In vitro Biocompatibility of New Silver(I) Coordination Compound Coated-Surfaces for Dental Implant Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Coating and Surface Characterization
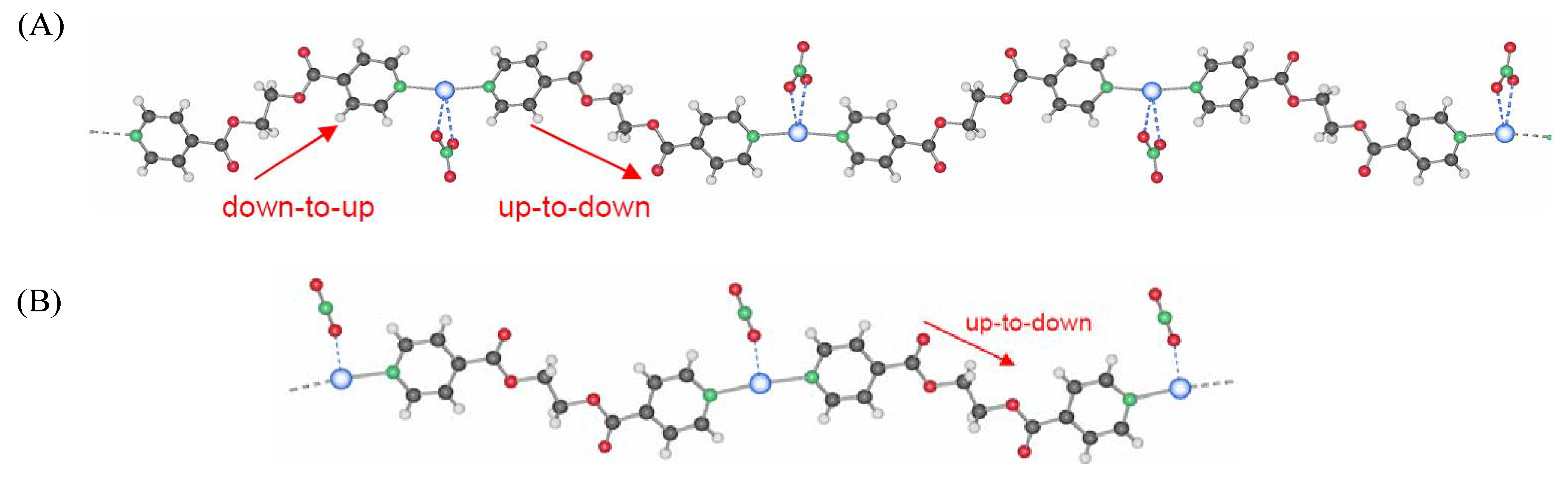
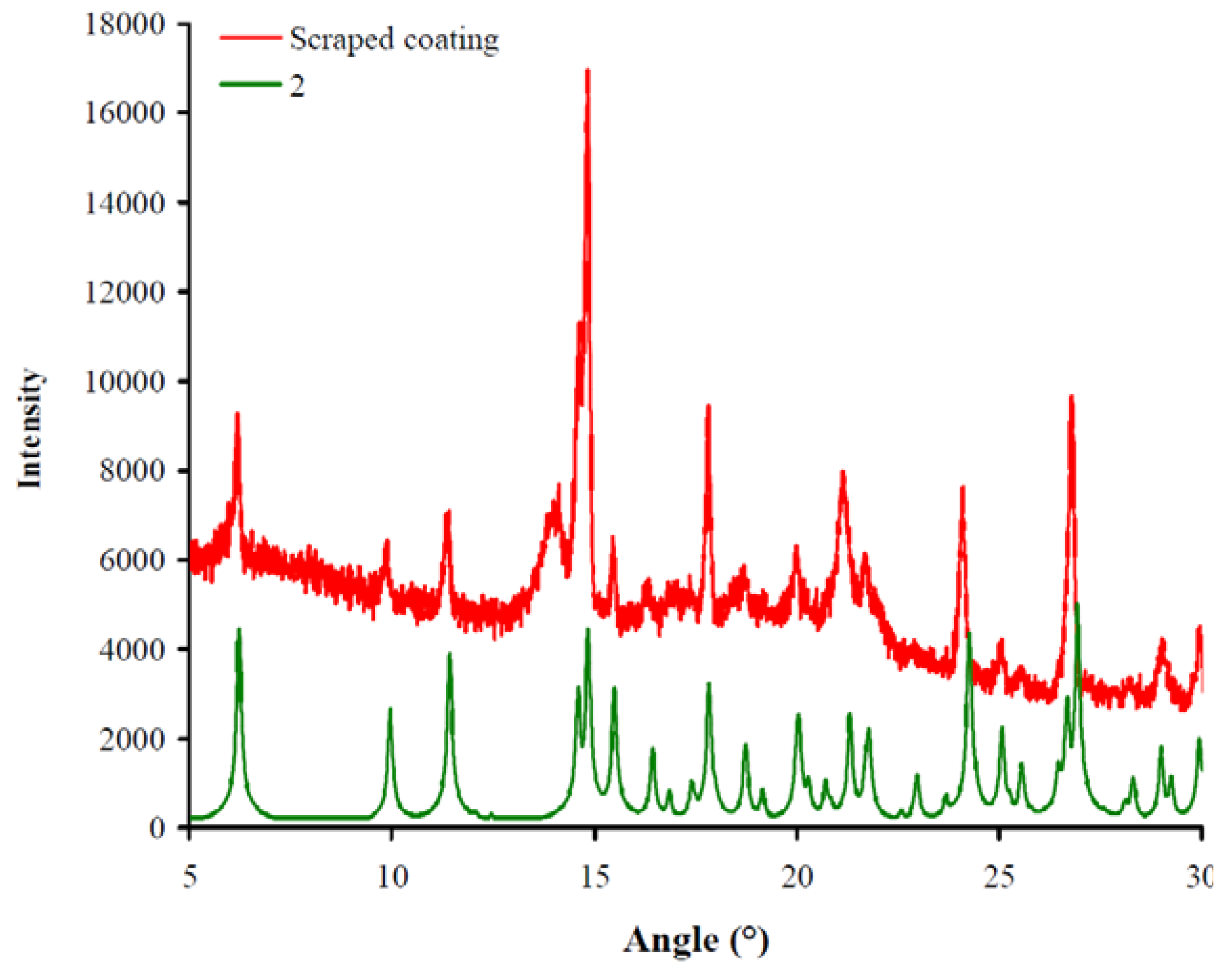
2.1.1. Powder X-ray diffraction
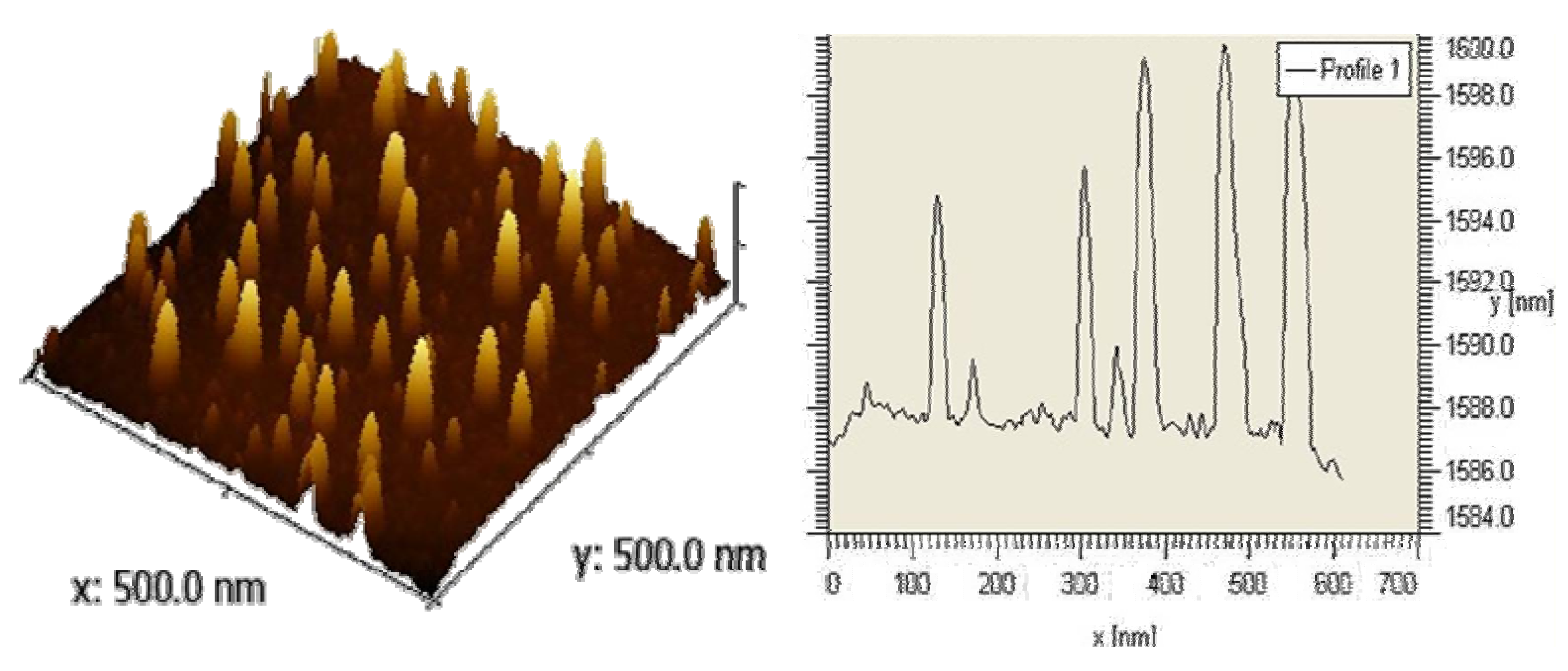
2.1.2. Topography
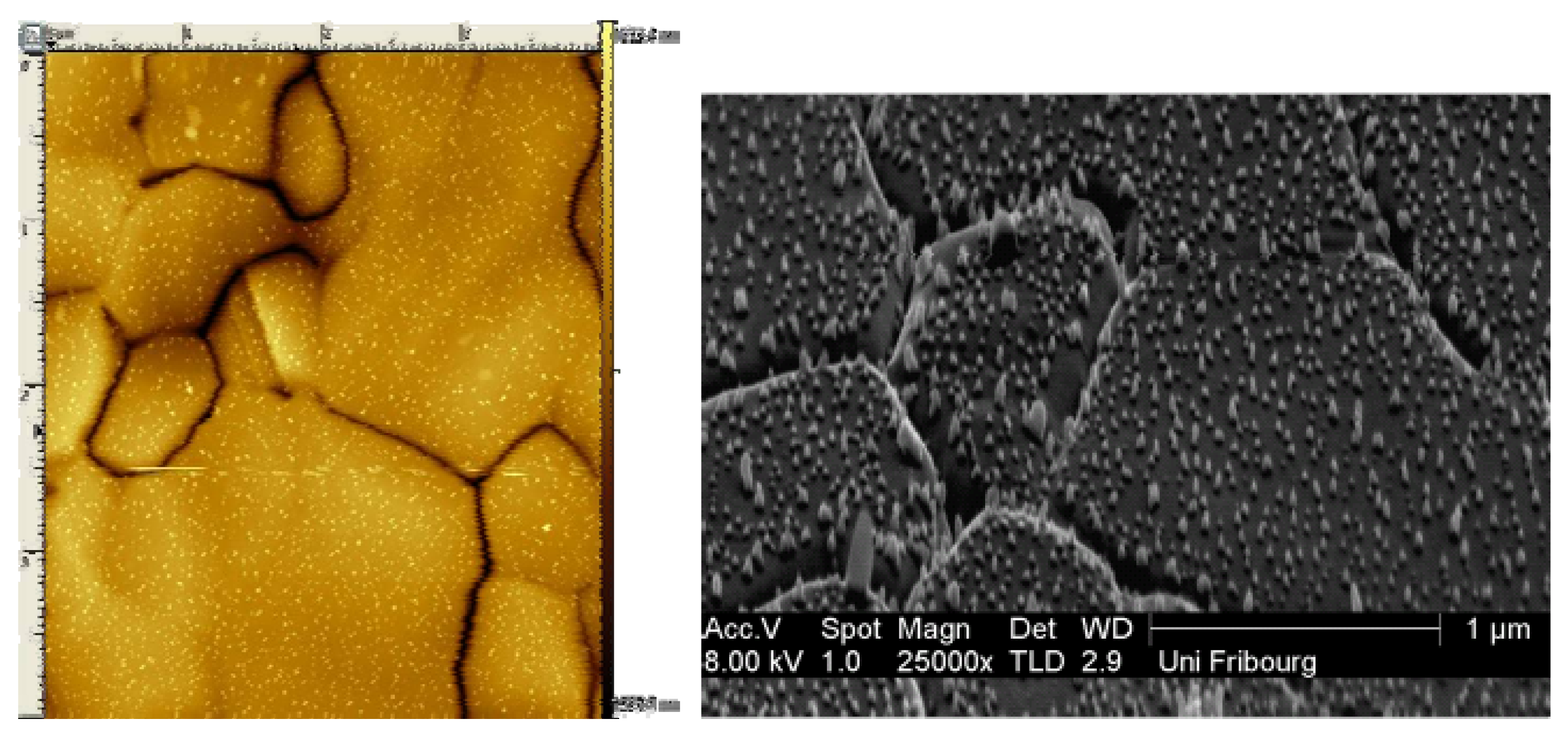
2.1.3. Surface wettability
2.2. Antimicrobial Properties
2.3. Cell Growth and Viability Evaluation

2.4. Morphological Examination
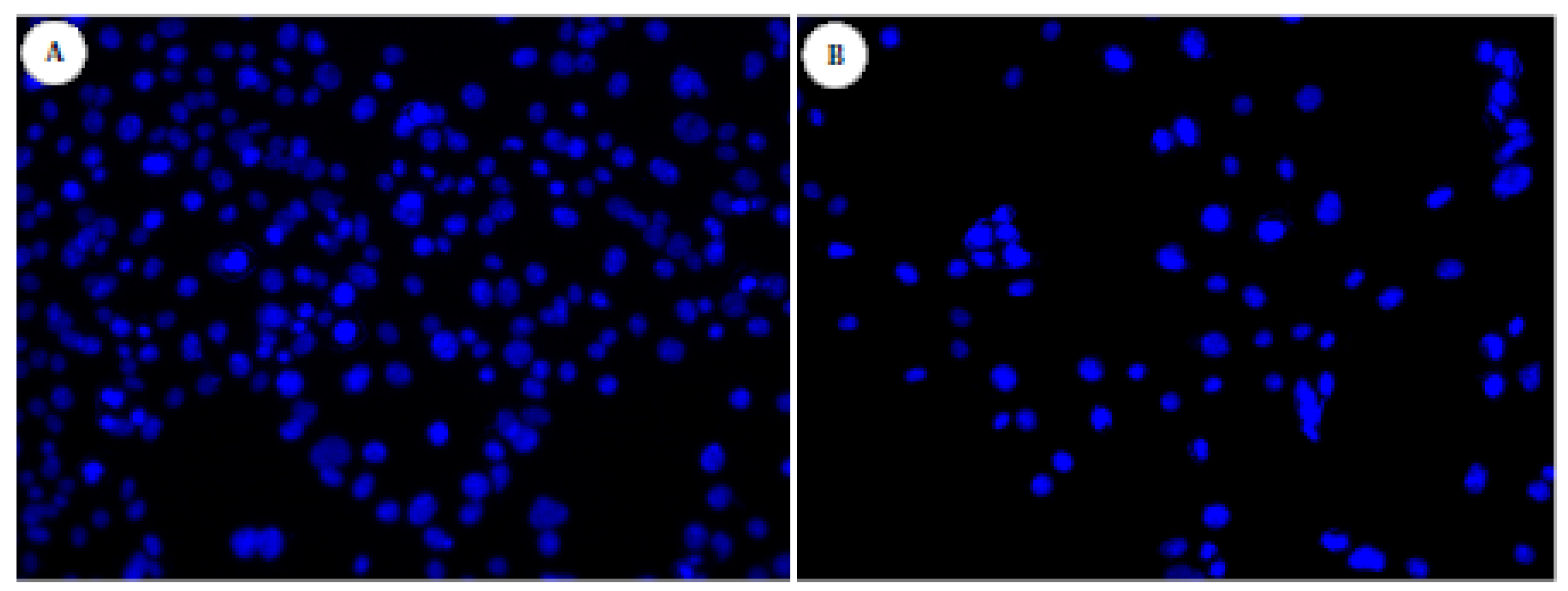
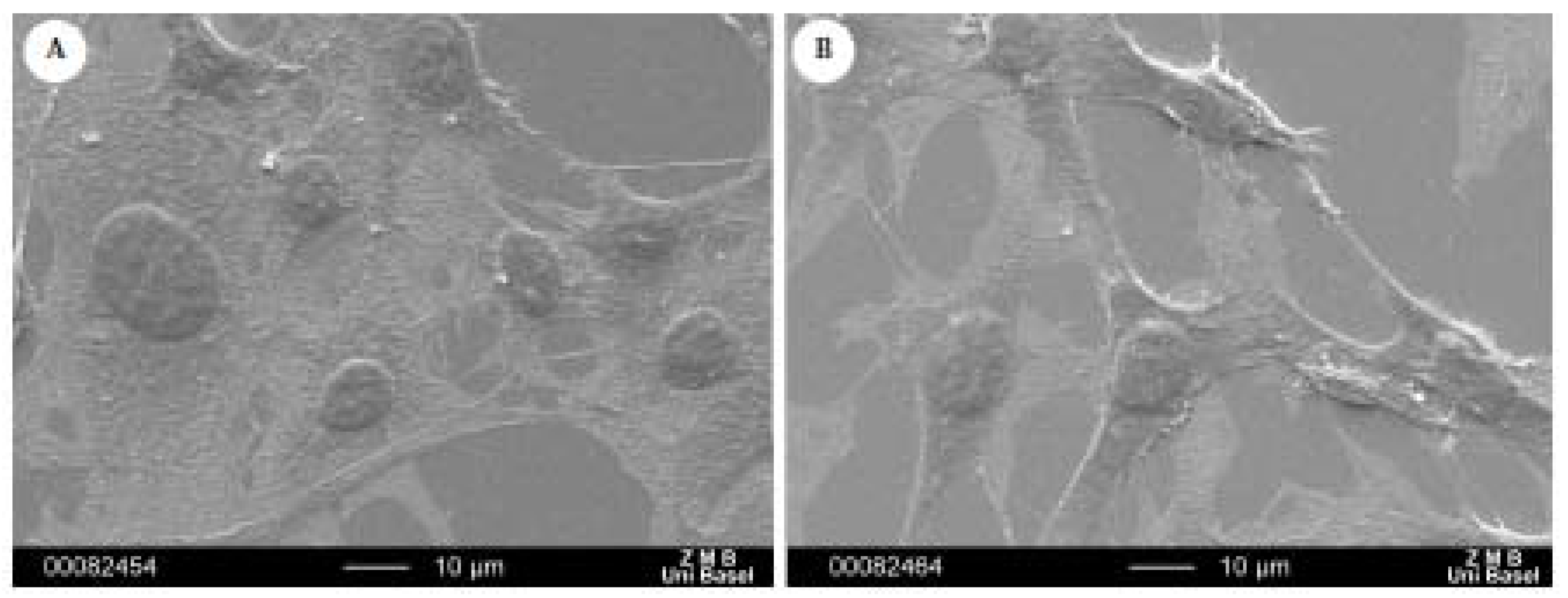
3. Experimental Section
3.1. Surfaces
3.2. Characterization of Surface Topography
3.2.1. X-Ray Powder Diffraction (XRPD)
3.2.2. Microscopy techniques
3.2.3. Wettability
3.3. Fibroblast Cell Culture
3.4. MTT Assay of Fibroblast Cells
3.5. Cell Spreading and Morphology
3.5.1. Fluorescence microscopy of fibroblast cells (DAPI staining)
3.5.2. Scanning electron microscopy (SEM)
4. Conclusions
Acknowledgements
References and Notes
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilm: A common cause of persistent infectious. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, B.D.; Costerton, J.W. Bacterial resistance to antibiotics: The role of biofilms. Prog. Drug Res. 1991, 37, 91–107. [Google Scholar] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27(11), 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Katsikogianni, M.; Missirlis, Y.F. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cells Mater. 2004, 8, 37–57. [Google Scholar]
