The Utility of Nanocomposites in Fire Retardancy
Abstract
:1. Introduction
2. Nanocomposite Formation and Characterization
2.1. Formation of Nanocomposites
2.1.1. In-situ Template Synthesis (sol-gel technique)
2.1.2. Polymerization Techniques
2.1.3. Solvent-Assisted Blending
2.1.4. Melt Blending
2.2. Characterization of Nanocomposites
2.2.1. X-ray Diffraction
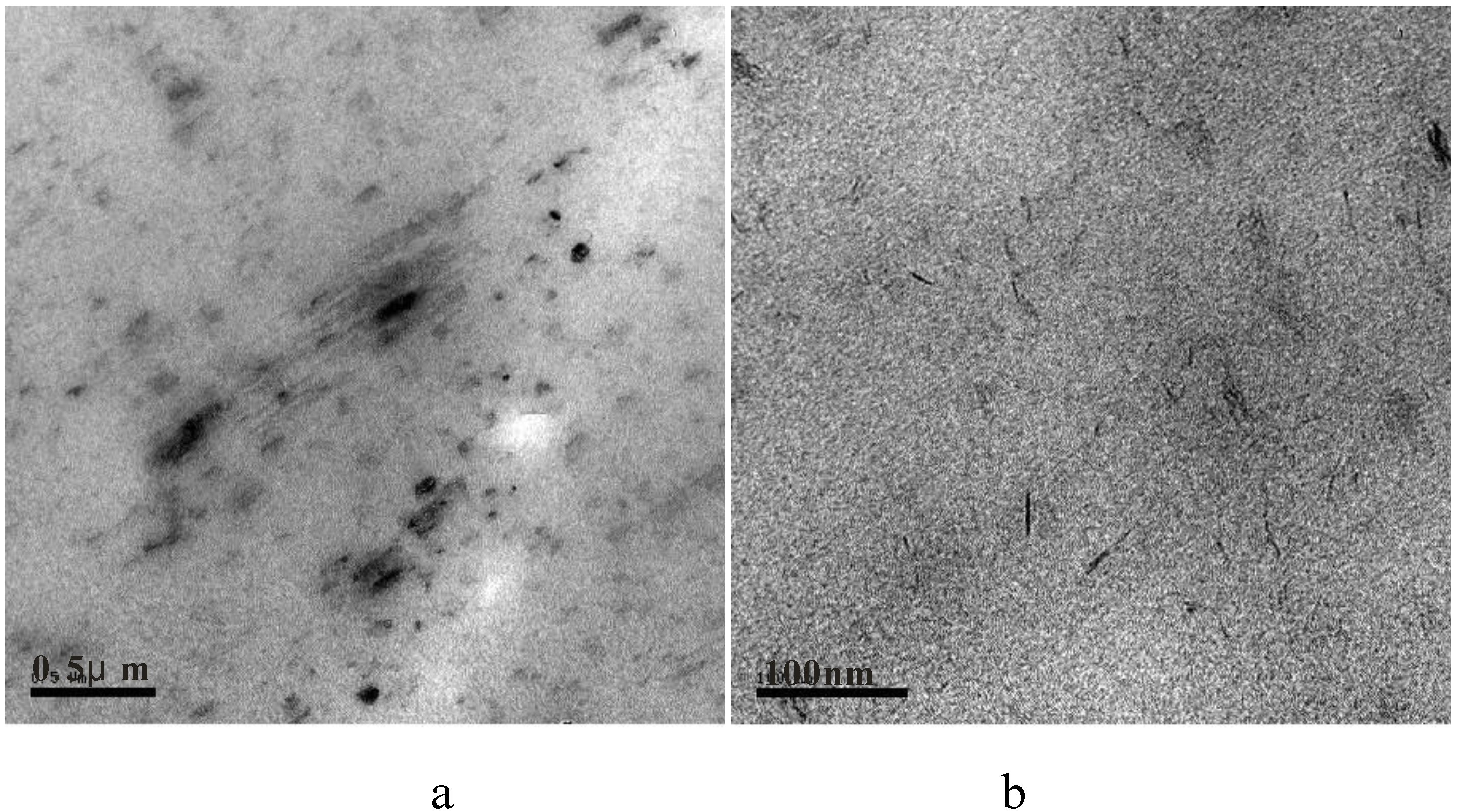
2.2.2. Transmission Electron Microscopy
2.2.3. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.2.4. Other Characterization Techniques for Nanocomposites
2.3. Nanocomposite Description

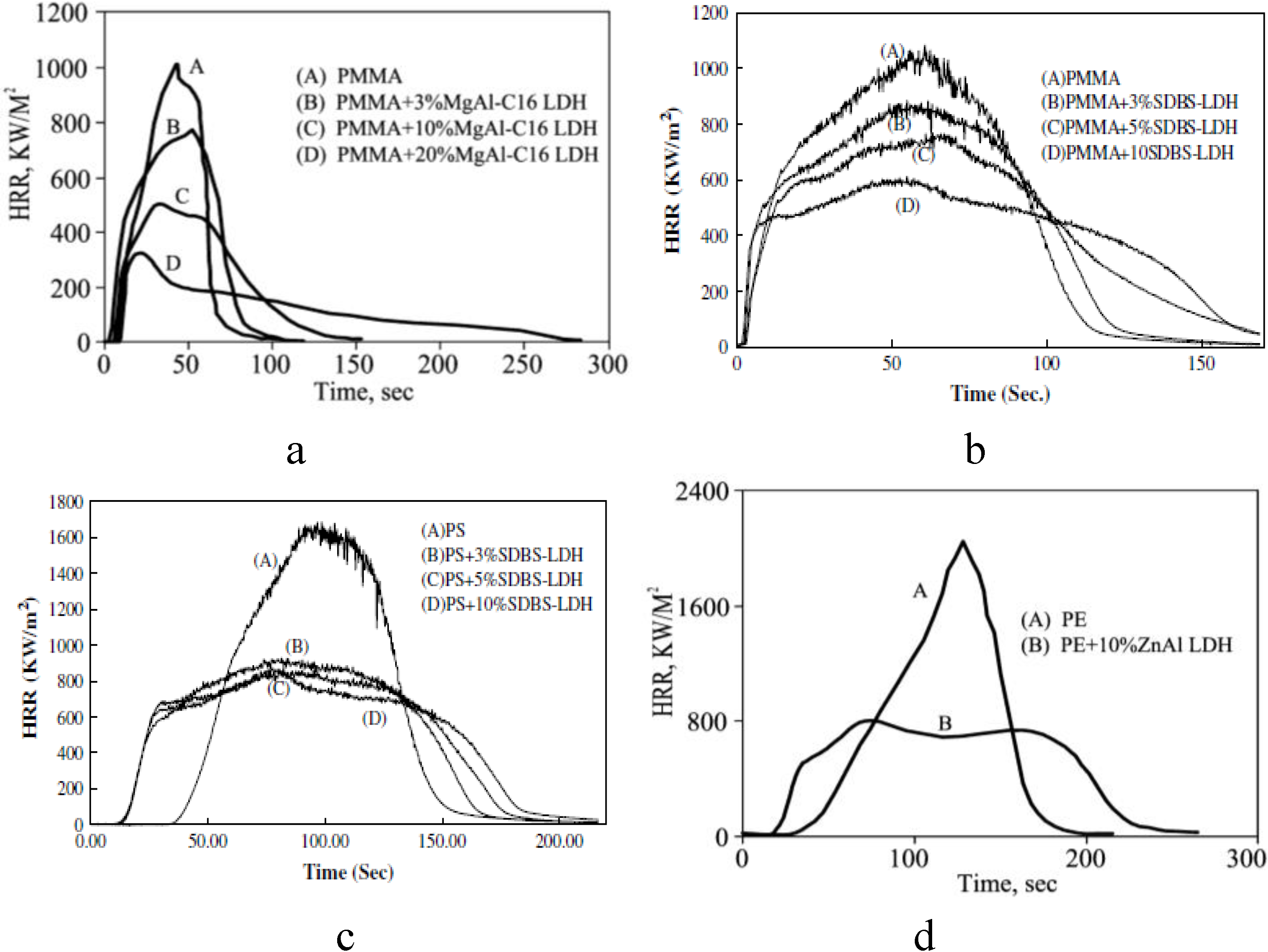
3. Evaluation of Fire Retardancy
3.1. Thermal Stability
3.2. Cone Calorimetry
3.3. Limiting Oxygen Index (LOI) and UL-94 Protocol
4. Fire Retardancy of Polymer-Clay Nanocomposites
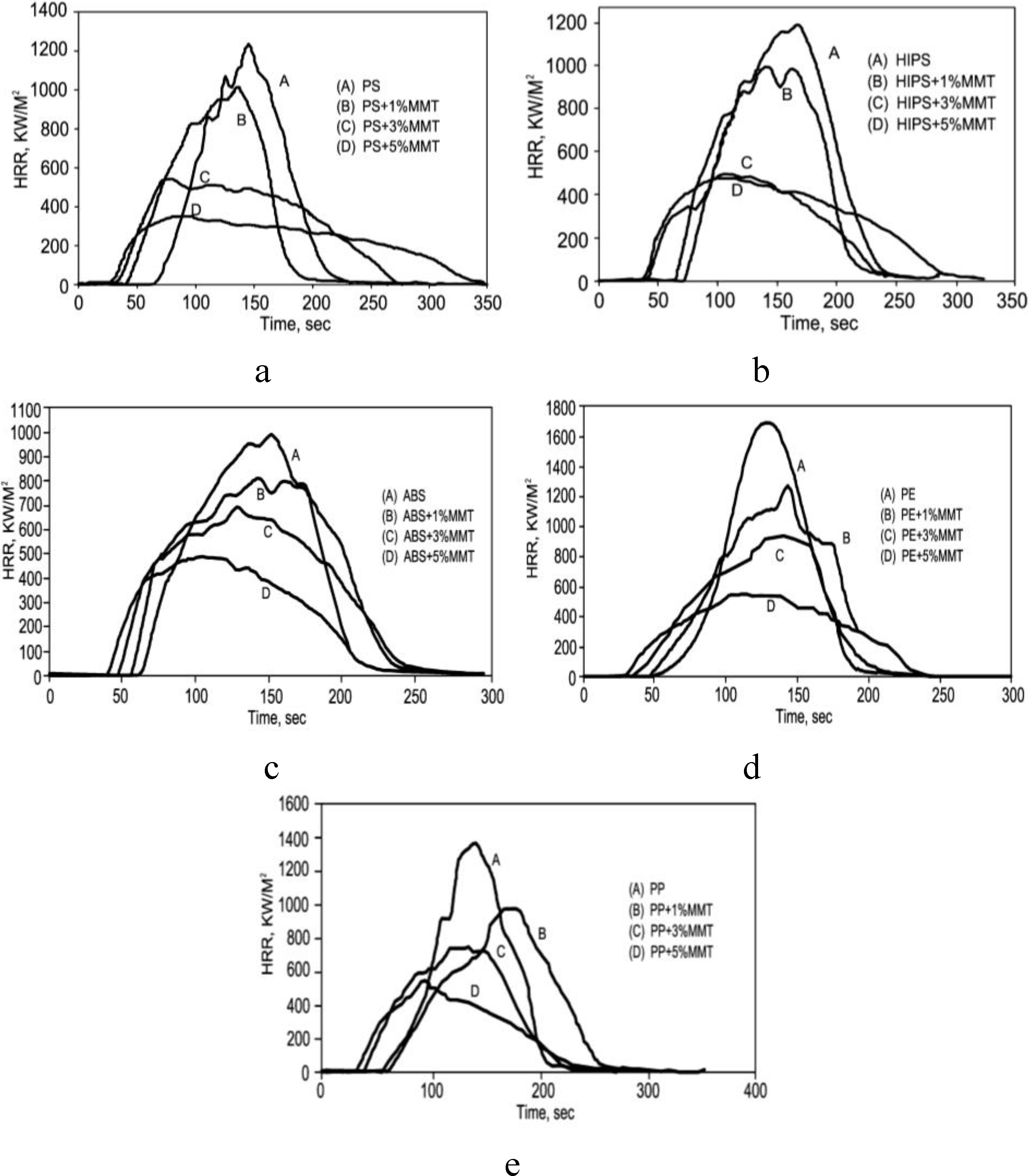
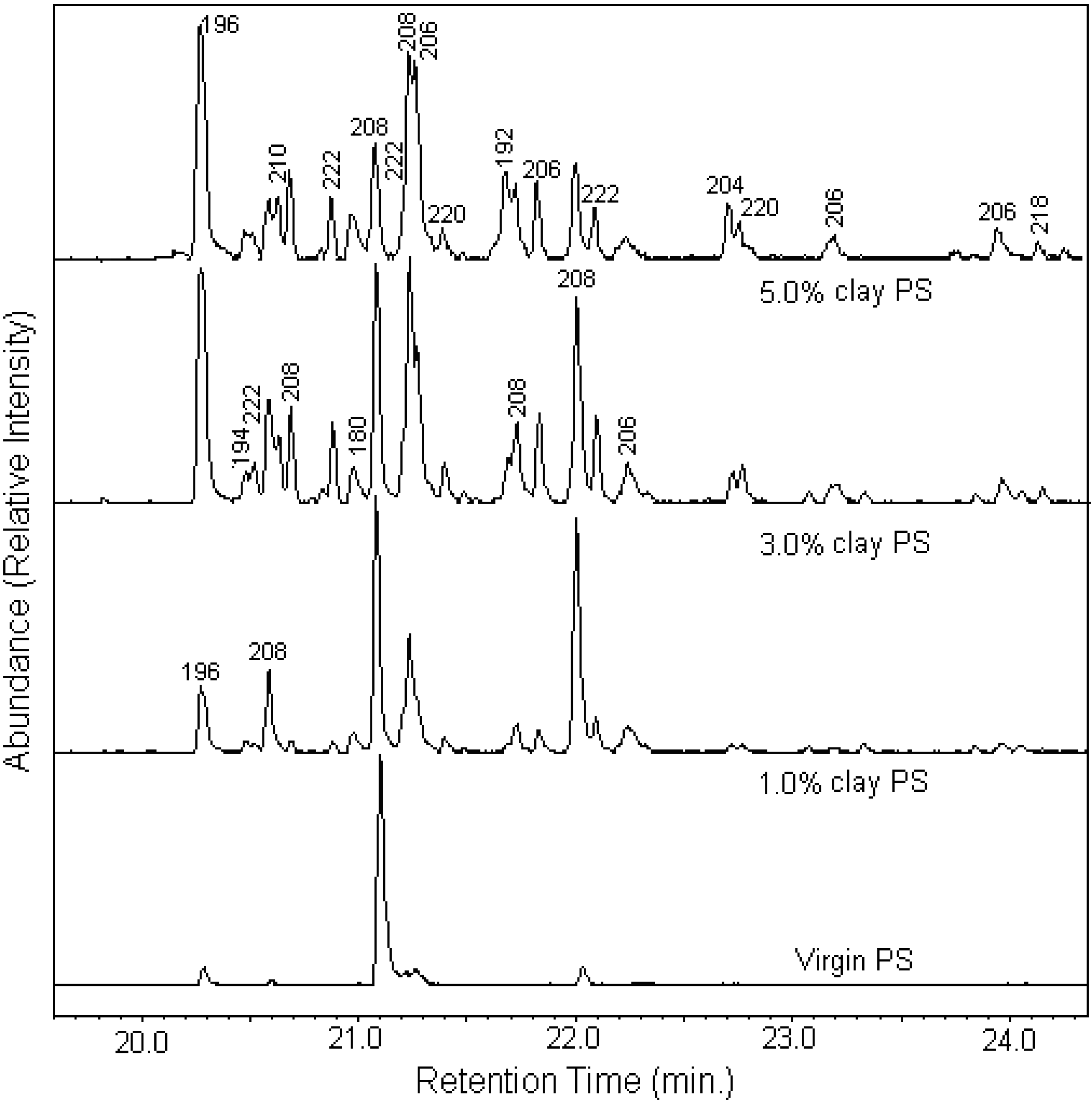
5. Mechanisms by Which Polymer-MMT Nanocomposites Reduce the PHRR
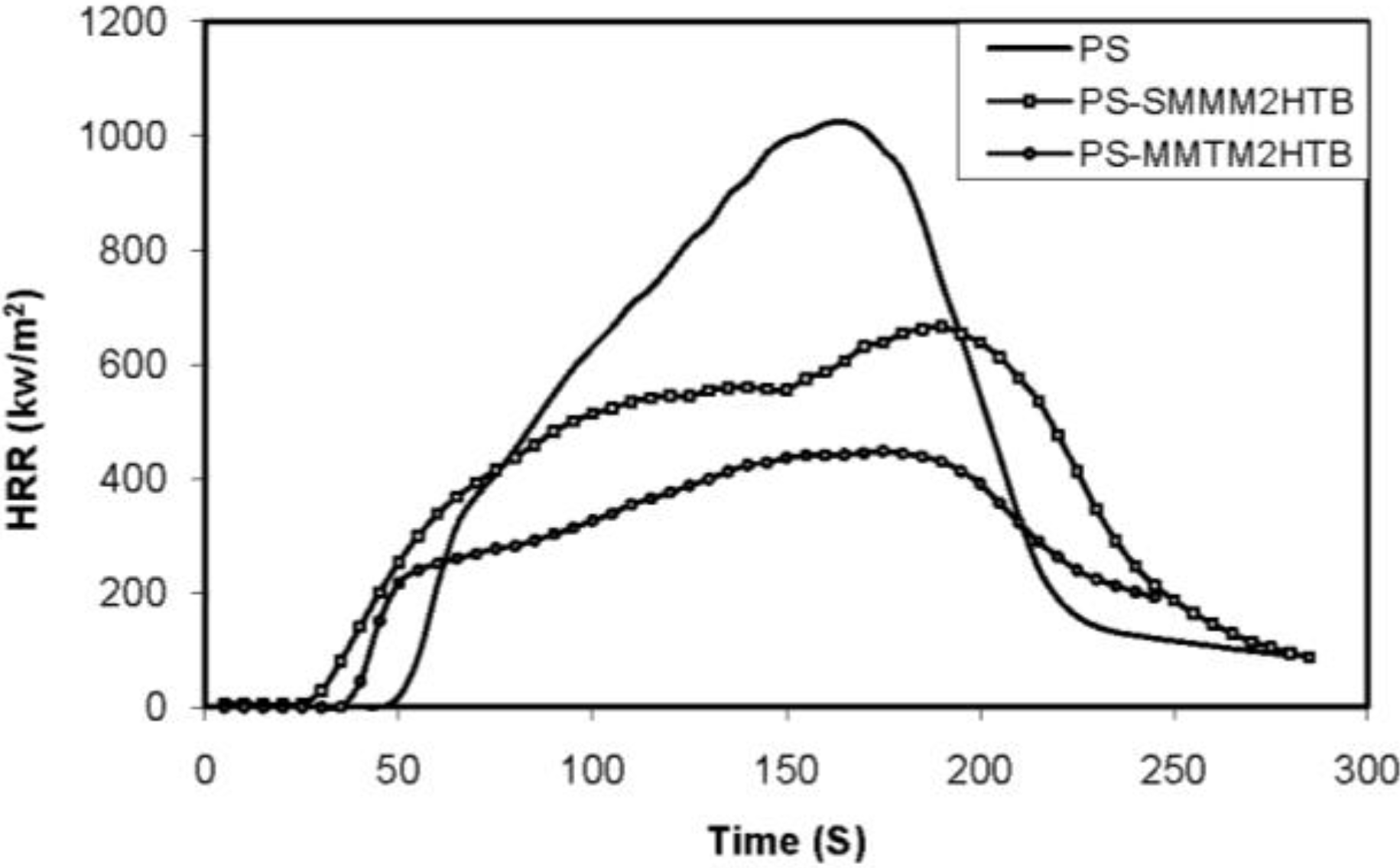
6. Extension to Other Nanomaterials
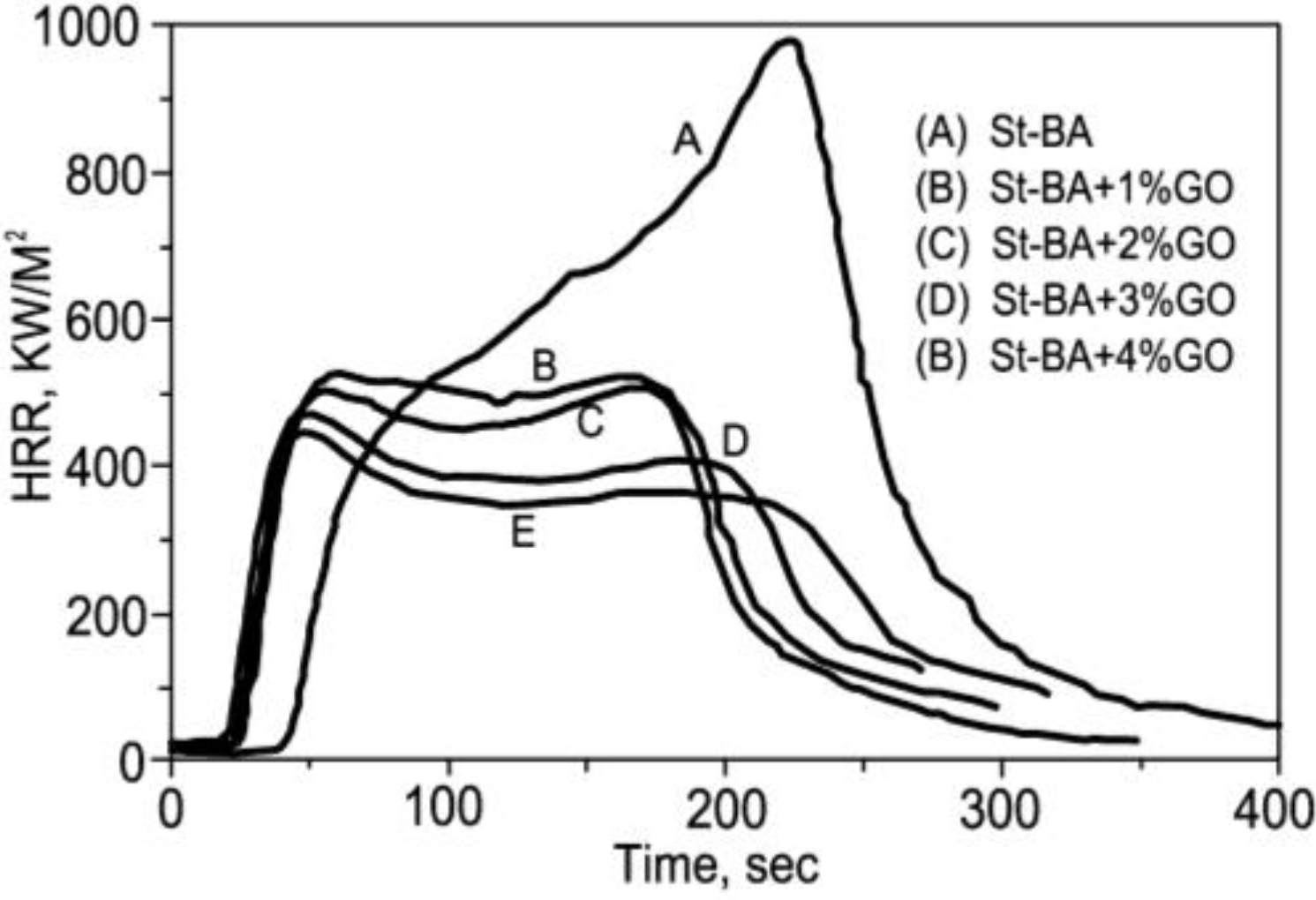
6.1. Graphite and Graphite Oxide
6.2. Nanoscale Silica Particles
6.3. Metal Oxides
6.4. Metal Hydroxides
7. Combinations of Nanomaterials with Conventional Fire Retardants
8. The Future of Nano-Dimensional Materials in Fire Retardancy
References and Notes
- Gilman, J.W. Flammability and thermal stability studies of polymer layered-silicate (clay) nanocomposites. Appl. Clay Sci. 1999, 15, 31–49. [Google Scholar] [CrossRef]
- Zhu, J.; Wilkie, C.A. Thermal and fire studies on polystyrene-clay nanocomposites. Polym. Int. 2000, 49, 1158–1163. [Google Scholar] [CrossRef]
- Fu, X.; Qutubuddin, S. Polymer-clay nanocomposites: exfoliation of organophilic montmorillonite nanolayers in polystyrene. Polymer 2001, 42, 807–813. [Google Scholar] [CrossRef]
- Zhu, J.; Start, P.; Mauritz, K.A.; Wilkie, C.A. Thermal stability and flame retardancy of poly(methyl methacrylate)-clay nanocomposites. Polym. Degrad. Stab. 2002, 77, 253–258. [Google Scholar] [CrossRef]
- Meneghetti, P.; Qutubuddin, S. Synthesis, thermal properties and applications of polymer-clay nanocomposites. Thermochim. Acta 2006, 442, 74–77. [Google Scholar] [CrossRef]
- Fahmy, T.Y.A.; Mobarak, F. Nanocomposites from natural cellulose fibers filled with kaolin in presence of sucrose. Carbohyd. Polym. 2008, 72, 751–755. [Google Scholar] [CrossRef]
- Li, L.Y.; Li, C.Y.; Ni, C.Y.; Rong, L.X.; Hsiao, B. Structure and crystallization behavior of nylon 66/multi-walled carbon nanotube nanocomposites at low carbon nanotube contents. Polymer 2007, 48, 3452–3460. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, J.S.; Lim, B.K.; Kim, S.O. Polymer/carbon nanotube nanocomposites via noncovalent grafting with end-functionalized polymers. J. Appl. Polym. Sci. 2008, 110, 2345–2351. [Google Scholar] [CrossRef]
- Wang, D.Y.; Parlow, D.; Yao, Q.; Wilkie, C.A. PVC-clay nanocomposites: preparation, thermal and mechanical properties. J. Vinyl Addit. Techn. 2004, 7, 203–213. [Google Scholar] [CrossRef]
- Su, S.P.; Wilkie, C.A. The thermal degradation of nanocomposites that contain an oligomeric ammonium cation on the clay. Polym. Degrad. Stab. 2004, 83, 347–362. [Google Scholar] [CrossRef]
- Zhang, J.; Manias, E.; Wilkie, C.A. Polymerically modified layered silicates: An effective route to nanocomposites. J. Nanosci. Nanotechno. 2008, 8, 1597–1615. [Google Scholar] [CrossRef]
- Leroux, F.; Besse, J. Polymer interleaved layered double hydroxide: a new emerging class of nanocomposites. Chem. Mater. 2001, 13, 3507–3515. [Google Scholar] [CrossRef]
- Manzi-Nshuti, C.; Chen, D.; Su, S.P.; Wilkie, C.A. The effects of intralayer metal composition of layered double hydroxides on glass transition, dispersion, thermal and fire properties of their PMMA nanocomposites. Thermochim. Acta 2009, 495, 63–71. [Google Scholar] [CrossRef]
- Manzi-Nshuti, C.; Songtipya, P.; Manias, E.; Jimenez-Gasco, M.M.; Hossenlopp, J.M.; Wilkie, C.A. Polymer nanocomposites using zinc aluminum and magnesium aluminum oleate layered double hydroxides: effects of LDH divalent metals on dispersion, thermal, mechanical and fire performance in various polymers. Polymer 2009, 50, 3564–3574. [Google Scholar] [CrossRef]
