Fluorescence and FTIR Spectra Analysis of Trans-A2B2-Substituted Di- and Tetra-Phenyl Porphyrins
Abstract
:1. Introduction
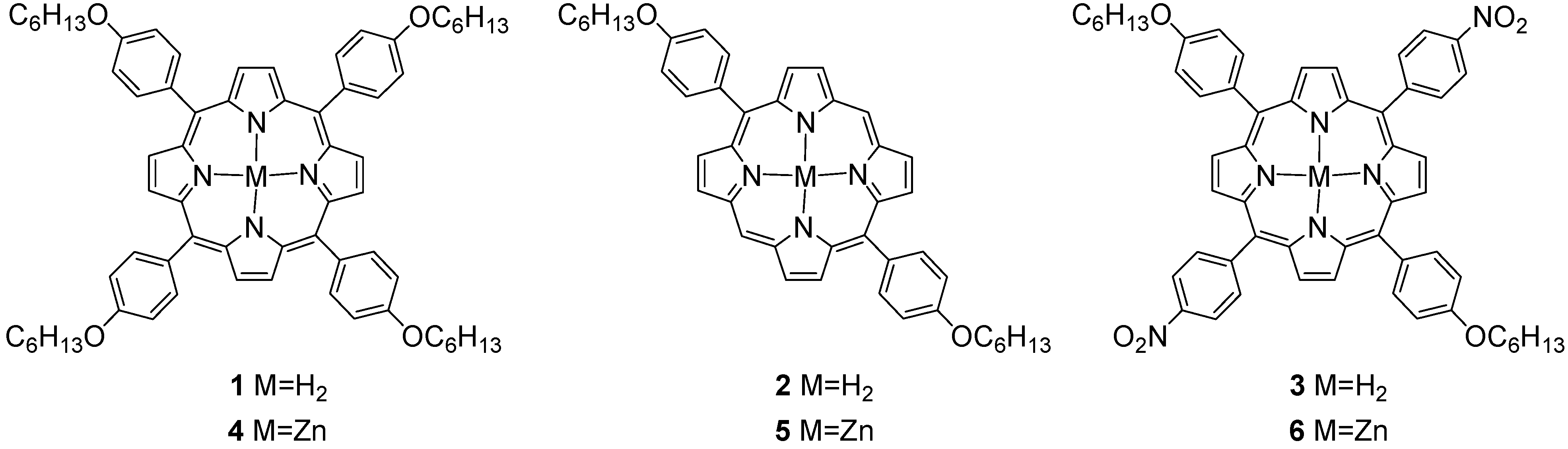
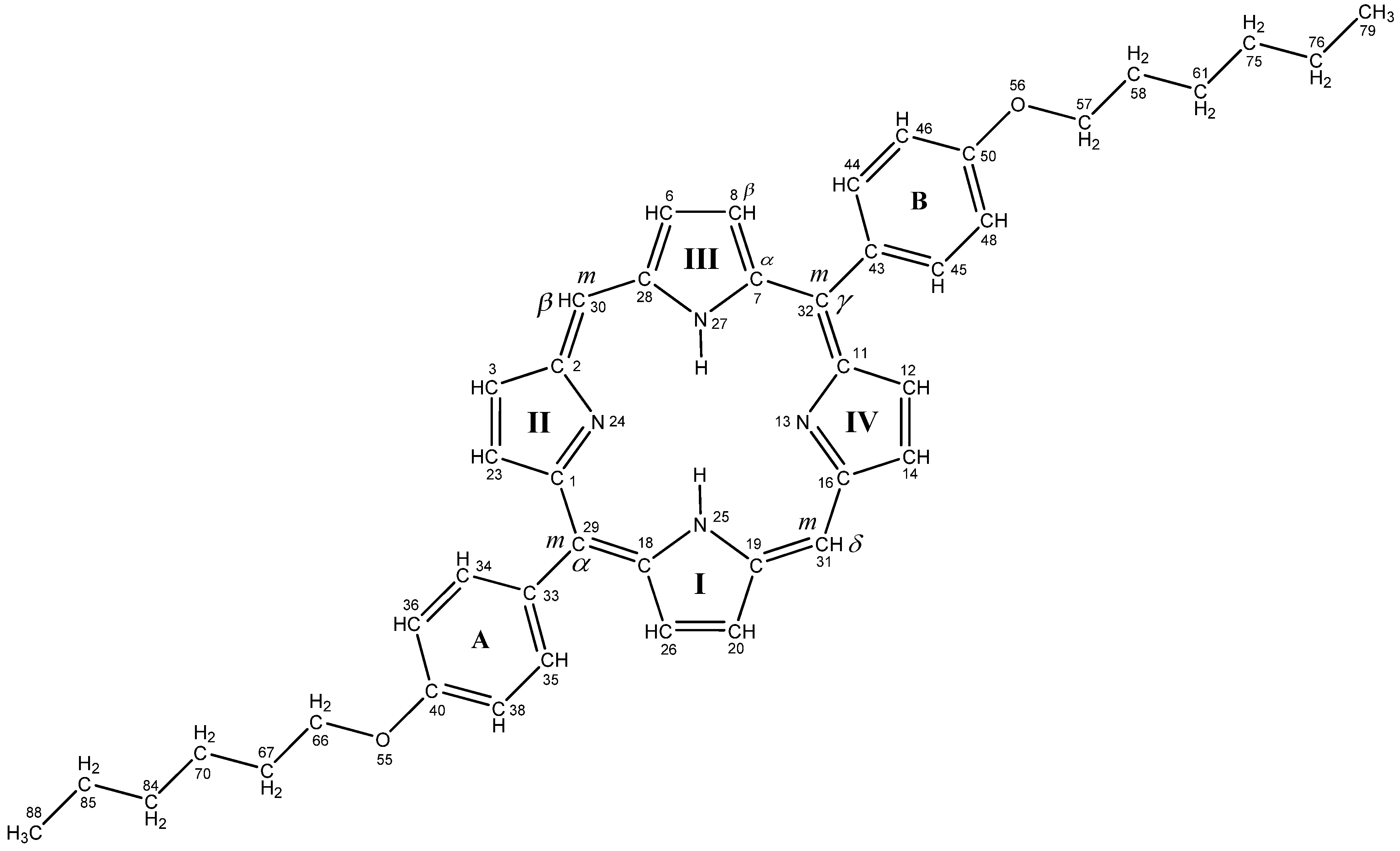
2. Results and Discussion
2.1. Preparation of Asymmetric Di- and Tetra-phenyl Porphyrin Compounds
| 4 | 5 | 6 | |
|---|---|---|---|
| Formula | C68H76N4O4Zn1 | C22H22N2OZn0.50.5 | C58H52Cl6N6O6Zn |
| f.w. (g.mol-1) | 1078.77 | 363.12 | 1207.18 |
| Cryst. Syst. | Orthorhombic | Triclinic | Triclinic |
| Space group | Pbcn (No. 60) | P-1 (No. 2) | P-1 (No. 2) |
| a (Å) | 18.923 (5) | 9.721 (5) | 10.877 (5) |
| b (Å) | 11.138 (5) | 10.593 (5) | 13.726 (5) |
| c (Å) | 28.542 (5) | 10.679 (5) | 18.728 (5) |
| α (o) | 90 | 64.838 (5) | 88.050 (5) |
| β (o) | 90 | 67.222 (5) | 87.781 (5) |
| γ (o) | 90 | 89.723 (5) | 84.908 (5) |
| V (Å3) | 6016 (3) | 900.7 (8) | 2782 (2) |
| Z | 4 | 2 | 2 |
| T (K) | 300 | 293 | 120 |
| Dx (g.cm-3) | 1.191 | 1.339 | 1.441 |
| μ (mm-1) | 0.46 | 0.73 | 3.73 |
| R(F) a, I>2σ(Fo) | 0.064 | 0.117 | 0.139 |
| Rw(F2) b, I>2σ(Fo) | 0.201 | 0.247 | 0.143 |
| S | 0.95 | 1.03 | 1.05 |
| Rint | 0.033 | 0.044 | 0.066 |
| θmax | 29.2° | 29.4 | 62.8° |
| Δρmin | -0.84 e Å-1 | -1.32 e Å-1 | -0.19 e Å-1 |
| Δρmax | 0.85 e Å-1 | 4.70 e Å-1 | 0.19 e Å-1 |

2.2. FTIR Spectra and Analysis of Vibration Structure
2.1.1. General appearance of FTIR spectra
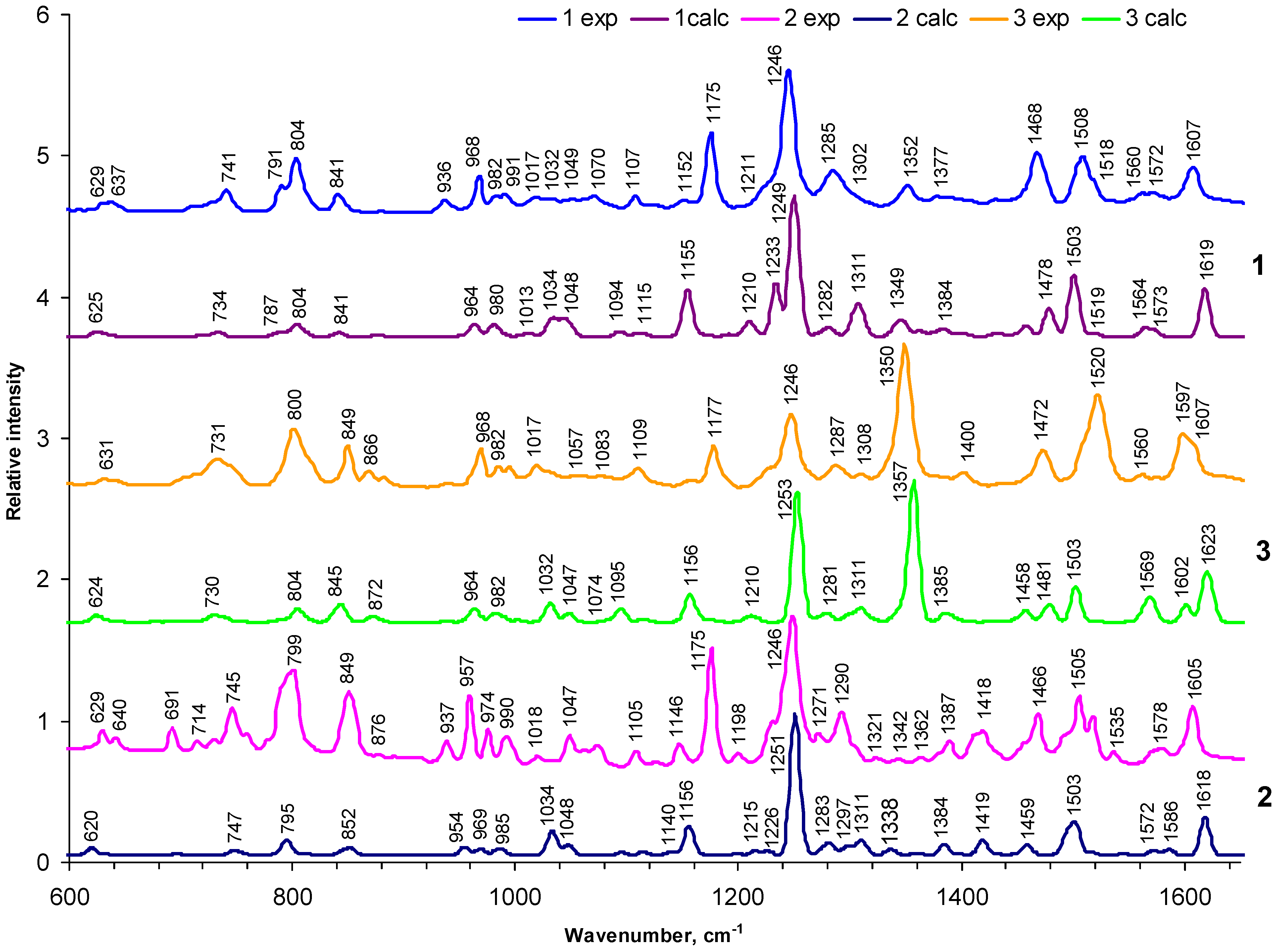

2.2.2. Vibrations of phenyl and pyrrole rings
2.2.3. Deformation vibrations
2.2.4. The influence of the nitro-group
2.2.5. Specific porphyrin ring modes in comparison with other studies
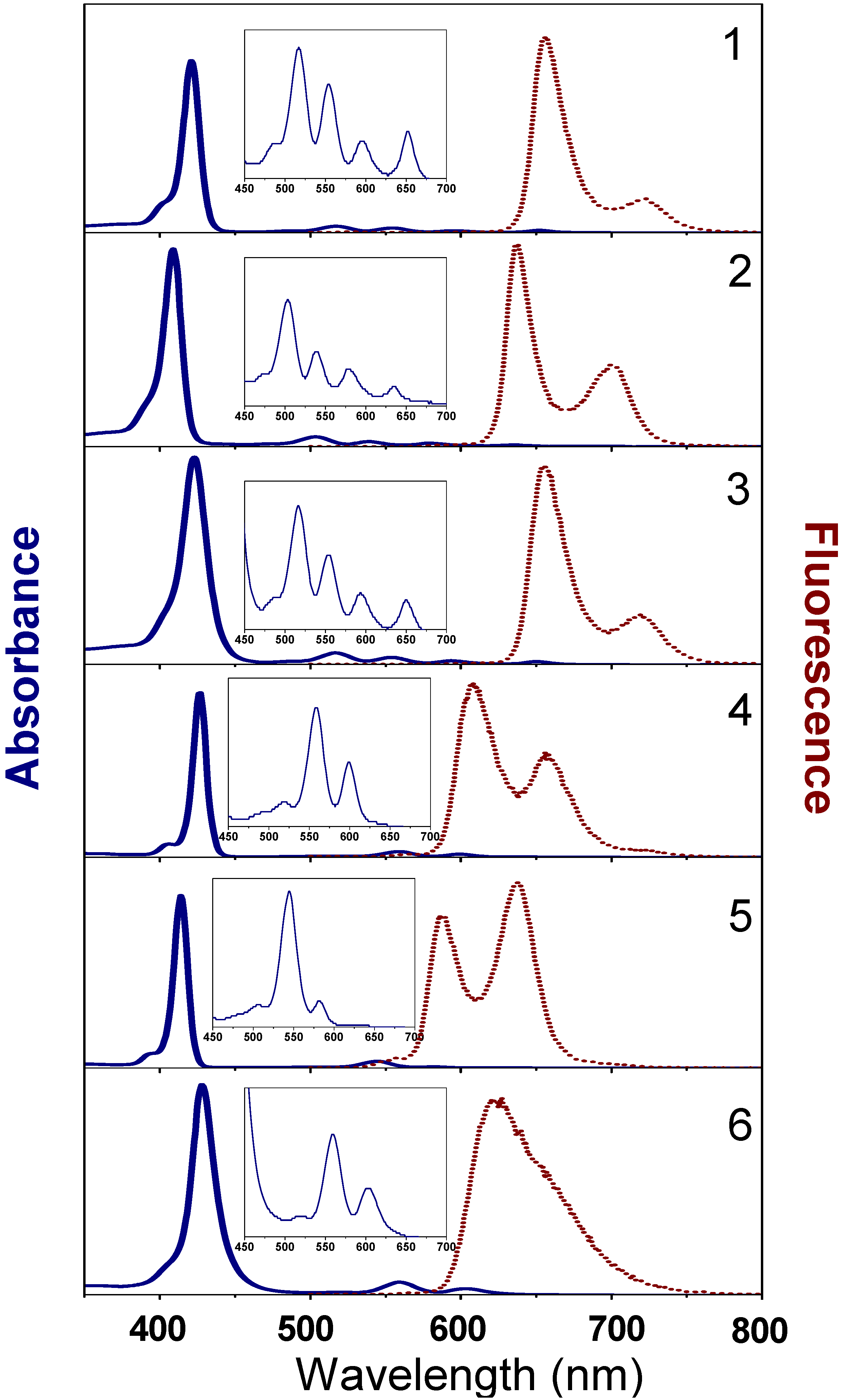
2.3. Optical Absorption, Excited States and Fluorescence
2.3.1. Theoretical and experimental absorption spectra
| Sample | Absorbance (nm) | Emission wavelength (nm) [decay time (ns)] | Quantum Efficiency a |
|---|---|---|---|
| 1 | S: 421; Q: 517; 554; 596; 652 | 656 [4.5]; 721 [4.5] | 0.13 |
| 4 | S: 427; Q: 558; 595 | 607 [1.3]; 657 [1.3 and 8.7] | 0.05 |
