43Ni10Cu27P20 | 802 | 585 | −0.271 | 452 | 372 | 1.729 | 1.594 | 436 | [29,38,39] | |
| 7 | O-Terphenil | 329 | 240 | −0.271 | 208 | 153 | 1.729 | 1.593 | 179 | [3,26,40] | |
| 8 | Pd40Cu30Ni10P20 | 823 | 578 | −0.298 | 447 | 366 | 1.702 | 1.553 | 432 | [29,31,39] | |
| 9 | Salol | 315 | 220 | −0.302 | 183 | 139 | 1.698 | 1.548 | 165 | [3,26,35] | |
| 10 | As2Se3 | 645 | 450 | −0.302 | 335 | 285 | 1.698 | 1.547 | 337 | [41] | |
| 11 | B2O3 | 783 | 545 | −0.304 | 402 | 345 | 1.696 | 1.544 | 408 | [3,11,32] | |
| 12 | B2O3 | 783 | 521 | −0.335 | 263 | 330 | 1.665 | 1.498 | 393 | [3,11,32] | |
| 13 | Bromopentane | 158 | 107 | −0.323 | 74 | 68 | 1.677 | 1.516 | 80 | [26] | |
| 14 | ZnCl2 | 565 | 380 | −0.327 | 274 | 241 | 1.673 | 1.509 | 286 | [2,26] | |
| 15 | Butene 1 | 88 | 59 | −0.330 | 37 | 1.670 | 1.506 | 44 | [2] | ||
| 16 | Zr41.2Ti13.8Cu12.5Ni10Be22.5 | 937 | 625 | −0.333 | 413 | 396 | 1.667 | 1.501 | 472 | [39,42] | |
| 17 | La55Al25Ni10Cu10 | 662 | 441 | −0.334 | 255 | 280 | 1.666 | 1.499 | 333 | [43,44,45] | |
| 18 | 2 Methylpentane | 120 | 80 | −0.338 | 58 | 50 | 1.663 | 1.494 | 60 | [2,26,46] | |
| 19 | Toluene | 178 | 117 | −0.343 | 104 | 74 | 1.657 | 1.485 | 89 | [47] | |
| 20 | Glycerol | 293 | 190 | −0.352 | 128 | 121 | 1.648 | 1.473 | 144 | [26,34,35] | |
| 21 | La55Al25Ni20 | 728 | 470 | −0.354 | 307 | 299 | 1.646 | 1.469 | 358 | [43,44,45] | |
| 22 | La55Al25Ni20 | 925 | 470 | −0.492 | 309 | 330 | 1.508 | 1.262 | 394 | [43,44,45] | |
| 23 | PET = (C10H8O4)n | 542 | 342 | −0.369 | 219 | 1.631 | 1.446 | 262 | [2] | ||
| 24 | Pd40Ni40P20 | 884 | 554 | −0.373 | 356 | 355 | 1.627 | 1.440 | 425 | [48,49] | |
| 25 | Pd40Ni40P20 | 987 | 554 | −0.439 | 356 | 369 | 1.561 | 1.342 | 442 | [48,49] | |
| 26 | Pt57.5Cu14.7Ni5.3P22.5 | 813 | 509 | −0.374 | 336 | 326 | 1.626 | 1.439 | 390 | [50] | |
| 27 | Pd0.775Cu0.06Si0.165 | 1,015 | 632 | −0.377 | 515 | 405 | 1.623 | 1.434 | 486 | [51] | |
| 28 | La55Al25Ni5Cu15 | 700 | 436 | −0.378 | 286 | 279 | 1.622 | 1.433 | 335 | [43,44,45] | |
| 29 | Zr52.5Cu17.9Ni14.6Al10Ti5 | 1,090 | 675 | −0.381 | 521 | 434 | 1.619 | 1.429 | 519 | [52] | |
| 30 | Pt57.3Cu14.6Ni5.3P22.8 | 788 | 487 | −0.382 | 336 | 313 | 1.618 | 1.427 | 375 | [50] | |
| 31 | Se | 491 | 303 | −0.383 | 220 | 195 | 1.617 | 1.426 | 233 | [2,53] | |
| 32 | La55Al25Ni15Cu5 | 729 | 449 | −0.384 | 273 | 289 | 1.616 | 1.424 | 346 | [43,44,45] | |
| 33 | Au49Ag5.5Pd2.3Cu26.9Si16.3 | 655 | 403 | −0.385 | 250 | 259 | 1.615 | 1.423 | 311 | [39] | |
| 34 | Ethanol | 159 | 97 | −0.390 | 78 | 58 | 63 | 1.610 | 1.415 | 75 | [2,11,32] |
| 35 | Zr58.5Cu15.6Ni12.8Al10.3Nb2.8 | 1,110 | 673 | −0.394 | 437 | 435 | 1.606 | 1.409 | 522 | [54] | |
| 36 | Zr57Cu15.4Ni12.6Al10.3Nb5 | 1,120 | 678 | −0.395 | 525 | 438 | 1.605 | 1.408 | 526 | [52] | |
| 37 | Pr55Ni25Al20 | 820 | 494 | −0.398 | 296 | 320 | 1.602 | 1.404 | 384 | [55] | |
| 38 | Ti41.5Cu37.5Ni7.5Zr2.5Hf5Sn5Si1 | 1,176 | 693 | −0.411 | 452 | 1.589 | 1.384 | 543 | [56] | ||
| 39 | Cu47Ti34Zr11Ni8 | 1,144 | 673 | −0.412 | 500 | 439 | 1.588 | 1.382 | 527 | [57] | |
| 40 | Y56Al24Co20 | 1,085 | 636 | −0.414 | 614 | 416 | 1.586 | 1.379 | 499 | [58] | |