- Manzi-Nshuti, C.; Wang, D.Y.; Hossenlopp, J.M.; Wilkie, C.A. The role of the trivalent metal in an LDH: synthesis, characterization and fire properties of thermally stable PMMA/LDH systems. Polym. Degrad. Stab. 2009, 94, 705–711. [Google Scholar] [CrossRef]
- Breuer, O.; Sundararaj, U. Big returns from small fibers: a review of polymer/carbon nanotube composites. Polym. Composite 2004, 25, 630–645. [Google Scholar] [CrossRef]
- An, K.H.; Jeong, S.Y.; Hwang, H.R.; Lee, Y.H. Enhanced sensitivity of a gas sensor incorporating single-walled carbon nanotube-polypyrrole nanocomposites. Adv. Mater. 2004, 16, 1005–1009. [Google Scholar] [CrossRef]
- Ounaies, Z.; Park, C.; Wise, K.E.; Siochi, E.J.; Harrison, J.S. Electrical properties of single wall carbon nanotube reinforced polyimide composites. Compos. Sci. Technol. 2003, 63, 1637–1646. [Google Scholar] [CrossRef]
- Gojny, F.H.; Wichmann, M.H.G.; Köpke, U.; Fiedler, B.; Schulte, K. Carbon nanotube-reinforced epoxy-composites: enhanced stiffness and fracture toughness at low nanotube content. Compos. Sci. Technol. 2004, 64, 2363–2371. [Google Scholar] [CrossRef]
- Gojny, F.H.; Wichmann, M.H.G.; Fiedler, B.; Kinloch, I.A.; Bauhofer, W.; Windle, A.H.; Schulte, K. Evaluation and identification of electrical and thermal conduction mechanisms in carbon nanotube/epoxy composites. Polymer 2006, 47, 2036–2045. [Google Scholar] [CrossRef]
- Barraza, H.J.; Pompeo, F.; Edgar, A.O.; Resasco, D.E. SWNT-filled thermoplastic and elastomeric composites prepared by miniemulsion polymerization. Nano Lett. 2002, 2, 797–802. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Grulke, E.; Hilding, J.; Groth, K.; Harris, R.; Butler, K.; Shields, J.; Kharchenko, S.; Douglas, J. Thermal and flammability properties of polypropylene/carbon nanotube nanocomposites. Polymer 2004, 45, 4227–4239. [Google Scholar] [CrossRef]
- Pötschke, P.; Fornes, T.D.; Paul, D.R. Rheological behavior of multiwalled carbon nanotube/polycarbonate composites. Polymer 2002, 43, 3247–3255. [Google Scholar] [CrossRef]
- McNally, T.; Pötschke, P.; Halley, P.; Murphy, M.; Martin, D.; Bell, S.E.J.; Brennan, G.P.; Bein, D.; Lemoine, P.; Quinn, J.P. Polyethylene multiwalled carbon nanotube composites. Polymer 2005, 46, 8222–8232. [Google Scholar] [CrossRef]
- Schartel, B.; Pötschke, P.; Knoll, U.; Abdel-Goad, M. Fire behaviour of polyamide 6/multiwall carbon nanotube nanocomposites. Eur. Polym. J. 2005, 41, 1061–1070. [Google Scholar] [CrossRef]
- Carter, L.W.; Hendricks, J.G.; Bolley, D.S. (National Lead Co.). Elastomer reinforced with a modified clay. U.S. Patent 2,531,396, 1950. [Google Scholar]
- Fujiwara, S.; Sakamoto, T. Method for manufacturing a clay-polyamide composite. Kokai Patent Application no. 109998, 1976. [Google Scholar]
- Dennis, H.R.; Hunter, D.L.; Chang, D.; Kim, S.; White, J.L.; Cho, J.W.; Paul, D.R. Effect of melt processing conditions on the extent of exfoliation in organoclay-based nanocomposites. Polymer 2001, 42, 9513–9522. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R: Reports 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Polymer/layered silicate nanocomposites: a review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Pinnavaia, T.J.; Beall, G.W. Polymer-clay nanocomposites. John Wiley and Sons: New York, NY, USA, 2000. [Google Scholar]
- Utracki, L.A. Clay-containing polymeric nanocomposites. Rapra Technology, Ltd.: UK, 2004; p. 786. [Google Scholar]
- Zeng, Q.H.; Yu, A.B.; Lu, G.Q.; Paul, D.R. Clay-based polymer nanocomposites: research and commercial development. J. Nanosci. Nanotechno. 2005, 5, 1574–1592. [Google Scholar] [CrossRef]
- Fermeglia, M.; Ferrone, M.; Pricl, S. Computer simulation of nylon-6/organoclay nanocomposites: prediction of the binding energy. Fluid Phase Equilibr. 2003, 212, 315–329. [Google Scholar] [CrossRef]
- Lebaron, P.C.; Wang, Z.; Pinnavaia, T.J. Polymer-layered silicate nanocomposites: an overview. Appl. Clay Sci. 1999, 15, 11–29. [Google Scholar] [CrossRef]
- Luo, J.J.; Daniel, I.M. Characterization and modeling of mechanical behavior of polymer/clay nanocomposites. Compos. Sci. Technol. 2003, 63, 1607–1616. [Google Scholar] [CrossRef]