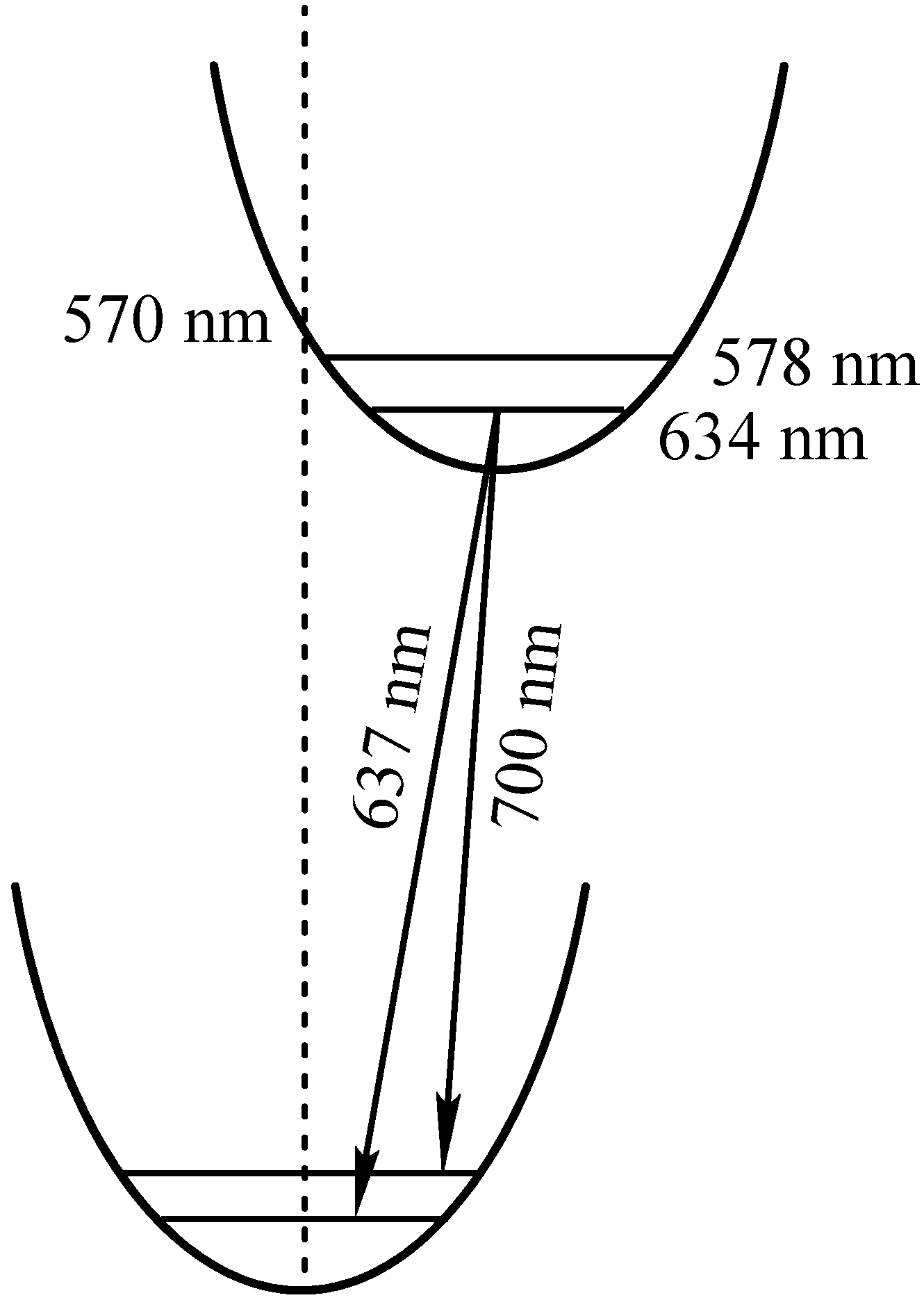
| 2 | S: 409; Q: 503; 538; 578; 634 | 637 [5.2]; 700 [5.2] | 0.06 |
| 5 | S: 414; Q: 545; 580(w) | 587 [2.4]; 638 [2.4] | 0.03 |
| 3 | S: 423; Q: 517; 554; 593; 650 | 655 [5.0]; 720 [5.1] | 0.13 |
| 6 | S: 428; Q: 559; 602 | 622 [1.40] | 0.07 |

| Compound | № | λ | E | F | Assignment |
|---|---|---|---|---|---|
| 2 | 1 | 569.9 | 2.18 | 0.0206 | HOMO→LUMO+1(+45%) HOMO-1→LUMO(+30%) |
| 2 | 535.5 | 2.32 | 0.0525 | HOMO→L+0(+44%) HOMO-1→LUMO+1(27%) | |
| 3 | 391.6 | 3.17 | 1.1557 | HOMO-1→LUMO+1(+24%) HOMO-3→LUMO(18%) | |
| 4 | 384.0 | 3.23 | 0.5538 | HOMO-1→LUMO(+21%) HOMO-3→LUMO(+12%) | |
| 5 | 381.3 | 3.25 | 0.0513 | HOMO-2→LUMO(+85%) | |
| 5 | 1 | 529.5 | 2.34 | 0.0001 | HOMO→LUMO(+56%) HOMO-1→LUMO+1(47%) |
| 2 | 528.4 | 2.35 | 0.0164 | HOMO→LUMO+1(+57%) HOMO-1→LUMO(+45%) | |
| 3 | 386.5 | 3.21 | 1.2378 | HOMO-3→LUMO+1(+38%) HOMO-1→LUMO(+25%) | |
| 4 | 379.7 | 3.27 | 0.3924 | HOMO-3→LUMO(31%) HOMO-1→LUMO+1(+24%) | |
| 5 | 376.1 | 3.30 | 0.0201 | HOMO-2→LUMO+1(+92%) | |
| 6 | 371.9 | 3.33 | 0.0003 | HOMO-2→LUMO(+98%) | |
| 7 | 358.8 | 3.46 | 0.0000 | HOMO-4→LUMO(+95%) | |
| 8 | 357.4 | 3.47 | 0.0000 | HOMO-4→LUMO+1(+94%) | |
| 9 | 352.0 | 3.52 | 0.5981 | HOMO-3→LUMO+1(+57%) HOMO-1→LUMO(13%) | |
| 10 | 350.0 | 3.54 | 0.3016 | HOMO-3→LUMO(+61%) HOMO-1→LUMO+1(+9%) | |
| 3 | 1 | 590.3 | 2.10 | 0.0457 | HOMO→LUMO(+43%) HOMO→LUMO+1(+25%) |
| 2 | 554.4 | 2.24 | 0.0689 | HOMO→LUMO+1(+36%) HOMO→LUMO(26%) | |
| 3 | 492.5 | 2.52 | 0.0061 | HOMO→LUMO+2(+96%) | |
| 4 | 481.1 | 2.58 | 0.0961 | HOMO→LUMO+3(+83%) HOMO-1→LUMO+1(+9%) | |
| 5 | 441.1 | 2.81 | 0.1676 | HOMO-1→LUMO+3(+44%) HOMO-1→LUMO(+24%) | |
| 6 | 438.3 | 2.83 | 0.0366 | HOMO-1→LUMO+2(+80%) HOMO-1→LUMO+3(+12%) | |
| 7 | 409.5 | 3.03 | 0.0004 | HOMO-2→LUMO(+97%) | |
| 8 | 405.8 | 3.06 | 0.1798 | HOMO-3→LUMO(+72%) HOMO-1→LUMO+1(10%) | |
| 9 | 403.0 | 3.08 | 0.5780 | HOMO-3→LUMO+1(+44%) HOMO-1→LUMO+3(22%) | |