- Liljemark, W.F.; Bloomquist, C. Human oral microbial ecology and dental caries and periodontal diseases. Crit. Rev. Oral. Med. 1996, 7, 180–198. [Google Scholar] [CrossRef]
- Ten Cate, J.M. Biofilms, a new approach to the microbiology of dental plaque. Odontology 2006, 94, 1–9. [Google Scholar]
- Illingworth, B.L.; Tweden, K.; Schroeder, R.F.; Cameron, J.D. In vivo efficacy of silver-coated (Silzone) infection-resistant polyester fabric against a biofilm-producing bacteria, Staphylococcus epidermidis. J. Heart Valve Dis. 1998, 7, 524–530. [Google Scholar] [PubMed]
- Bosetti, M.; Massè, A.; Tobin, E.; Cannas, M. Silver coated materials for external fixation devices: In vitro biocompatibility and genotoxicity. Biomaterials 2002, 23, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Courtney, H.S.; Bettenga, M.; Agrawal, C.M.; Bumgardner, J.D. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 2006, 27, 5512–5517. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S.J.; Spadaro, J.A.; Webster, D.A. Antibacterial and osteoinductive properties of demeralized bone matrix treated with silver. Clin. Orthop. Relat. Res. 1981, 161, 154–162. [Google Scholar] [PubMed]
- Pissiotis, E.; Spangberg, L. Reaction of bony tissue too implanted silver glass ionomer and a reinforced zinc oxide-eugenol cement. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000, 89(5), 623–629. [Google Scholar] [CrossRef]