- Park, C.I.; Park, O.O.; Lim, J.G.; Kim, H.J. The fabrication of syndiotactic polystyrene/organophilic clay nanocomposites and their properties. Polymer 2001, 42, 7465–7475. [Google Scholar] [CrossRef]
- Lu, C.; Mai, Y. Influence of aspect ratio on barrier properties of polymer-clay nanocomposites. Phys. Rev. Lett. 2005, 95, 88303. [Google Scholar] [CrossRef]
- Xu, B.; Zheng, Q.; Song, Y.; Shangguan, Y. Calculating barrier properties of polymer/clay nanocomposites: effects of clay layers. Polymer 2006, 47, 2904–2910. [Google Scholar] [CrossRef]
- Kim, B.K.; Seo, J.W.; Jeong, H.M. Morphology and properties of waterborne polyurethane/clay nanocomposites. Eur. Polym. J. 2003, 39, 85–91. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, Y.H.; Yeh, J.T.; Fan, Z.Q. Study on solvent permeation resistance properties of nylon6/clay nanocomposite. Eur. Polym. J. 2005, 41, 459–466. [Google Scholar] [CrossRef]
- Morgan, A.B.; Harris, R., Jr.; Kashiwagi, T. Flammability of polystyrene layered silicate (clay) nanocomposites: carbonaceous char formation. Fire Mater. 2002, 26, 247–253. [Google Scholar] [CrossRef]
- Zhao, C.G.; Qin, H.L.; Gong, F.L.; Feng, M.; Zhang, S.M.; Yang, M.S. Mechanical, thermal and flammability properties of polyethylene/clay nanocomposites. Polym. Degrad. Stab. 2005, 87, 183–189. [Google Scholar] [CrossRef]
- Morgan, A.B.; Wilkie, C.A. Practical issues and future trends in polymer nanocomposites flammability research. In Flame Retardant Polymer Nanocomposites; Morgan, A.B., Wilkie, C.A., Eds.; John Wiley & Sons: New York, NY, USA, 2007; pp. 362–372. [Google Scholar]
- Leszczynska, A.; Njuguna, J.; Pielichowski, K.; Banerjee, J.R. Polymer/montmorillonite nanocomposites with improved thermal properties: Part I. Factors influencing thermal stability and mechanisms of thermal stability improvement. Thermochim. Acta 2007, 453, 75–96. [Google Scholar] [CrossRef] [Green Version]
- Horrocks, A.R.; Price, D. Fire Retardant Materials; Woodhead Publishing Limited: Cambridge, England, 2001; pp. 203–205. [Google Scholar]
- Lee, J.; Takekoshi, T.; Giannelis, E. Fire retardant polyetherimide nanocomposites. In Nanophase Nanocomposite Materials II; Komarneni, S., Parker, J.C., Wollenberger, H.J., Eds.; MAR Symp. Proc.457: Pittsburgh, PA, USA, 1997; pp. 513–518. [Google Scholar]
- Jang, B.; Wang, D.Y.; Wilkie, C.A. Relationship between the solubility parameter of polymers and the clay dispersion in polymer/clay nanocomposites and the role of the surfactant. Macromol. 2005, 38, 6533–6543. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C. A review on polymer-layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Fornes, T.D.; Yoon, P.J.; Keskkula, H.; Paul, D.R. Nylon 6 nanocomposites: the effect of matrix molecular weight. Polymer 2001, 42, 9929–9940. [Google Scholar] [CrossRef]
- Carrado, K. Synthetic organo-and polymer-clays: preparation, characterization, and materials applications. Appl. Clay Sci. 2000, 17, 1–23. [Google Scholar] [CrossRef]
- Wang, D.Y.; Zhu, J.; Yao, Q.; Wilkie, C.A. A Comparison of various methods for the preparation of polystyrene and poly(methyl methacrylate) clay nanocomposites. Chem. Mater. 2002, 14, 3837–3843. [Google Scholar]
- Tasdelen, M.; Kreutzer, J.; Yagci, Y. In situ synthesis of polymer/clay nanocomposites by living and controlled/living polymerization. Macromol. Chem. Phys. 2009, 211, 279–285. [Google Scholar] [CrossRef]
- Peng, H.D.; Tjiu, W.C.; Shen, L.; Huang, S.; He, C.B.; Liu, T.X. Preparation and mechanical properties of exfoliated CoAl layered double hydroxide (LDH)/polyamide 6 nanocomposites by in situ polymerization. Compos. Sci. Technol. 2009, 69, 991–996. [Google Scholar] [CrossRef]
- Park, C.; Ounaies, Z.; Watson, K.A.; Crooks, R.E.; Smith, J. Dispersion of single wall carbon nanotubes by in situ polymerization under sonication. Chem. Phys. Lett. 2002, 364, 303–308. [Google Scholar] [CrossRef]
- Beyer, G. Nanocomposites: a new class of flame retardants for polymers. Plast. Addit. Compound. 2002, 4, 22–28. [Google Scholar] [CrossRef]