| 10 | 400.2 | 3.10 | 0.0256 | HOMO-2→LUMO+1(+92%) | |
| 6 | 1 | 552.3 | 2.24 | 0.0469 | HOMO→LUMO(+65%) HOMO-1→LUMO+1(36%) |
| 2 | 544.0 | 2.28 | 0.0100 | HOMO→LUMO+1(+54%) HOMO-1→LUMO(+46%) | |
| 3 | 476.7 | 2.60 | 0.0095 | HOMO→LUMO+2(+95%) | |
| 4 | 468.1 | 2.65 | 0.1128 | HOMO→LUMO+3(+77%) HOMO→LUMO(9%) | |
| 5 | 447.4 | 2.77 | 0.0164 | HOMO-1→LUMO+2(+85%) HOMO-1→LUMO+3(9%) | |
| 6 | 444.6 | 2.79 | 0.0870 | HOMO-1→LUMO+3(+58%) HOMO-1→LUMO(18%) | |
| 7 | 403.3 | 3.07 | 0.0039 | HOMO-2→LUMO(+96%) | |
| 1 | 1 | 581.4 | 2.13 | 0.0245 | HOMO→LUMO+1(+61%) HOMO-1→LUMO(30%) |
| 2 | 544.3 | 2.28 | 0.0440 | HOMO→LUMO(+59%) HOMO-1→LUMO+1(+32%) | |
| 3 | 399.7 | 3.10 | 0.9878 | HOMO-1→LUMO(+48%) HOMO→LUMO+1(+22%) | |
| 4 | 390.6 | 3.17 | 1.1135 | HOMO-1→LUMO+1(+43%) HOMO→LUMO(23%) | |
| 5 | 385.3 | 3.22 | 0.0247 | HOMO-2→LUMO+1(+55%) HOMO-2→LUMO(39%) | |
| 6 | 382.8 | 3.24 | 0.0034 | HOMO-2→LUMO(+51%) HOMO-2→LUMO+1(+34%) | |
| 7 | 378.8 | 3.27 | 0.0145 | HOMO-3→LUMO+1(+69%) HOMO-6→LUMO+1(8%) | |
| 4 | 1 | 540.7 | 2.29 | 0.0162 | HOMO→LUMO(+57%) HOMO-1→LUMO+1(+38%) |
| 2 | 540.5 | 2.29 | 0.0157 | HOMO→LUMO+1(+57%) HOMO-1→LUMO(39%) | |
| 3 | 391.9 | 3.16 | 1.1281 | HOMO-1→LUMO+1(+34%) HOMO→LUMO(23%) | |
| 4 | 390.7 | 3.17 | 1.1551 | HOMO-1→LUMO(+35%) HOMO→LUMO+1(+25%) | |
| 5 | 380.1 | 3.26 | 0.0156 | HOMO-2→LUMO(+52%) HOMO-2→LUMO+1(43%) | |
| 6 | 377.4 | 3.28 | 0.0002 | HOMO-2→LUMO+1(+44%) HOMO-2→LUMO(+36%) | |
| 7 | 372.2 | 3.33 | 0.0140 | HOMO-3→LUMO(+87%) HOMO-3→LUMO+1(+6%) |

3. Methods
3.1. Quantum Chemical Calculations
3.2. Optical and Luminescence Spectroscopy
3.3. FTIR Spectroscopy
3.4. X-Ray Diffraction
3.5. NMR Characterization
3.6. Synthesis of Compounds
3.6.1. Synthesis of 1: 5,10,15,20-tetra(4-hexyloxyphenyl) porphyrin
3.6.2. General procedure for the preparation of trans-A2B2 meso-tetraarylporphyrins
3.6.3. Synthesis of 2: 5,15-bis(4-hexyloxyphenyl) porphyrin
3.6.4. Synthesis of 3: 5,15-bis-(4-nitrophenyl)-10,20-bis(4-hexyloxyphenyl) porphyrin