- Robin, A.Y.; Fromm, K.M. Coordination polymer networks with O-and N-donors: What they are, why and how they are made. Coord. Chem. Rev. 2006, 250, 2127–2157. [Google Scholar] [CrossRef]
- Robin, A.Y.; Meuwly, M.; Fromm, K.M.; Goesmann, H.; Bernardinelli, G. Structural Diversity of coordination polymers with the ligand ethanediyl bis(isonicotinate): Part 4. Coordination compounds of Ag(I) and dependency of the co-crystallizing water molecules. Cryst. Eng. Comm. 2004, 6(60), 336–343. [Google Scholar] [CrossRef]
- Sagué, J.L.; Robin, A.Y.; Fromm, K.M. Concomitant crystallization of two polymorphs––A ring and a helix: Concentration effect on supramolecular polymorphism. Chem. Commun. 2005, 36, 4548–4550. [Google Scholar]
- Robin, A.Y.; Sagué, J.L.; Fromm, K.M. On the formation of coordination polymer networks between AgNO3 and L (L = ethanediyl bis(isonicotinate) as a function of solvent. Cryst. Eng. Comm. 2006, 8, 403–416. [Google Scholar] [CrossRef]
- Sagué, J.L.; Fromm, K.M. The First Two-Dimensional Polycatenane: A New Type of Robust Network Obtained by Ag-Connected One-Dimensional Polycatenanes. Cryst. Growth Des. 2006, 6(7), 1566–1568. [Google Scholar] [CrossRef]