- Pourabas, B.; Raeesi, V. Preparation of ABS/montmorillonite nanocomposite using a solvent/non-solvent method. Polymer 2005, 46, 5533–5540. [Google Scholar] [CrossRef]
- Qiu, L.; Chen, W.; Qu, B. Structural characterisation and thermal properties of exfoliated polystyrene/ZnAl layered double hydroxide nanocomposites prepared via solution intercalation. Polym. Degrad. Stab. 2005, 87, 433–440. [Google Scholar] [CrossRef]
- Su, S.P.; Jiang, D.D.; Wilkie, C.A. Poly(methyl methacrylate), polypropylene and polyethylene nanocomposite formation by melt blending using novel polymerically-modified clays. Polym. Degrad. Stab. 2004, 83, 321–331. [Google Scholar] [CrossRef]
- McNally, T.; Murphy, W.R.; Lew, C.Y.; Turner, R.J.; Brennan, G.P. Polyamide-12 layered silicate nanocomposites by melt blending. Polymer 2003, 44, 2761–2772. [Google Scholar] [CrossRef]
- Wang, L.J.; Xie, X.L.; Su, S.P.; Feng, J.X.; Wilkie, C.A. A comparison of the fire retardancy of poly(methyl methacrylate) using montmorillonite, layered double hydroxide and kaolinite. Polym. Degrad. Stab. 2010, 95, 572–578. [Google Scholar] [CrossRef]
- Zhang, Q.; Rastogi, S.; Chen, D.; Lippits, D.; Lemstra, P.J. Low percolation threshold in single-walled carbon nanotube/high density polyethylene composites prepared by melt processing technique. Carbon 2006, 44, 778–785. [Google Scholar] [CrossRef]
- Schmidt, D.; Shah, D.; Giannelis, E.P. New advances in polymer/layered silicate nanocomposites. Curr. Opin. Solid ST.M. 2002, 6, 205–212. [Google Scholar] [CrossRef]
- Wang, L.J.; Su, S.P.; Chen, D.; Wilkie, C.A. Variation of anions in layered double hydroxides: effects on dispersion and fire properties. Polym. Degrad. Stab. 2009, 94, 770–781. [Google Scholar] [CrossRef]
- VanderHart, D.L.; Asano, A.; Gilman, J.W. NMR measurements related to clay-dispersion quality and organic-modifier stability in nylon-6/clay nanocomposites. Macromol. 2001, 34, 3819–3822. [Google Scholar] [CrossRef]
- VanderHart, D.L.; Asano, A.; Gilman, J.W. Solid-state NMR investigation of paramagnetic nylon-6 clay nanocomposites. 1. Crystallinity, morphology, and the direct influence of Fe3+ on nuclear spins. Chem. Mater. 2001, 13, 3781–3795. [Google Scholar] [CrossRef]
- Vanderhart, D.L.; Asano, A.; Gilman, J.W. Solid-state NMR investigation of paramagnetic nylon-6 clay nanocomposites. 2. Measurement of clay dispersion, crystal stratification, and stability of organic modifiers. Chem. Mater. 2001, 13, 3796–3809. [Google Scholar] [CrossRef]
- Gilman, J.W.; Bourbigot, S.; Shields, J.R.; Nyden, M.; Kashiwagi, T.; Davis, R.D.; VanderHart, D.L.; Demory, W.; Wilkie, C.A.; Morgan, A.B.; Harris, J.; Lyon, R.E. High throughput methods for polymer nanocomposites research: Extrusion, NMR characterization and flammability property screening. J. Mater. Sci. 2003, 38, 4451–4460. [Google Scholar] [CrossRef]
- Bourbigot, S.; VanderHart, D.L.; Gilman, J.W.; Awad, W.H.; Davis, R.D.; Morgan, A.B.; Wilkie, C.A. Investigation of nanodispersion in polystyrene-montmorillonite nanocomposites by solid-state NMR. J. Polym. Sci.: Part B: Polym. Phys. 2003, 41, 3188–3213. [Google Scholar] [CrossRef]
- Bourbigot, S.; VanderHart, D.L.; Gilman, J.W.; Bellayer, S.; Stretz, H.; Paul, D.R. Solid state NMR characterization and flammability of styrene-acrylonitrile copolymer montmorillonite nanocomposite. Polymer 2004, 45, 7627–7638. [Google Scholar] [CrossRef]
- Fian, A.; Haase, A.; Stadlober, B.; Jakopic, G.; Matsko, N.; Grogger, W.; Leising, G. AFM, ellipsometry, XPS and TEM on ultra-thin oxide/polymer nanocomposite layers in organic thin film transistors. Anal. Bioanal. Chem. 2008, 390, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Start, P.; Mauritz, K.A.; Wilkie, C.A. Silicon-methoxide-modified clays and their polystyrene nanocomposites. J. Polym. Sci. Part A: Polym. Chem. 2002, 40, 1498–1503. [Google Scholar] [CrossRef]
- Liu, Y.L.; Hsu, C.Y.; Wei, W.L.; Jeng, R.J. Preparation and thermal properties of epoxy-silica nanocomposites from nanoscale colloidal silica. Polymer 2003, 44, 5159–5167. [Google Scholar] [CrossRef]
- Wang, J.; Du, J.; Zhu, J.; Wilkie, C.A. An XPS study of the thermal degradation and flame retardant mechanism of polystyrene-clay nanocomposites. Polym. Degrad. Stab. 2002, 77, 249–252. [Google Scholar] [CrossRef]