3.6.5. Preparation of Zn-porphyrins: method A
3.6.6. Preparation of Zn-porphyrins: method B
3.6.7. Synthesis of 4: [5,10,15,20-tetra(4-hexyloxyphenyl) porphyrinato] zinc(II)
3.6.8. Synthesis of 5: [5,15-bis(4-hexyloxyphenyl) porphyrinato] zinc(II)
3.6.9. Synthesis of 6: [5,15-bis-(4-nitrophenyl)-10,20-bis(4-hexyloxyphenyl) porphyrinato] zinc(II)
4. Conclusions
Acknowledgements
Supporting information
References and Notes
- The Porphyrin Handbook; Kadish, K.M.; Smith, K.M.; Guilard, R. (Eds.) Academic Press: New York, NY, USA, 1999.
- Minaev, B.F.; Minaeva, V.A. Spin-dependent binding of dioxygen to heme. Ukr. Bioorg. Acta 2008, 2, 56–64. [Google Scholar]
- Rogers, J.; Nguyen, K.; Hufnagle, D.C.; McLean, D.G.; Su, W.; Gossett, K.M.; Burke, A.R.; Vinogradov, S.A.; Pachter, R.; Fleitz, P.A. Observation and interpretation of annulated porphyrins: studies on the photophysical properties of meso-tetraphenylmetalloporphyrins. J. Phys. Chem. A. 2003, 107, 11331–11339. [Google Scholar] [CrossRef]
- Krasnovskii, A.A.; Bashtanov, M.E.; Drozdova, N.N.; Yuzhakova, O.A.; Luk'yanets, E.A. Laser induced singlet-oxygen-sensitised delayed fluorescence of dyes in aqueous solutions. Quantum Electron. 2002, 32, 83–86. [Google Scholar] [CrossRef]
- Henderson, B.W.; Dougherty, T.J. How does photodynamic therapy work? Photochem. Photobiol. 1992, 55, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Eguchi, M.; Tryk, D.A.; Inoue, H. Porphyrin photochemistry in inorganic/organic hybrid materials: Clays, layered semiconductors, nanotubes, and mesoporous materials. Photochem. Rev. 2006, 7, 104–126. [Google Scholar] [CrossRef]
- Calvete, M.; Yang, G.Y.; Hanack, M. Porphyrins and phthalocyanines as materials for optical limiting. Synth. Met. 2004, 141, 231–243. [Google Scholar] [CrossRef]
- Zhao, Z.; Poon, C.-T.; Wong, W.-K.; Wong, W.-Y.; Tam, H.-L.; Cheah, K.-W.; Xie, T.; Wang, D. Synthesis, photophysical characterization, and surface photovoltage spectra of windmill-shaped phthalocyanine–porphyrin heterodimers and heteropentamers. Eur. J. Inorg. Chem. 2008, 119–128. [Google Scholar]