- Robin, A.Y.; Sagué, J.L.; Neels, A.; Vig Slenters, T.; Fromm, K.M. Structure–property relationships: Polymorphism, solvates, and clay behavior in the one-dimensional coordination polymer chains [Ag(L)(NO3)](H2O)n, L = ethanediyl bis(isonicotinate), n = 0, and 2. Inorg. Chim. Acta 2007, 360(1), 212–220. [Google Scholar] [CrossRef]
- Slenters, T.V.; Sagué, J.L.; Brunetto, P.S.; Zuber, S.; Fleury, A.; Mirolo, L.; Robin, A.Y.; Meuwly, M.; Gordon, O.; Landmann, R.; Daniels, A.U.; Fromm, K.M. Of Chains and Rings: Synthetic Strategies and Theoretical Investigations for Tuning the Structure of Silver Coordination Compounds and Their Applications. Materials 2010, 3, 3407–3429. [Google Scholar] [CrossRef]
- Sodhi, R.N.S. Application of surface analytical and modification techniques to biomaterial research. J. Electron Spectros. Relat. Phenom. 1996, 81, 269–284. [Google Scholar] [CrossRef]
- Werner, C.; Jacobasch, H.J. Surface characterization of polymers for medical devices. Int. J. Artif. Organs 1999, 22, 160–176. [Google Scholar] [PubMed]
- Vig Slenters, T.; Hauser-Gerspach, I.; Daniels, A.U.; Fromm, K.M. Silver coordination compounds as light stable, nano-structured and anti-bacterial coatings for dental implant and restorative materials. J. Mater. Chem. 2008, 18, 5359–5362. [Google Scholar] [CrossRef]
- Vig Slenters, T. Novel silver containing antimicrobial coatings for implant materials: New applications of Ag(I) coordination networks. Ph.D. Thesis, University of Basel, Basel, Switzerland, 2009. [Google Scholar]