- Jing, C.J.; Chen, L.G.; Shi, Y.; Jin, X.G. Solid state NMR and fluorescence studies of conjugated polymer nanocomposties. Chin. Chem. Lett. 2005, 16, 1519–1522. [Google Scholar]
- Jing, C.J.; Chen, L.S.; Shi, Y.; Jin, X.G. Synthesis and characterization of exfoliated MEH-PPV/clay nanocomposites by in situ polymerization. Eur. Polym. J. 2005, 41, 2388–2394. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Mu, M.; Winey, K.; Cipriano, B.; Raghavan, S.R.; Pack, S.; Rafailovich, M.; Yang, Y.; Grulke, E.; Shields, J.; Harris, R.; Douglas, J. Relation between the viscoelastic and flammability properties of polymer nanocomposites. Polymer 2008, 49, 4358–4368. [Google Scholar] [CrossRef]
- Lee, K.M.; Han, C.D. Effect of hydrogen bonding on the rheology of polycarbonate/organoclay nanocomposites. Polymer 2003, 44, 4573–4588. [Google Scholar] [CrossRef]
- Chin, I.J.; Thurn-Albrecht, T.; Kim, H.C.; Russell, T.P.; Wang, J. On exfoliation of montmorillonite in epoxy. Polymer 2001, 42, 5947–5952. [Google Scholar] [CrossRef]
- Porter, D.; Metcalfe, E.; Thomas, M. Nanocomposite fire retardants-a review. Fire Mater. 2000, 24, 45–52. [Google Scholar] [CrossRef]
- Krishnamoorti, R.; Giannelis, E.P. Rheology of end-tethered polymer layered silicate nanocomposites. Macromol. 1997, 30, 4097–4102. [Google Scholar] [CrossRef]
- Bhiwankar, N.N.; Weiss, R.A. Melt-intercalation of sodium-montmorillonite with alkylamine and quartenized ammonium salts of sulfonated polystyrene ionomers. Polymer 2005, 46, 7246–7254. [Google Scholar]
- Wang, L.J.; Su, S.P.; Chen, D.; Wilkie, C.A. Fire retardancy of bis [2-(methacryloyloxy)ethyl] phosphate modified poly(methyl methacrylate) nanocomposites containing layered double hydroxide and montmorillonite. Polym. Degrad. Stab. 2009, 94, 1110–1118. [Google Scholar] [CrossRef]
- Becker, O.; Varley, R.J.; Simon, G.P. Thermal stability and water uptake of high performance epoxy layered silicate nanocomposites. Eur. Polym. J. 2004, 40, 187–195. [Google Scholar] [CrossRef]
- Chigwada, G.; Wang, D.Y.; Jiang, D.D.; Wilkie, C.A. Styrenic nanocomposites prepared using a novel biphenyl-containing modified clay. Polym. Degrad. Stab. 2006, 91, 755–762. [Google Scholar] [CrossRef]
- Liu, L.; Ye, Z. Effects of modified multi-walled carbon nanotubes on the curing behavior and thermal stability of boron phenolic resin. Polym. Degrad. Stab. 2009, 94, 1972–1978. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials-Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Huggett, C. Estimation of rate of heat release by means of oxygen consumption measurements. Fire Mater. 2004, 4, 61–65. [Google Scholar] [CrossRef]
- Gann, R.G.; Averill, J.D.; Butler, K.M.; Jones, W.W.; Mulholland, G.W.; Neviaser, J.L.; Ohlemiller, T.J.; Peacock, R.D.; Reneke, P.A.; Hall, J.R. International study of the sublethal effects of fire smoke on survivability and health (SEFS): Phase I Final Report. 2001; National Institute of Standards and Technology. [Google Scholar]
- Zheng, X.X.; Jiang, D.D.; Wang, D.Y.; Wilkie, C.A. Flammability of styrenic polymer clay nanocomposites based on a methyl methacrylate oligomerically-modified clay. Polym. Degrad. Stab. 2006, 91, 289–297. [Google Scholar] [CrossRef]
- Su, S.P.; Wilkie, C.A. Exfoliated poly (methyl methacrylate) and polystyrene nanocomposites occur when the clay cation contains a vinyl monomer. J. Polym. Sci. Part A: Polym. Chem. 2003, 41, 1124–1135. [Google Scholar] [CrossRef]
- Jash, P.; Wilkie, C.A. Effects of surfactants on the thermal and fire properties of poly (methyl methacrylate)/clay nanocomposites. Polym. Degrad. Stab. 2005, 88, 401–406. [Google Scholar]
- Costache, M.C.; Jiang, D.D.; Wilkie, C.A. Thermal degradation of ethylene-vinyl acetate coplymer nanocomposites. Polymer 2005, 46, 6947–6958. [Google Scholar] [CrossRef]
- Jang, B.N.; Wilkie, C.A. The effect of clay on the thermal degradation of polyamide 6 in polyamide 6/clay nanocomposites. Polymer 2005, 46, 3264–3274. [Google Scholar] [CrossRef]
- Su, S.P.; Jiang, D.D.; Wilkie, C.A. Novel polymerically-modified clays permit the preparation of intercalated and exfoliated nanocomposites of styrene and its copolymers by melt blending. Polym. Degrad. Stab. 2004, 83, 333–346. [Google Scholar] [CrossRef]