- Li, L.; Kang, S.W.; Hardena, J.; Sunc, Q.; Zhoua, X.; Daic, L.; Jaklia, A.; Kumarb, S.; Li, Q. Nature-inspired light-harvesting liquid crystalline porphyrins for organic photovoltaics. Liq. Cryst. 2008, 35, 233–239. [Google Scholar] [CrossRef]
- Mitra, S.; Foster, T.H. Photochemical oxygen consumption sensitized by a porphyrin phosphorescent probe in two model systems. Biophys. J. 2000, 78, 2597–2605. [Google Scholar] [CrossRef] [PubMed]
- Biesaga, M.; Pyrzynska, K.; Trojanowicz, M. Porphyrins in analytical chemistry a review. Talanta 2000, 51, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Vestberg, R.; Nystrom, A.; Lindgren, M.; Malmstrom, E.; Hult, A. Porphyrin-cored 2,2-bis(methylol)propionic acid dendrimers. Chem. Mater. 2004, 16, 2794–2804. [Google Scholar] [CrossRef]
- Minaev, B.; Lindgren, M. Vibration and fluorescence spectra of porphyrin-cored 2,2-bis(methylol)- propionic acid dendrimers. Sensors 2009, 9, 1937–1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chukharev, V.; Vuorinen, T.; Efimov, A.; Tkachenko, N.V.; Kimura, M.; Fukuzumi, S.; Imahori, H.; Lemmetyinen, H. Photoinduced electron transfer in self-assembled monolayers of porphyrin-fullerene dyads on ITO. Langmuir 2005, 21, 6385–6391. [Google Scholar] [CrossRef] [PubMed]
- Yasseri, A.A.; Syomin, D.; Malinovskii, V.L.; Loewe, S.L.; Lindsey, J.S.; Zaera, F.; Bocian, D.F. Characterization of self-assembled monolayers of porphyrins bearing multiple thiol-derivatized rigid-rod tethers. J. Am. Chem. Soc. 2004, 126, 11944–11953. [Google Scholar] [CrossRef]
- Minaev, B.; Agren, H. Theoretical DFT study of phosphorescence from porphyrins. Chem. Phys. 2005, 315, 215–239. [Google Scholar] [CrossRef]
- Mohammed, A.; Minaev, B.; Agren, H.; Lindgren, M.; Norman, P. Classification of raman active modes of Platinum (II) acetylides: A combined experimental and theoretical study. Chem. Phys. Lett. 2009, 481, 209–213. [Google Scholar] [CrossRef]