- Marmur, A. Equilibrium contact angles: Theory and measurement. Colloids Surf. 1996, 116, 55–61. [Google Scholar] [CrossRef]
- Carman, M.L.; Estes, T.G.; Feinberg, A.W.; Schumacher, J.F.; Wilkerson, W.; Wilson, L.H.; Callow, M.E.; Callow, J.A.; Brennan, A.B. Engineered antifouling microtopographies––Correlating wettability with cell attachment. Biofouling 2006, 22(1), 11–21. [Google Scholar] [CrossRef]
- Jonsson, U.; Ivarsson, B.; Lundstrom, I.; Berghem, L. Adsorption behaviour of fibronectin on well characterized silica surfaces. J. Colloid. Interface Sci. 1982, 90, 148–163. [Google Scholar] [CrossRef]
- Absolom, D.R.; Zingg, W.; Neumann, A.W. Protein adsorption to polymer poarticles: Role of surface properties. J. Biomed. Mater. Res. 1987, 21, 161–171. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, D.E.; Deo, N.; Markovic, B.; Stranick, M.; Somasundaran, P. Adsorption and dissolution behaviour of human plasma fibronectin on thermally and chemically modified titanium dioxide particles. Biomaterials 2002, 23, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.; Vogler, E.A. Volumetric interpretation of protein adsorption: Mass and energy balance for albumin adsorption to particulate adsorbents with incrementally increasing hydrophilicity. Biomaterials 2006, 27(34), 5801–5812. [Google Scholar] [PubMed]