| 41 | La55Al25Ni5Cu10Co5 | 754 | 439 | −0.418 | 241 | 288 | 1.582 | 1.374 | 345 | [43,44,45] | |
| 42 | Mg59.5Cu22.9Ag6.6Gd11 | 734 | 425 | −0.421 | 249 | 279 | 1.579 | 1.369 | 335 | [59] | |
| 43 | Mg61Cu28Gd11 | 737 | 422 | −0.427 | 256 | 278 | 1.573 | 1.359 | 334 | [59] | |
| 44 | Mg65Cu25Y10 | 739 | 400 | −0.459 | 363 | 260 | 271 | 1.541 | 1.312 | 325 | [27] |
| 45 | Zr46.75Ti8.25Cu7.5Ni10Be27.5 | 1,070 | 595 | −0.444 | 372 | 398 | 1.556 | 1.334 | 477 | [60] | |
| 46 | Cyclo-octanol | 298 | 165 | −0.446 | 92 | 110 | 1.554 | 1.331 | 133 | [26] | |
| 47 | Zr65Al10Ni10Cu15 | 1,161 | 641 | −0.448 | 437 | 430 | 1.552 | 1.328 | 516 | [39] | |
| 48 | Ce60Al10Ni10Cu20 | 677 | 373 | −0.449 | 331 | 250 | 1.551 | 1.326 | 300 | [61] | |
| 49 | Al87Co4Ce9 | 1,104 | 573 | −0.481 | 397 | 1.519 | 1.279 | 475 | [62] | ||
| 50 | Hevea rubber | 421 | 200 | −0.525 | 136 | 147 | 1.475 | 1.213 | 174 | [32] | |
| 51 | Au0.77Ge0.136Si0.094 | 629 | 290 | −0.539 | 241 | 217 | 1.461 | 1.192 | 257 | [63] |
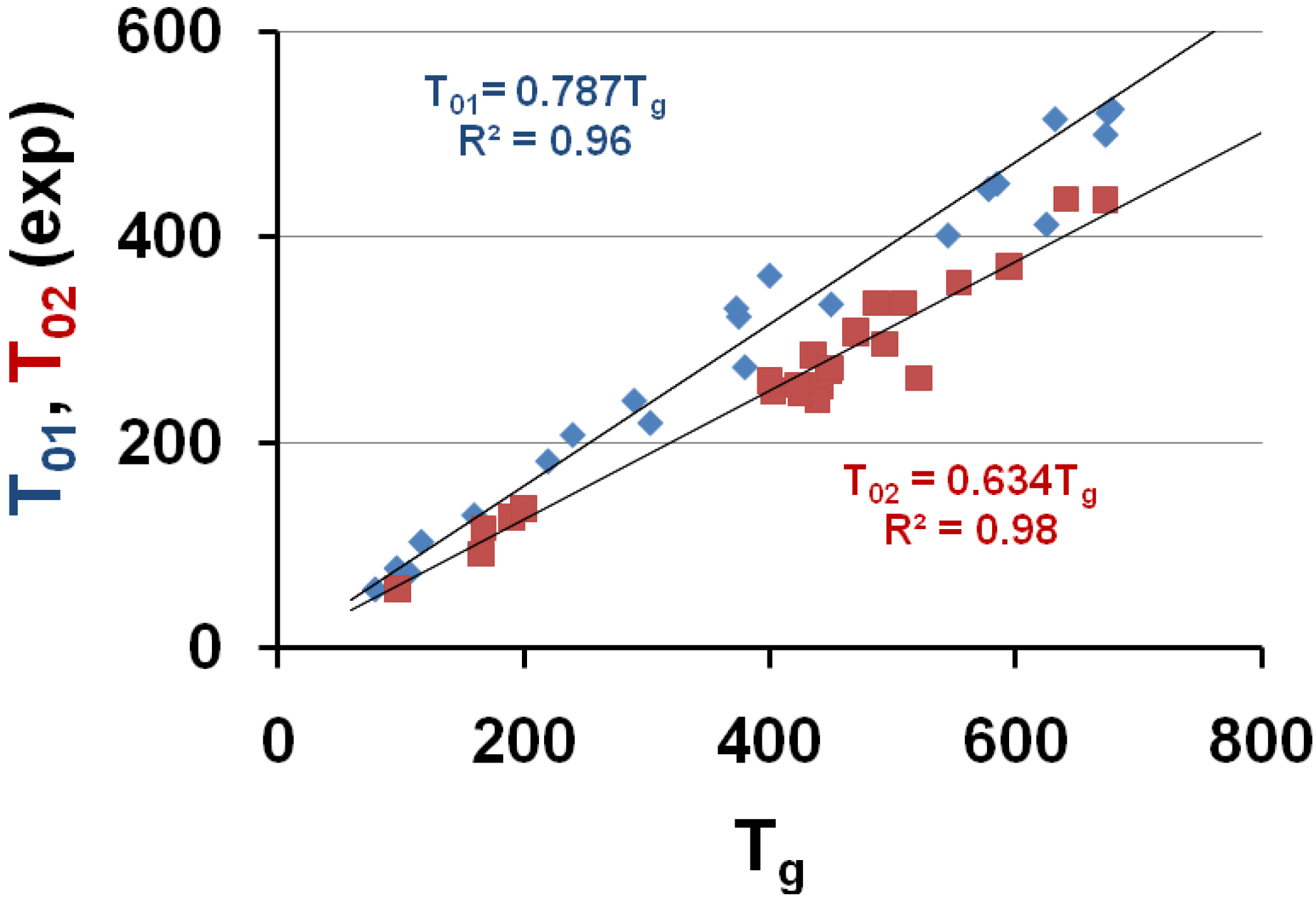
3.2.Homogeneous Nucleation Temperature of Vitreous Phase and Relaxation Time









 are nearly the same in all glass-forming melts at Tg.
are nearly the same in all glass-forming melts at Tg. is generally of the order of 100 s at
Tg. A value τ0 = 1.4 × 10−14 s is deduced with B/(Tg − T0g) = 36.5 and τ0 = 3.14 × 10−15 s with B/(Tg − T0g) = 38 in all glasses [3,9,27]. Equation (17) giving τ0 is used to determine lnAg only, depending on {ln(1 + θ2lgs] − ln[Vm × ΔSm].
is generally of the order of 100 s at
Tg. A value τ0 = 1.4 × 10−14 s is deduced with B/(Tg − T0g) = 36.5 and τ0 = 3.14 × 10−15 s with B/(Tg − T0g) = 38 in all glasses [3,9,27]. Equation (17) giving τ0 is used to determine lnAg only, depending on {ln(1 + θ2lgs] − ln[Vm × ΔSm].3.3. The Thermodynamic Vitreous Transition T*g at the Disappearance Temperature of the Fully-Relaxed Enthalpy
 . The following calculated values of ΔCpgl are in good agreement with the specific heat difference ΔCpls between solid and liquid; for propylene glycol, ΔCpgl = 52.3 and ΔCpls = 67.3 J/mole/K [68,69]; for glycerol, ΔCpgl = 69.9 and ΔCpls ≅ 79.4 J/mole/K [32,68,69]; for vit106a, ΔCpgl = 13.5 and ΔCpls ≅ 15.5 J/mole/K [54]; for As2Se3, ΔCpgl = 67 J/mole/K from the relaxed enthalpy and 67 J/mole/K from the reversible specific heat [67]. The specific heat jump ΔCpls is a little too large at Tg when it is measured at a too low heating rate because it still contains an endothermic contribution except when stepscan techniques are used [13].
. The following calculated values of ΔCpgl are in good agreement with the specific heat difference ΔCpls between solid and liquid; for propylene glycol, ΔCpgl = 52.3 and ΔCpls = 67.3 J/mole/K [68,69]; for glycerol, ΔCpgl = 69.9 and ΔCpls ≅ 79.4 J/mole/K [32,68,69]; for vit106a, ΔCpgl = 13.5 and ΔCpls ≅ 15.5 J/mole/K [54]; for As2Se3, ΔCpgl = 67 J/mole/K from the relaxed enthalpy and 67 J/mole/K from the reversible specific heat [67]. The specific heat jump ΔCpls is a little too large at Tg when it is measured at a too low heating rate because it still contains an endothermic contribution except when stepscan techniques are used [13].