- Jarzecki, A.; Kozlowski, P.; Pulay, P.; Ye, B.; Li, X. Scaled quantum mechanical and experimental vibrational spectra of Zn and Mg porphyrins. Spectrochim. Acta A. 1997, 53, 1195–1209. [Google Scholar] [CrossRef]
- Frish, M.J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M.A.; Cheeseman, J. R.; Montgomery, J. A., Jr.; Vreven, T.; Kudin, K. N.; Burant, J. C.; et al. Gaussian 03, Gaussian Inc. Pittsburg PA, USA, 2003. Gaussian 03: EM64L-G03RevE.01 11-Sep-2007; 14-Mar-2010. Availalble online: http://www.gaussian.com/.
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Litter, B.J.; Ciringh, Y.Z.; Lindsey, J.S. Investigation of Conditions Giving Minimal Scrambling in the Synthesis of trans-porphyrins from dipyrromethanes and aldehydes. J. Org. Chem. 1999, 64, 2864–2872. [Google Scholar] [CrossRef] [PubMed]
- Littler, B.J.; Miller, M.A.; Hung, C.-H.; Wagner, R.W.; O’Shea, D.F.; Boyle, P.D.; Lindsey, J.S. Refined synthesis of 5-substituted dipyrromethanes. J. Org. Chem. 1999, 64, 1391–1396. [Google Scholar] [CrossRef]
- Sobral, A.J.F.N.; Rebanda, N.G.C.L.; da Silva, M.; Lampreia, S.H.; Ramos Silva, M.; Matos Beja, A.; Paixao, J.A; Rocha Gonsalves, A.M.D. One-step synthesis of dipyrromethanes in water. Tetrahedron Lett. 2003, 44, 3971–3973. [Google Scholar] [CrossRef]
- Mamardashvili, N.Z.; Golubchikov, O.A. Spectral Properties of porphyrins and their precursors and derivatives. Russ. Chem. Rev. 2001, 70, 577–606. [Google Scholar] [CrossRef]
- Raymond, J.; Abraham, G.; Hawkes, E.; Smith, K.M. The nuclear magnetic resonance spectra of porphyrins. Part VIII. The 13C nuclear magnetic resonance spectra of some porphyrins and metalloporphyrins. J. Chem. Soc. Perkin Trans. 1974, 2, 627–634. [Google Scholar]
- Mrinalini, G.; Walawalkar, M.; Krisjnan, V. Structure of a Non-Mesogen Porphyrin, 5,10,15,20-Tetrakis[4-(1-hexyloxy)phenyl]porphyrin. Acta Cryst. 1993, C49, 479–481. [Google Scholar]