- Costache, M.C.; Heidecker, M.J.; Manias, E.; Camino, G.; Frache, A.; Beyer, G.; Gupta, R.K.; Wilkie, C.A. The influence of carbon nanotubes, organically modified montmorillonites and layered double hydroxides on the thermal degradation and fire retardancy of polyethylene, ethylene-vinyl acetate copolymer and polystyrene. Polymer 2007, 48, 6532–6545. [Google Scholar] [CrossRef]
- Nyambo, C.; Kandare, E.; Wang, D.Y.; Wilkie, C.A. Flame-retarded polystyrene: Investigating chemical interactions between ammonium polyphosphate and MgAl layered double hydroxide. Polym. Degrad. Stab. 2008, 93, 1656–1663. [Google Scholar] [CrossRef]
- Nyambo, C.; Wang, D.; Wilkie, C.A. Will layered double hydroxides give nanocomposites with polar or non-polar polymers? Polym. Advan. Technol. 2008, 20, 332–340. [Google Scholar] [CrossRef]
- Manzi-Nshuti, C.; Wang, D.Y.; Hossenlopp, J.M.; Wilkie, C.A. Aluminum-containing layered double hydroxides: the thermal, mechanical, and fire properties of (nano) composites of poly (methyl methacrylate). J. Mater. Chem. 2008, 18, 3091–3102. [Google Scholar] [CrossRef]
- Nyambo, C.; Songtipya, P.; Manias, E.; Jimenez-Gasco, M.M.; Wilkie, C.A. Effect of MgAl-layered double hydroxide exchanged with linear alkyl carboxylates on fire-retardancy of PMMA and PS. J. Mater. Chem. 2008, 18, 4827–4838. [Google Scholar] [CrossRef]
- Manzi-Nshuti, C.; Hossenlopp, J.M.; Wilkie, C.A. Fire retardancy of melamine and zinc aluminum layered double hydroxide in poly(methyl methacrylate). Polym. Degrad. Stab. 2008, 93, 1855–1863. [Google Scholar] [CrossRef]
- Nyambo, C.; Wilkie, C.A. Layered double hydroxides intercalated with borate anions: Fire and thermal properties in ethylene vinyl acetate copolymer. Polym. Degrad. Stab. 2009, 94, 506–512. [Google Scholar] [CrossRef]
- Nyambo, C.; Chen, D.; Su, S.P.; Wilkie, C.A. Variation of benzyl anions in MgAl-layered double hydroxides: Fire and thermal properties in PMMA. Polym. Degrad. Stab. 2009, 94, 496–505. [Google Scholar] [CrossRef]
- Manzi-Nshuti, C.; Hossenlopp, J.M.; Wilkie, C.A. Comparative study on the flammability of polyethylene modified with commercial fire retardants and a zinc aluminum oleate layered double hydroxide. Polym. Degrad. Stab. 2009, 94, 782–788. [Google Scholar] [CrossRef]
- Nyambo, C.; Kandare, E.; Wilkie, C.A. Thermal stability and flammability characteristics of ethylene vinyl acetate (EVA) composites blended with a phenyl phosphonate-intercalated layered double hydroxide (LDH), melamine polyphosphate and/or boric acid. Polym. Degrad. Stab. 2009, 94, 513–520. [Google Scholar] [CrossRef]
- Manzi-Nshuti, C.; Chen, D.; Su, S.P.; Wilkie, C.A. Structure-property relationships of new polystyrene nanocomposites prepared from initiator-containing layered double hydroxides of zinc aluminum and magnesium aluminum. Polym. Degrad. Stab. 2009, 94, 1290–1297. [Google Scholar] [CrossRef]
- Nyambo, C.; Chen, D.; Su, S.P.; Wilkie, C.A. Does organic modification of layered double hydroxides improve the fire performance of PMMA? Polym. Degrad. Stab. 2009, 94, 1298–1306. [Google Scholar] [CrossRef]
- Manzi-Nshuti, C.; Songtipya, P.; Manias, E.; Jimenez-Gasco, M.M.; Hossenlopp, J.M.; Wilkie, C.A. Polymer nanocomposites using zinc aluminum and magnesium aluminum oleate layered double hydroxides: effects of the polymeric compatibilizer and of composition on the thermal and fire properties of PP/LDH nanocomposites. Polymer 2009, 50, 3564–3574. [Google Scholar] [CrossRef]
- Wang, X.L.; Rathore, R.; Songtipya, P.; Jimenez-Gasco, M.M.; Manias, E.; Wilkie, C.A. EVA-layered double hydroxide (nano)composites: Mechanism of fire retardancy. Polym. Degrad. Stab. in press.
- Rakhimkulov, A.; Lomakin, S.; Dubnikova, I.; Shchegolikhin, A.; Davidov, E.; Kozlowski, R. The effect of multi-walled carbon nanotubes addition on the thermo-oxidative decomposition and flammability of PP/MWCNT nanocomposites. J. Mater. Sci. 2010, 45, 633–640. [Google Scholar] [CrossRef]