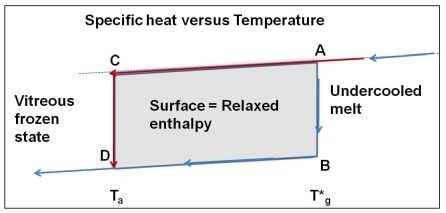
3.4. The Crystal Homogeneous Nucleation Temperature at T*g



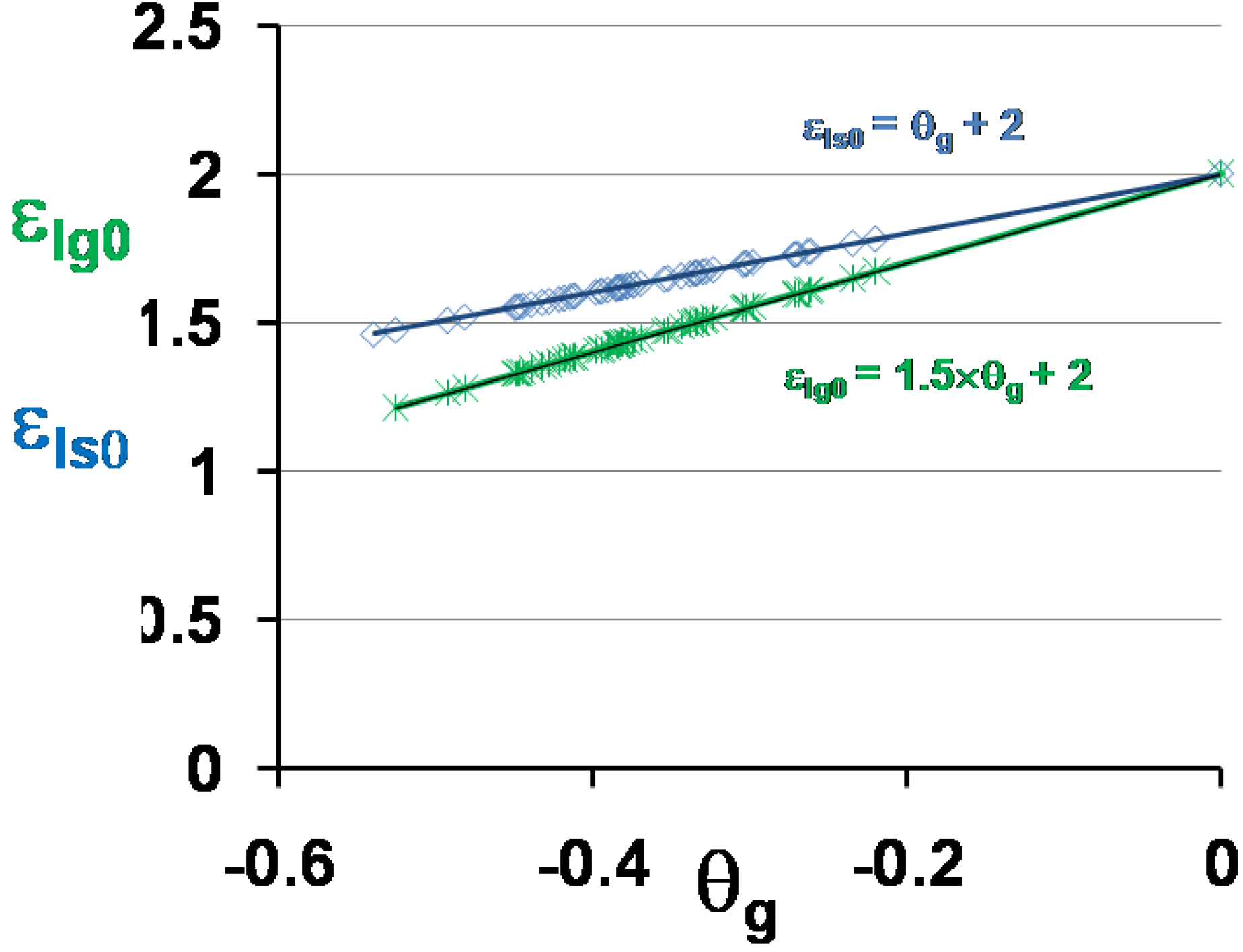
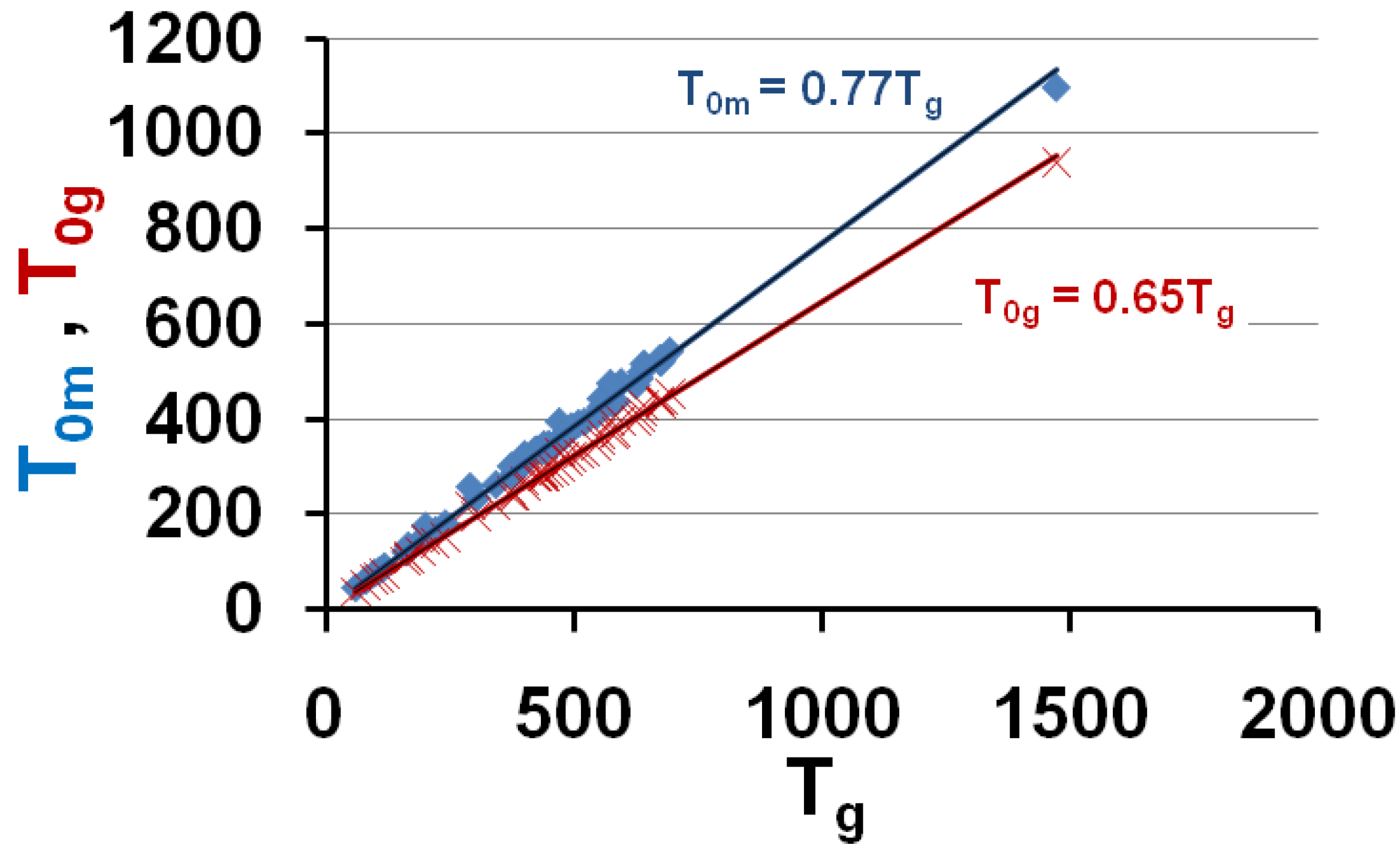
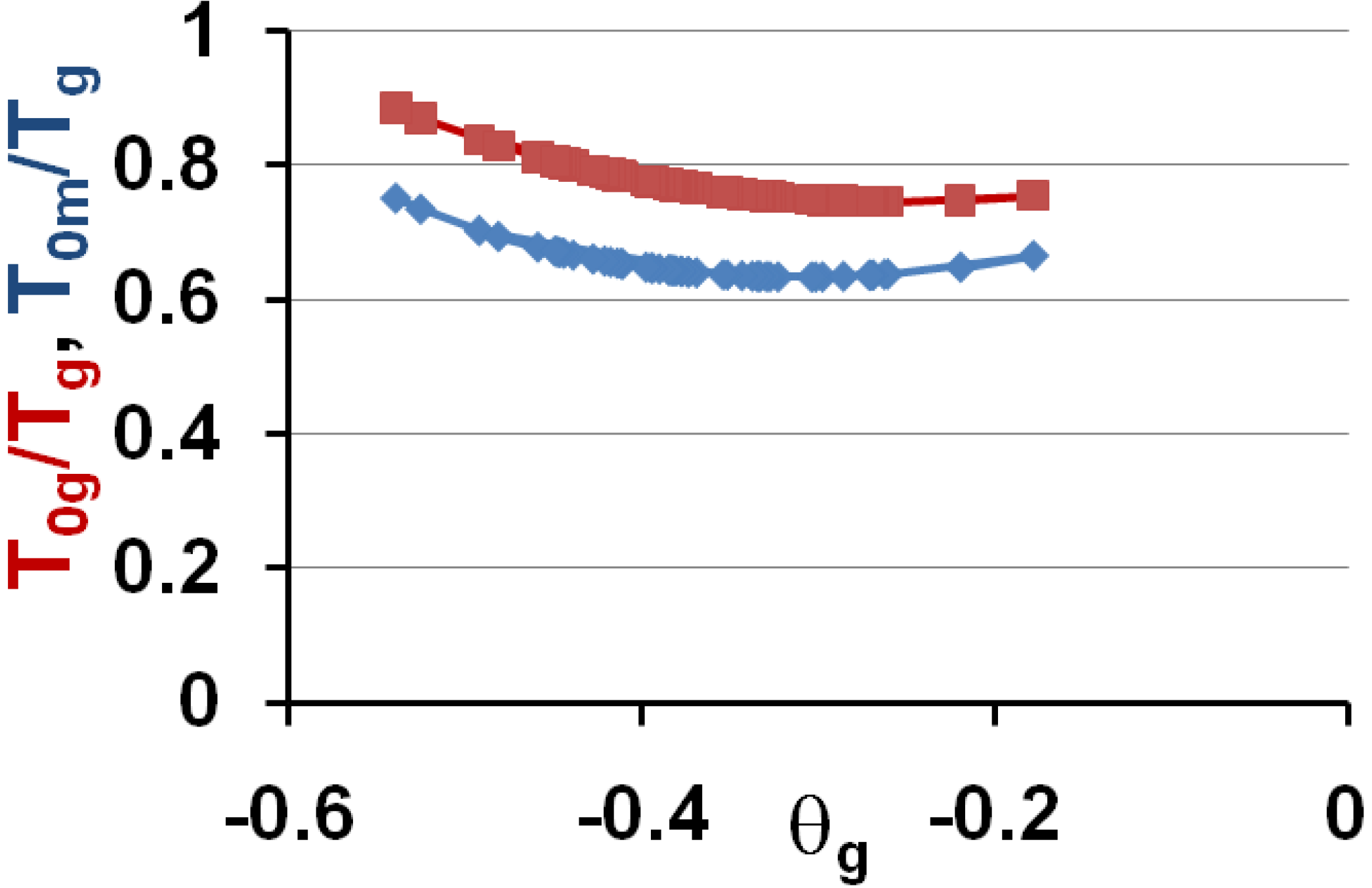
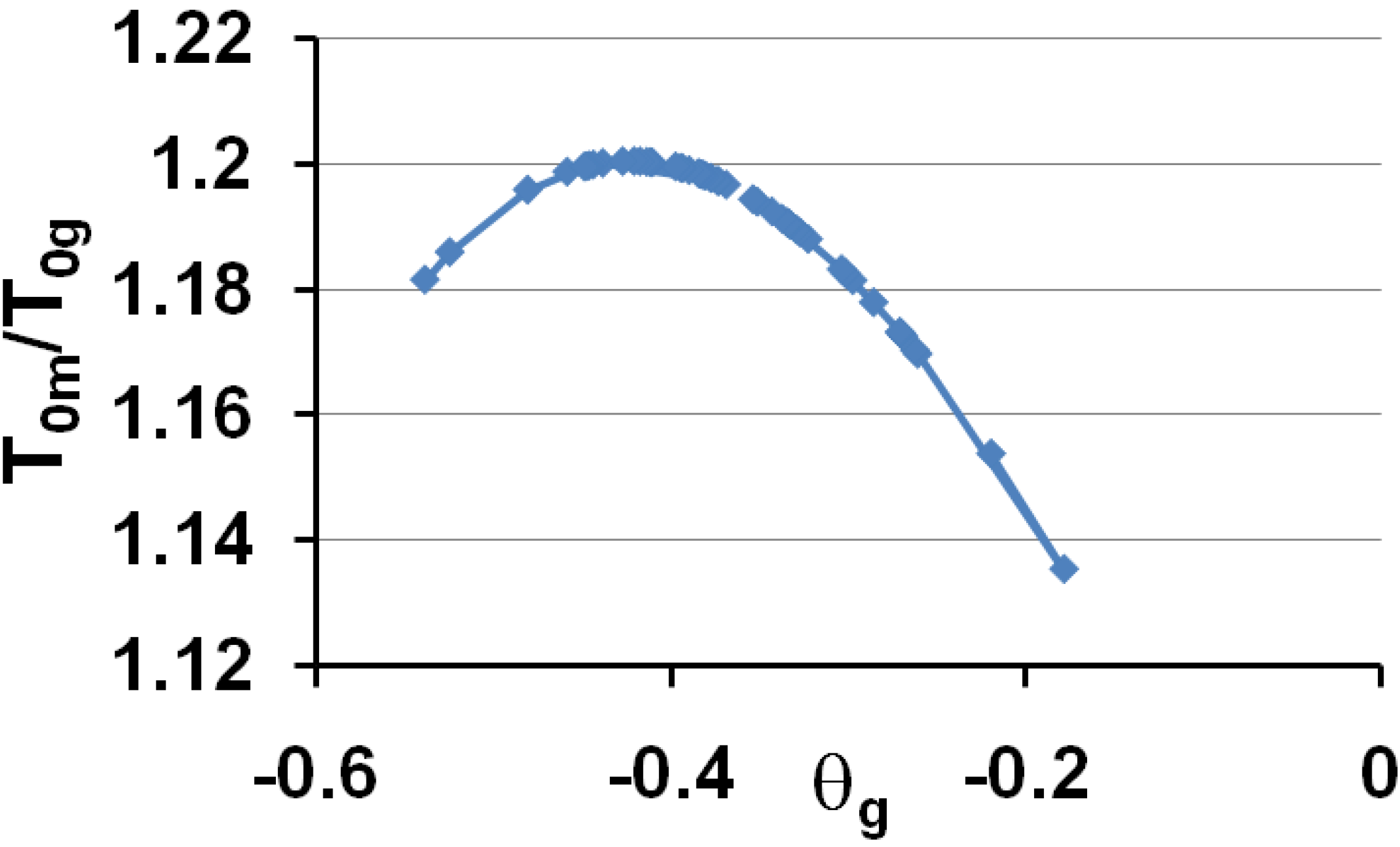
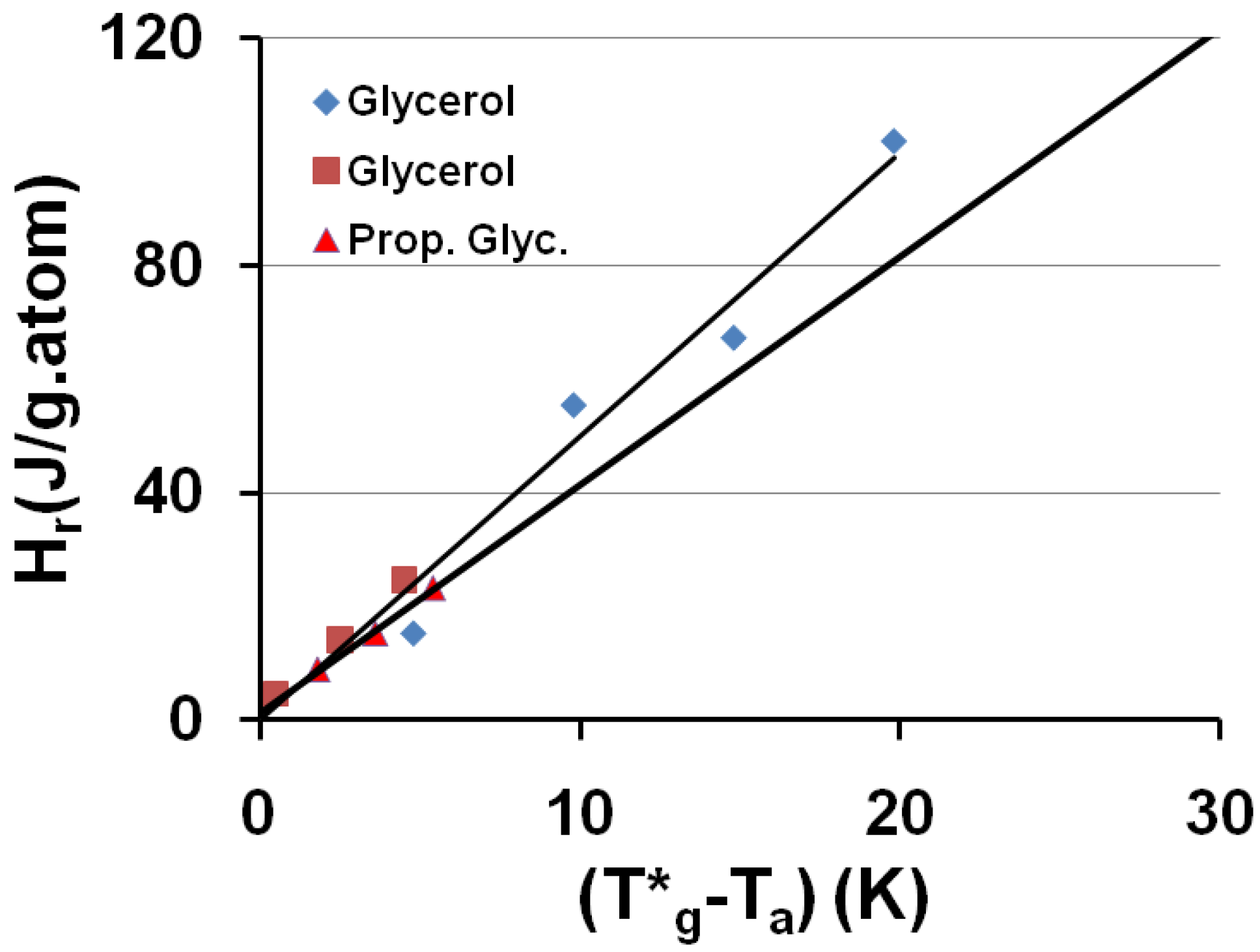
3.5. Volume Energy Saving Associated with Nascent Crystals in Non-Metallic Glass-Forming Melts