- Adilov, S.; Venkat, R.T. Layered Porphyrin Coordination Polymers Based on Zinc-Nitro Recognition: Reversible Intercalation of Nitrobenzene. Cryst. Growth Des. 2007, 7, 481–484. [Google Scholar] [CrossRef]
- Robert, W.; Jalal, S; Mondal, U.; Eigenbrot, C.W.; Adler, A.; Radonvich, L.J.; Hoard, J.L. Crystal and molecular structure of the silver(i1) and zinc(i1) derivatives of meso-tetraphenylporphyrin. an exploration of crystal-packing effects on bond distance. Inorg. Chem. 1986, 25, 795–799. [Google Scholar] [CrossRef]
- Kappa CCD Program Package: COLLECT, DENZO, SCALEPACK, SORTAV; Nonius, B.V.; Delft, The Netherlands, 1999
- Cascarano, G.; Altomare, A.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Siliqi, D.; Burla, M.C.; Polidori, G.; Camalli, M. X-Ray programs: SIR97. Acta Crystallogr. 1996, A52, C79. [Google Scholar]
- Watkin, D.J.; Prout, C.K.; Carruthers, J.R.; Betteridge, P.W. CRISTAL Issue 11; Chemical Crystallography Laboratory: Oxford, UK, 1999. [Google Scholar]
- Minaev, B.; Minaeva, V.; Baryshnikov, G.; Mohammed, A.; Agren, H.; Lindgren, M.; Lee, H.S. DFT study of infrared spectra of non-symmetrically substituted porphins. Visnik. Cherkas’kogo University Series Chem. Sci. 2010, 74, 3–14. [Google Scholar]
- Zhang, Y.H.; Zhao, W.; Jiang, P.; Zhang, L.J.; Zhang, T.; Wang, J. Structural parameters and vibrational spectra of a series of zinc meso-phenylporphyrins: a DFT and experimental study. Spectrochim. Acta A. 2010, 75, 880–887. [Google Scholar] [CrossRef]
- Solovyov, K.N.; Gladkov, L.L.; Gradyushko, A.T.; Ksenofontova, N.M.; Shulga, A.M.; Starukhin, A.S. Resonance Raman Spectra of deuterated metalloporphins. J. Mol. Struct. 1978, 45, 267–305. [Google Scholar] [CrossRef]
- Knyukshto, V.N.; Shulga, A.M.; Sagun, E.I.; Zenkevich, E.I. Spectral manifestations of the splitting of the lowest triplet levels in Pd complexes of nonsymmetric porphyrins. Opt. Spectrosc. 2006, 101, 895–901. [Google Scholar] [CrossRef]