- Gilman, J.W.; Kashiwagi, T.; Nyden, M.; Brown, J.E.T.; Jackson, C.L.; Lomakin, S.; Giannelis, E.P.; Manias, E. Flammability studies of polymer layered silicate nanocomposites: Polyolefin, epoxy and vinyl ester resins. In Chemistry and Technology of Polymer Additives; Al-Malaika, S., Golovoy, A., Wilkie, C.A., Eds.; Blackwell Scientific: Oxford, UK, 1999; pp. 249–265. [Google Scholar]
- Gilman, J.W.; Jackson, C.L.; Morgan, A.B.; Harris, R., Jr.; Manias, E.; Giannelis, E.P.; Wuthenow, M.; Hilton, D.; Phillips, S.H. Flammability properties of polymer-layered-silicate nanocomposites. polypropylene and polystyrene nanocomposites. Chem. Mater. 2000, 12, 1866–1873. [Google Scholar] [CrossRef]
- Zhu, J.; Uhl, F.M.; Morgan, A.B.; Wilkie, C.A. Studies on the mechanism by which the formation of nanocomposites enhances thermal stability. Chem. Mater. 2001, 13, 4649–4654. [Google Scholar] [CrossRef]
- Zheng, X.; Wilkie, C.A. Flame retardancy of polystyrene nanocomposites based on an oligomeric organically-modified clay containing phosphate. Polym. Degrad. Stab. 2003, 81, 539–550. [Google Scholar] [CrossRef]
- Kashiwagi, T. Polymer combustion and flammability: role of the condensed phase. In Symposium (International) on Combustion; 1994; Volume 25, pp. 1423–1437. [Google Scholar]
- Chen, K.; Wilkie, C.A.; Vyazovkin, S. Revealing nano-confinement in degradation and relaxation studies of two structurally different polystyrene-clay systems. J. Phys. Chem. B 2007, 111, 12685–12692. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.N.; Wilkie, C.A. The thermal degradation of polystyrene nanocomposite. Polymer 2005, 46, 2933–2942. [Google Scholar] [CrossRef]
- Jang, B.N.; Wilkie, C.A. The effects of clay on the thermal degradation behavior of poly(styrene-co-acrylonitirile). Polymer 2005, 46, 9702–9713. [Google Scholar] [CrossRef]
- Jang, B.N.; Costache, M.; Wilkie, C.A. The relationship between thermal degradation behavior of polymer and the fire retardancy of polymer/clay nanocomposites. Polymer 2005, 46, 10678–10687. [Google Scholar] [CrossRef]
- Costache, M.; Wang, D.; Heidecker, M.J.; Manias, E.; Wilkie, C.A. The thermal degradation of poly (methyl methacrylate) nanocomposites with montmorillonite, layered double hydroxides and carbon nanotubes. Polym. Advan. Techol. 2006, 17, 272–280. [Google Scholar] [CrossRef]
- Uhl, F.M.; Wilkie, C.A. Polystyrene/graphite nanocomposites: effect on thermal stability. Polym. Degrad. Stab. 2002, 76, 111–122. [Google Scholar] [CrossRef]
- Uhl, F.M.; Wilkie, C.A. Preparation of nanocomposites from styrene and modified graphite oxides. Polym. Degrad. Stab. 2004, 84, 215–226. [Google Scholar]
- Uhl, F.M.; Yao, Q.; Nakajima, H.; Manias, E.; Wilkie, C.A. Expandable graphite/polyamide-6 nanocomposites. Polym. Degrad. Stab. 2005, 89, 70–84. [Google Scholar] [CrossRef]
- Uhl, F.M.; Yao, Q.; Wilkie, C.A. Formation of nanocomposites of styrene and its copolymers using graphite as the nanomaterial. Polym. Advan. Techol. 2005, 16, 533–540. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, Y.; Xu, J.Y.; Fan, W.C.; Chen, Z.Y. Flammability and thermal stability studies of styrene-butyl acrylate copolymer/graphite oxide nanocomposite. Polym. Degrad. Stab. 2004, 85, 583–588. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/silica nanocomposites: preparation, characterization, properties, and applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef] [PubMed]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P.H. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng.: R: Reports 2009, 63, 100–125. [Google Scholar]
- Ji, Q.; Wang, X.L.; Zhang, Y.H.; Kong, Q.S.; Xia, Y.Z. Characterization of Poly (ethylene terephthalate)/SiO2 nanocomposites prepared by Sol-Gel method. Compos. Part A: Appl. Sci. Manuf. 2009, 40, 878–882. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Morgan, A.B.; Antonucci, J.M.; VanLandingham, M.R.; Harris, R.H., Jr.; Awad, W.H.; Shields, J.R. Thermal and flammability properties of a silica-poly (methylmethacrylate) nanocomposite. J. Appl. Polym. Sci. 2003, 89, 2072–2078. [Google Scholar]
- Yang, F.; Yngard, R.; Nelson, G. Flammability of polymer-clay and polymer-silica nanocomposites. J. Fire Sci. 2005, 23, 209–226. [Google Scholar] [CrossRef]