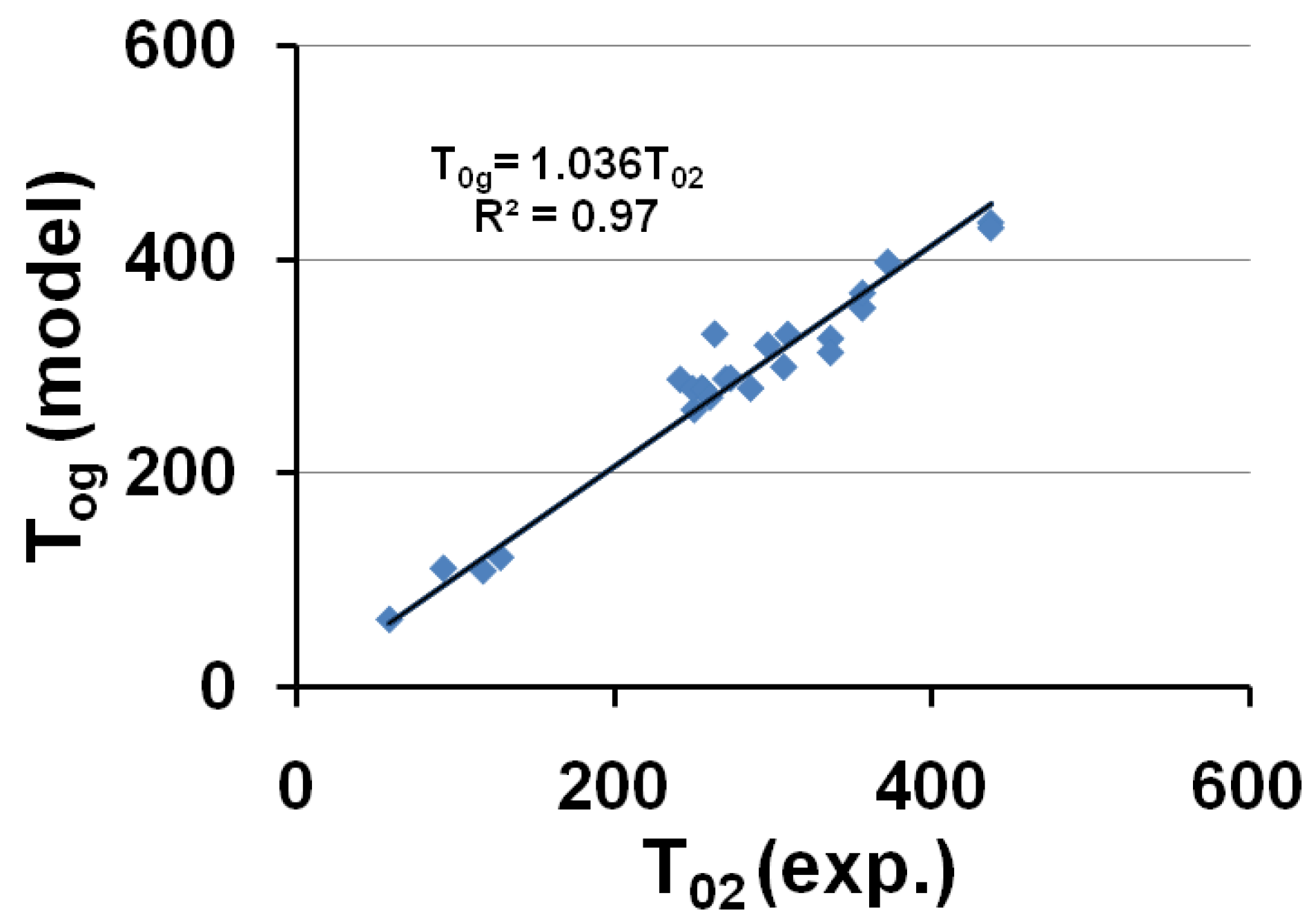
3.6. Thermodynamic Origin of Relaxed Enthalpy and of Out-of-Equilibrium Nucleation Temperatures Tg

4. Summary and Complementary Information on the Two Crystal Nucleation Temperatures
 , ΔCpgl being the specific heat jump at T*g. The apparent specific heat jump at Tg calculated from the heat flux measurement is equal to the reversible one. The specific heat jump deduced from heat flux measurement occurs at a temperature Tg depending on the heating rate. There is no visible anomaly at T*g in a DSC run. This phenomenon is schematized in Figure 10. The glass transition temperatures Tg determined by DSC correspond to out-of-equilibrium crystal homogeneous nucleation temperatures and to out-of-equilibrium values of the energy saving coefficient εlg0.