- Israelachvili, J.; Wennerstrom, H. Role of hydration and water structure in biological and colloidal interactions. Nature 1996, 379, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A survey of structure–property relationships of surfaces that resist the adsorption of protein. Langmuir 2001, 17(18), 5605–5620. [Google Scholar]
- Hanein, D.; Geiger, B.; Addadi, L. Fibronectin adsorption to surfaces of hydrated crystals: An analysis of the importance of bound water in protein substrate interactions. Langmuir 1993, 9, 1058–1065. [Google Scholar] [CrossRef]
- Harnett, E.M.; Aldermann, J.; Wood, T. The surface energy of various biomaterials coated with adhesion molecules used in cell culture. Colloid. Surface. B 2007, 55, 90–97. [Google Scholar] [CrossRef]
- Tamada, Y.; Ikada, Y. Effect of preadsorbed proteins on cell adhesion to polymer surfaces. J. Colloid Interface Sci. 1993, 155, 334–339. [Google Scholar] [CrossRef]
- Lee, J.H.; Khang, G.; Lee, J.W.; Lee, H.B. Interaction of different types of cells on polymer surfaces with wettability gradient. J. Colloid Interface Sci. 1998, 205, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Ruardy, T.G.; Schakenraad, J.M.; van der Mei, H.C.; Busscher, H.J. Adhesion and spreading of human skin fibroblasts on physicochemically characterized gradient surfaces. J. Biomed. Mater. Res. 1995, 29, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Gordon, O.; Vig Slenters, T.; Brunetto, P.S.; Villaruz, A.E.; Sturdevant, D.E.; Otto, M.; Landmann, R.; Fromm, K.M. Silver Coordination Polymers for Prevention of Implant Infection: Thiol Interaction, Impact on Respiratory Chain Enzymes, and Hydroxal Radical Induction. Antimicrob. Agents Chemother. 2010, 54(10), 4208–4218. [Google Scholar] [PubMed]