- Laachachi, A.; Leroy, E.; Cochez, M.; Ferriol, M.; Lopez Cuesta, J.M. Use of oxide nanoparticles and organoclays to improve thermal stability and fire retardancy of poly(methyl methacrylate). Polym. Degrad. Stab. 2005, 89, 344–352. [Google Scholar] [CrossRef]
- Rault, F.; Pleyber, E.; Campagne, C.; Rochery, M.; Giraud, S.; Bourbigot, S.; Devaux, E. Effect of manganese nanoparticles on the mechanical, thermal and fire properties of polypropylene multifilament yarn. Polym. Degrad. Stab. 2009, 94, 955–964. [Google Scholar] [CrossRef]
- Dzunuzovic, E.; Marinovic-Cincovic, M.; Jeremic, K.; Nedeljkovic, J. Influence of cubic α-Fe2O3 particles on the thermal stability of poly(methyl methacrylate) synthesized by in situ bulk polymerization. Polym. Degrad. Stab. 2009, 94, 701–704. [Google Scholar] [CrossRef]
- Qiu, L.Z.; Xie, R.C.; Ding, P.; Qu, B.J. Preparation and characterization of Mg(OH)2 nanoparticles and flame-retardant property of its nanocomposites with EVA. Compo. Struct. 2003, 62, 391–395. [Google Scholar]
- Zhang, X.; Guo, F.; Chen, J.; Wang, G.; Liu, H. Investigation of interfacial modification for flame retardant ethylene vinyl acetate copolymer/alumina trihydrate nanocomposites. Polym. Degrad. Stab. 2005, 87, 411–418. [Google Scholar] [CrossRef]
- Beyer, G. Flame retardant properties of EVA-nanocomposites and improvements by combination of nanofillers with aluminium trihydrate. Fire Mater. 2001, 25, 193–197. [Google Scholar] [CrossRef]
- Zhang, J.; Wilkie, C.A. Fire retardancy of polypropylene-metal hydroxide nanocomposites. In Fire and Polymers IV; ACS symposium series 922; Wilkie, C.A., Nelson, G.L., Eds.; Oxford University Press, 2006; pp. 61–74. [Google Scholar]
- Zhang, J.; Wilkie, C.A. Fire retardancy of polyethylene-alumina trihydrate containing clay as a synergist. Polym. Advan. Techol. 2005, 16, 549–553. [Google Scholar] [CrossRef]
- Chigwada, G.; Wilkie, C.A. Synergy between conventional phosphorus fire retardants and organically-modified clays can lead to fire retardancy of styrenics. Polym. Degrad. Stab. 2003, 81, 551–557. [Google Scholar] [CrossRef]
- Wang, D.Y.; Echols, K.; Wilkie, C.A. Cone calorimetric and thermogravimetric analysis evaluation of halogen-containing polymer nanocomposites. Fire Mater. 2005, 29, 283–294. [Google Scholar] [CrossRef]
- Bourbigot, S.; Samyn, F.; Turf, T.; Duquesne, S. Nanomorphology and reaction to fire of polyurethane and polyamide nanocomposites containing flame retardants. Polym. Degrad. Stab. 2010, 95, 320–326. [Google Scholar] [CrossRef]
Acronyms
| ABS | Acrylonitrile-butadiene-styrene terpolymer |
| AFM | Atomic force microscopy |
| ATH | Alumina trihydrate |
| BPR | Boron phenolic resin |
| BZn | Zinc borate |
| EVA | Poly(ethylene-co-vinyl acetate) |
| GC-MS | Gas chromatograph-mass spectrometer |
| HIPS | High impact polystyrene |
| HRR/RHR | Heat release rate |
| LDH | Layered double hydroxide |
| LOI | Limiting oxygen index |
| MH | Magnesium hydroxide |
| MMT | Montmorillonite |
| MWNT/MWCNT | Multi-wall carbon nanotubes |
| m-MWCNT | Modified multi-wall carbon nanotubes |
| NMR | Nuclear magnetic resonance |
| OMMT | Organically modified montmorillonite |
| PA-6 | Polyamide-6 |
| PE | Polyethylene |
| PET | Poly(ethylene terephthalate) |
| PHRR | Peak heat release rate |
| PMMA | Poly(methyl methacrylate) |
| PP | Polypropylene |
| PS | Polystyrene |
| SEM | Scanning electron microscopy |
| SWNT/SWCNT | Single-wall carbon nanotubes |
| TEM | Transmission electron microscopy |
| TGA | Thermogravimetric analysis |
| THR | Total heat released |
| TPP | Triphenylphosphate |
| UL-94 | Underwriter’ Laboratory Test #94 |
| XRD | X-ray diffraction |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, L.; He, X.; Wilkie, C.A. The Utility of Nanocomposites in Fire Retardancy. Materials 2010, 3, 4580-4606. https://doi.org/10.3390/ma3094580
Wang L, He X, Wilkie CA. The Utility of Nanocomposites in Fire Retardancy. Materials. 2010; 3(9):4580-4606. https://doi.org/10.3390/ma3094580
Chicago/Turabian StyleWang, Linjiang, Xuejun He, and Charles A. Wilkie. 2010. "The Utility of Nanocomposites in Fire Retardancy" Materials 3, no. 9: 4580-4606. https://doi.org/10.3390/ma3094580