, ΔCpgl being the specific heat jump at T*g. The apparent specific heat jump at Tg calculated from the heat flux measurement is equal to the reversible one. The specific heat jump deduced from heat flux measurement occurs at a temperature Tg depending on the heating rate. There is no visible anomaly at T*g in a DSC run. This phenomenon is schematized in Figure 10. The glass transition temperatures Tg determined by DSC correspond to out-of-equilibrium crystal homogeneous nucleation temperatures and to out-of-equilibrium values of the energy saving coefficient εlg0.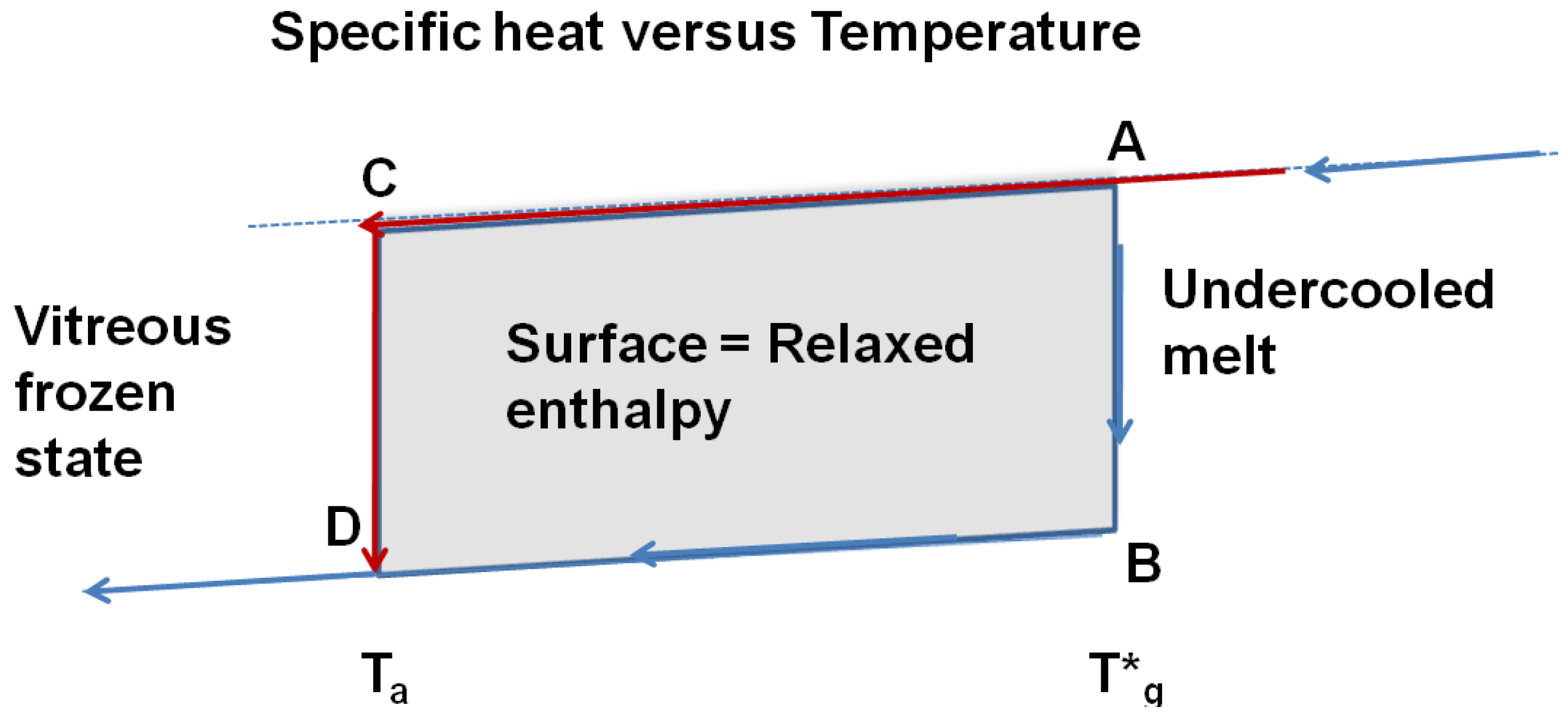
5. Conclusions
Acknowledgements
References
- Zarzycki, J. Les Verres et l’Etat Vitreux; Masson: Paris, France, 1982. [Google Scholar]
- Gutzov, I.; Schmeltzer, J. The Vitreous State; Springer-Verlag: Berlin, Germany, 1995. [Google Scholar]
- Angell, C.A. Formation of glasses from liquids and biopolymers. Science 1995, 267, 1924–1935. [Google Scholar] [CrossRef]
- Berthier, L.; Biroli, G.; Bouchaud, J.; Cipelletti, L.; Masri, D.E.; L’Hôte, D.; Ladieu, F.; Pierno, M. Direct experimental evidence of a growing length scale accompanying the glass transition. Science 2005, 310, 1797–1800. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Awasaki, T.; Shintani, H.; Watanabe, K. Critical-like behavior of glass-forming liquids. Nature Mater. 2010, 9, 324–331. [Google Scholar] [CrossRef]
- Kauzmann, W. The nature of the glassy state and the behavior of liquids at low temperatures. Chem. Rev. 1948, 43, 219–256. [Google Scholar] [CrossRef]
- Liu, C.-Y.; He, J.; Keunings, R.; Bailly, C. New linearized relation for the universal viscosity-temperature behavior of polymer melts. Macromolecules 2006, 39, 8867–8869. [Google Scholar] [CrossRef]
- Williams, M.L.; Landel, R.F.; Ferry, J.D. Temperature dependence of relaxation mechanisms in amorphous polymers and other glass-forming melts. J. Am. Chem. Soc. 1955, 77, 3701–3707. [Google Scholar] [CrossRef]
- Takeuchi, A.; Kato, H.; Inoue, A. Vogel-Fulcher-Tammann plot for viscosity scaled with temperature interval between actual and ideal glass transitions for metallic glasses in liquid and supercooled liquid states. Intermetallics 2010, 18, 406–411. [Google Scholar]
- Doolittle, A.K. Studies in Newtonian Flow. II. The dependence of the viscosity of liquids on free-space. J. Appl. Phys. 1951, 22, 1471–1475. [Google Scholar] [CrossRef]
- Ramachandrarao, P.; Dubey, K.S. A thermodynamic approach to the viscous behaviour of glass-forming liquids. Mat. Sc. Eng. B 1995, 32, 285–293. [Google Scholar] [CrossRef]
- Gibbs, J.H.; DiMarzio, E.A. Nature of the glass transition and the glassy state. J. Chem. Phys. 1958, 28, 373–383. [Google Scholar] [CrossRef]
- Holubovà, J.; Cernozek, Z.; Cernoskovà, E. The study of the glass transition by the stepscan DSC technique. J. Optoelectron. Adv. Mat. 2005, 7, 2671–2676. [Google Scholar]
- Tournier, R.F. Crystal growth nucleation and Fermi energy equalization of intrinsic spherical nuclei in glass-forming melts. Sci. Technol. Adv. Mater. 2009, 10, 014607:1–014607:12. [Google Scholar]
- Tournier, R.F. Crystal nucleation and equalization of Fermi energies of intrinsic nuclei and glass-forming melts. J. Phys. Confer. Ser. 2009, 144, 012116:1–012116:4. [Google Scholar] [CrossRef]
- Tournier, R.F. Presence of intrinsic growth nuclei in overheated and undercooled liquid elements. Phys. B 2007, 392, 79–93. [Google Scholar] [CrossRef]
- Vinet, B.; Magnusson, L.; Fredriksson, H.; Desré, P.J. Correlations between surface and interface energies with respect to crystal nucleation. J. Coll. Interf. Sci. 2002, 255, 363–374. [Google Scholar] [CrossRef]
- Turnbull, D.; Cech, R.E. Microscopic observation of the solidification of small metal droplets. J. Appl. Phys. 1950, 21, 804–810. [Google Scholar] [CrossRef]
- Bosio, L.; Defrain, A.; Epelboin, I. Changements de phase du gallium à la pression atmosphérique. J. Phys. France 1966, 27, 61–71. [Google Scholar] [CrossRef]
- Hays, C.C.; Johnson, W.L. Undercooling of bulk metallic glasses processed by electrostatic levitation. J. Non-Cryst. Solids 1999, 250-252, 596–600. [Google Scholar] [CrossRef]
- Tournier, R.F. Expected properties of gold melt containing intrinsic nuclei. In Proceedings of the 6th International Conference on Electromagnetic Processing of Materials, EPM 2009, Dresden, Germany, 19–23 October 2009; pp. 304–307.