- Sinha, R.K.; Morris, F.; Shah, S.A.; Tuun, R.S. Surface composition of othopaedic implant metals regulates cell attachment, spreading and cytoskeletal organisation of primary human osteoblasts in vitro. Clin. Orthop. 1994, 305, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Hunter, A.; Archer, C.W.; Walker, P.S.; Blunn, G.W. Attachement and proliferation of osteoblasts and fibroblasts on biomaterials for orthopaedic use. Biomaterials 1995, 16, 287–295. [Google Scholar] [CrossRef] [PubMed]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Brunetto, P.S.; Slenters, T.V.; Fromm, K.M. In vitro Biocompatibility of New Silver(I) Coordination Compound Coated-Surfaces for Dental Implant Applications. Materials 2011, 4, 355-367. https://doi.org/10.3390/ma4020355
Brunetto PS, Slenters TV, Fromm KM. In vitro Biocompatibility of New Silver(I) Coordination Compound Coated-Surfaces for Dental Implant Applications. Materials. 2011; 4(2):355-367. https://doi.org/10.3390/ma4020355
Chicago/Turabian StyleBrunetto, Priscilla S., Tünde Vig Slenters, and Katharina M. Fromm. 2011. "In vitro Biocompatibility of New Silver(I) Coordination Compound Coated-Surfaces for Dental Implant Applications" Materials 4, no. 2: 355-367. https://doi.org/10.3390/ma4020355