- Tournier, R.F. Undercooling versus Overheating of Liquid Elements Containing Intrinsic Nuclei: Application to Magnetic Texturing. In Presented at the 4th International Workshop on Materials Analysis and Processing in Magnetic Fields Organized by Hamid Garmestani, Atlanta, GA, USA, 10–12 May 2010.
- Tournier, R.F. Nucleation of crystallization in titanium and vitreous state in glass-forming melt. In Proceedings of the 12th World Conference on Titanium, Beijing, China, 19–24 June 2011.
- Hirata, A.; Hirotsu, Y.; Ohkubo, T.; Hanada, T.; Bengus, V.Z. Compositional dependence of local atomic structures in amorphous Fe100−xBx alloys studied by electron diffraction and high-resolution electron microscopy. Phys. Rev. B 2006, 74, 214206:1–214206:9. [Google Scholar]
- Sidorov, V.; Popel, P.; Calvo-Dahlborg, M.; Dahlborg, U.; Manov, V. Heat treatment of iron-based melts before quenching. Mater. Sc. Eng. A 2001, 304-306, 480–486. [Google Scholar] [CrossRef]
- Kokshenev, V.B. Characteristic temperatures of liquid-glass transition. Phys. A 1999, 262, 88–97. [Google Scholar] [CrossRef]
- Busch, R.; Liu, W.; Johnson, W.L. Thermodynamics and kinetics of the Mg65Cu25Y10 bulk metallic glass forming liquid. J. Appl. Phys. 1998, 83, 4134–4441. [Google Scholar] [CrossRef]
- Shadowspeaker, L.; Busch, R. On the fragility of Nb-Ni-based and Zr-based bulk metallic glasses. Appl. Phys. Lett. 2004, 85, 2508–2510. [Google Scholar] [CrossRef]
- Lu, I.-R.; Görler, G.P.; Willnecker, R. Specific volume of glass-forming liquid Pd43Cu27Ni10P20 and related thermodynamic aspects of the glass transition. Appl. Phys. Lett. 2002, 80, 4534–4536. [Google Scholar] [CrossRef]
- Yavari, A.R.; Le Moulec, A.; Nishiyama, N.; Inoue, A.; Vaughan, G.; Kvick, A.; Botta, W.J. Glass Transition Tg and quenched-in free volume in bulk metallic glasses measured by X-ray diffraction. J. Metast. Nanocryst. Mater. 2004, 20-21, 23–28. [Google Scholar] [CrossRef]
- Nishiyama, N.; Inoue, A. Glass transition behavior and viscous flow working of Pd40Cu30Ni10P20 amorphous alloy. Mater. Trans. JIM 1999, 40, 64–71. [Google Scholar] [CrossRef]
- Dubey, K.S.; Ramachandrarao, P.; Lele, S. Thermodynamic and viscous behavior of undercooled liquids. Thermochim. Acta 1996, 280/281, 25–62. [Google Scholar] [CrossRef]
- Kouamé, N.; Sei, J.; Houphouët-Boigny, D.; Kra, G.; Jumas, J.C.; Olivier-Fourcade, J. Propriétés thermiques et optiques des verres du système Sb2S3–As2S3. C. R. Chimie 2007, 10, 498–501. [Google Scholar] [CrossRef]
- Birge, N.O. Specific heat spectroscopy of glycerol and propylene glycol near the glass transition. Phys. Rev. B. 1986, 34, 1631–1642. [Google Scholar] [CrossRef]
- Schönhals, A.; Kremer, F.; Hofmann, A.; Fischer, E.W. Anomalies in the scaling of the α-relaxation studied by dielectric spectroscopy. Phys. A 1993, 201, 263–269. [Google Scholar] [CrossRef]
- Mishra, R.K.; Dubey, K.S. Glass-forming ability of materials: A thermodynamic approach. J. Non-Cryst. Sol. 2009, 355, 2199–2204. [Google Scholar] [CrossRef]
- Yang, Z.; Lam, C.H.; Dimasi, E.; Bouet, N.; Jordan-Sweet, J.; Tsui, O.K.C. Method to measure the viscosity of nanometer liquid films from the surface fluctuations. Appl. Phys. Lett. 2009, 94, 251906:1–251906:3. [Google Scholar]
- Lu, I.-R.; Wilde, G.; Görler, G.P.; Willnecker, R. Thermodynamic properties of Pd-based glass-forming alloys. J. Non-Cryst. Sol. 1999, 250-252, 577–581. [Google Scholar] [CrossRef]
- Schroers, J. On the formability of bulk metallic glass in its supercooled liquid state. Acta Mater. 2008, 56, 471–478. [Google Scholar] [CrossRef]
- Johari, G.P. Heat capacity and entropy of an equilibrium liquid from Tg to 0 K, and examining the conjectures of an underlying thermodynamic transition. Chem. Phys. 2001, 265, 217–231. [Google Scholar] [CrossRef]
- Henderson, D.W.; Ast, D.G. Viscosity and crystallization kinetics of As2Se3. J. Non-Cryst. Sol. 1984, 64, 43–70. [Google Scholar] [CrossRef]
- Waniuk, T.A.; Busch, R.; Masuhr, A.; Johnson, W.L. Equilibrium viscosity of the Zr41.2Ti13.8Cu12.5Ni10Be22.5 bulk metallic glass-forming liquid and viscous flow during relaxation, phase separation, and primary crystallization. Acta Mater. 1998, 46, 5229–5236. [Google Scholar] [CrossRef]
- Lu, Z.P.; Li, Y.; Liu, C.T. Glass-forming tendency of bulk La–Al–Ni–Cu–(Co) metallic glass-forming melts. J. Appl. Phys. 2003, 93, 286–290. [Google Scholar] [CrossRef]
- Lu, Z.P.; Liu, C.T. A new glass-forming ability criterion for bulk metallic glasses. Acta Mater. 2002, 50, 3501–3512. [Google Scholar] [CrossRef]
- Lu, Z.P.; Li, Y.; Ng, S.C. Reduced glass transition temperature and glass forming ability of bulk glass forming alloys. J. Non-Cryst. Sol. 2000, 270, 103–114. [Google Scholar] [CrossRef]
- Angell, C.A.; Rao, K.J. Configurational excitations in condensed matter, and the “bond lattice” model for liquid-glass transition. J. Chem. Phys. 1971, 57, 470–481. [Google Scholar] [CrossRef]
- Hatase, M.; Hanaya, M.P.; Hikima, T.; Oguni, M. Discovery of homogeneous-nucleation-based crystallization in simple glass-forming liquid of toluene below its glass-transition temperature. J. Non-Cryst. Sol. 2003, 307-310, 257–263. [Google Scholar]
- Kato, H.; Wada, T.; Hasegawa, M.; Saida, J.; Inoue, A. Fragility and thermal stability of Pt- and Pd-based bulk glass forming liquids and their correlation with deformability. Scripta Materialia 2006, 54, 2023–2027. [Google Scholar] [CrossRef]
- Kui, H.-W.; Turnbull, D. The heat capacity of Ni40Pd40P20 in the liquid, glass and crystallized states. J. Non-Cryst. Sol. 1987, 94, 62–69. [Google Scholar] [CrossRef]
- Legg, B.A.; Schroers, J.; Busch, R. Thermodynamics, kinetics, and crystallization of Pt57.3 Cu14.6 Ni5.3 P22.8 bulk metallic glass. Acta Mater. 2007, 55, 1109–1116. [Google Scholar] [CrossRef]
- Chen, H.S.; Goldstein, M. Anomalous visco-elastic behavior of metallic-glasses of Pd–Si-based alloys. J. Appl. Phys. 1972, 43, 1642–1648. [Google Scholar] [CrossRef]
- Mukherjee, S.; Schroers, J.U.; Zhou, Z.; Johnson, W.L.; Rhim, W.-K. Viscosity and specific volume of bulk metallic glass-forming alloys and their correlation with glass-forming stability. Acta Mater. 2004, 52, 3689–3695. [Google Scholar] [CrossRef]
- Màlek, J.; Svoboda, R.; Pustkova, P.; Cicmanec, P. Volume and enthalpy relaxation of a-Se in the glass transition region. J. Non-Cryst. Sol. 2009, 355, 264–272. [Google Scholar] [CrossRef]
- Gallino, I.; Shah, M.B.; Busch, R. Enthalpy relaxation and its relation to the thermodynamics and crystallization of the Zr58.5Cu15.6Ni12.8Al10.3Nb2.8 bulk metallic glass-forming alloy. Acta Mater. 2007, 55, 1367–1376. [Google Scholar] [CrossRef]
- Meng, Q.G; Zhang, S.G.; Li, J.G.; Bian, X.F. Strong liquid behavior of Pr55Ni25Al20 bulk metallic glass. J. Alloys Compounds 2007, 431, 191–196. [Google Scholar] [CrossRef]
- Huang, H.J.; Shen, J.; Sun, X.B.; Yu, J.F. A new Ti–Zr–Hf–Cu–Ni–Si–Sn bulk amorphous alloy with high glass-forming ability. J. Alloys Compounds 2007, 427, 171–175. [Google Scholar] [CrossRef]
- Glade, S.C.; Johnson, W.L. Viscous flow of the Cu47Ti34Zr11Ni8 glass forming alloy. J. Appl. Phys. 2000, 87, 7249–7251. [Google Scholar] [CrossRef]
- He, S.; Liu, Y.; Huang, B.; Li, Z.; Wu, H. Effect of Zr on glass-forming ability and crystallization kinetics of Y56Al24Co20 metallic glass. J. Mater. Proc. Techn. 2008, 204, 179–183. [Google Scholar] [CrossRef]
- Zheng, Q.; Xu, J.; Ma, E. High glass-forming ability correlated with fragility of Mg–Cu(Ag)–Gd alloys. J. Appl. Phys. 2007, 102, 113519:1–113519:5. [Google Scholar]
- Busch, R.; Bakke, E.; Johnson, W.L. Viscosity of the supercooled liquid and relaxation at the glass transition of the Zr46.75Ti8.25Cu7.5Ni10Be27.5 bulk metallic glass forming alloy. Acta Metall. 1998, 46, 4725–4732. [Google Scholar]
- Zhang, B.; Wang, R.J.; Zhao, D.Q.; Pan, M.X.; Wang, W.H. Properties of Ce-based bulk metallic glass-forming alloys. Phys. Rev. B 2004, 70, 224208:1–224208:7. [Google Scholar]
- Bian, X.F.; Sun, B.A.; Hu, L.N.; Jia, Y.B. Fragility of superheated melts and glass-forming ability in Al-based alloys. Phys. Lett. A 2005, 335, 61–67. [Google Scholar] [CrossRef]
- Chen, H.; Turnbull, D.J. Evidence of a glass-liquid transition in gold-germanium-silicon. J. Chem. Phys. 1968, 48, 2560–2571. [Google Scholar] [CrossRef]
- Adam, G.; Gibbs, J.H. On the temperature dependence of cooperative relaxation properties in glass-forming liquids. J. Chem. Phys. 1965, 43, 139–146. [Google Scholar] [CrossRef]
- Turnbull, D.; Fisher, J.C. Rate of nucleation in condensed systems. J. Chem. Phys. 1949, 17, 71–73. [Google Scholar] [CrossRef]
- Cernosek, Z.; Holubova, J.; Cernoskova, E. Kauzmann temperature and the glass transition. J. Optoelectron. Adv. Mater. 2005, 7, 2941. [Google Scholar]
- Cernosek, Z.; Holubova, J.; Cernoskova, E.; Liska, M. Enthalpic relaxation and the glass transition. J. Optoel. Adv. Mater. 2002, 4, 489–503. [Google Scholar]
- Mehl, P.M. Determination of enthalpy relaxation times using traditional differential scanning calorimetry for glycerol and for propylene glycol. Thermochem. Acta 1996, 272, 201–209. [Google Scholar] [CrossRef]
- Fransson, A.; Bäckström, G. Isothermal enthalpy relaxation of glycerol. Int. J. Thermophys. 1987, 8, 351–362. [Google Scholar] [CrossRef]
- Néel, L. Superparamagnétisme de grains très fins antiferromagnétiques. C. R. Am. Sci. 1961, 252, 4075–4080. [Google Scholar]
- Wu, D.T.; Granasy, L.; Spaepen, F. Nucleation and the solid-liquid interfacial free energy. MRS Bulletin. Available online: http://www.mrs.org/publications/bulletin (accessed on December 2004).
- Berton, A.; Chaussy, J.; Odin, J.; Rammal, R.; Tournier, R. Magnetocaloric investigation of (H,T) phase diagram of Cu-Mn spin glass. J. Physique-Lett. 1982, 43, L153–L158. [Google Scholar] [CrossRef]
- Tholence, J.L; Tournier, R. Susceptibility and remanent magnetization of a spin glass. J. Phys. Suppl. Coll. 1974, 35, C4:229–C4:235. [Google Scholar]
- Mydosh, J.A. Spin Glasses: An Experimental Introduction; Taylor & Francis: London, UK, 1993. [Google Scholar]
- Mei, J.N.; Soubeyroux, J.L.; Blandin, J.J.; Li, J.S.; Kou, H.C.; Fu, H.Z.; Zhou, L. Structural relaxation of Ti40Zr25Ni8Cu9Be18 bulk metallic glass. J. Non-Cryst. Sol. 2011, 357, 110–115. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tournier F., R. Thermodynamic Origin of the Vitreous Transition. Materials 2011, 4, 869-892. https://doi.org/10.3390/ma4050869
Tournier F. R. Thermodynamic Origin of the Vitreous Transition. Materials. 2011; 4(5):869-892. https://doi.org/10.3390/ma4050869
Chicago/Turabian StyleTournier F., Robert. 2011. "Thermodynamic Origin of the Vitreous Transition" Materials 4, no. 5: 869-892. https://doi.org/10.3390/ma4050869