- Solovyov, K.N; Gladkov, L.L.; Starukhin, A.S.; Shkirman, S.F. Spectroscopy of Porphyrins: Vibrational States; Science and Technics: Minsk, The Republic of Byelorussia, 1985; pp. 124–260. [Google Scholar]
- Gladkov, L.; Gradyushko, A.; Solovyov, K.; Shulga, A.; Starukhin, A. Experimental and theoretical investigation of IR spectra of porphin, its deuterated derivatives and their metal complexes. J. Mol. Struc-Theochem 1978, 45, 463–493. [Google Scholar] [CrossRef]
- Bykovskaya, L.; Gradyushko, A.; Personov, R.; Romanovskij, Y.B.; Solovyov, K.; Shulga, A.; Starukhin, A. Method for determining of the polarization of vibronic transitions of polyatomic molecules in isotropic media upon selective laser excitation (in Russian). Izv. AN SSSR, Ser. Fiz. 1980, 44, 822–826. [Google Scholar]
- Li, X.Y.; Zgierski, M. Porphine force field: in-plane normal modes of free-base porphine. Comparison with metalloporphines and structural implications. J. Phys. Chem. 1991, 95, 4268–4287. [Google Scholar] [CrossRef]
- Glimsdal, E.; Carlsson, M.; Eliasson, B.; Minaev, B.; Lindgren, M. Excited states and two-photon absorption of some novel thiophenyl Pt(II)-ethynyl derivatives. J. Phys. Chem. A 2007, 111, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Minaev, B.; Wang, Y.H.; Wang, C.K.; Luo, Y.; Agren, H. DFT study of vibronic structure of the first absorption Q(x) band in free-base porphin. Spectrochim. Acta A 2006, 65, 308–323. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infra-Red Spectra of Complexes Molecules; Methuen & Co. Ltd: London, UK, 1954; p. 323. [Google Scholar]
- Silverstein, R.M.; Bassler, G.C.; Morrill, T.C. Spectrometric Identification of Organic Compounds; John Wiley and Sons Inc.: New York, NY, USA, 1999; p. 419. [Google Scholar]
- Oakes, R.E.; Bell, S.E. DFT studies of the resonance raman spectra of ground and excited triplet state free base meso-tetraphenylporphyrin (H2TPP). J. Phys. Chem. A 2003, 107, 10953–10959. [Google Scholar]
- Kozlovski, P.M.; Jarzecki, A.A.; Pulay, P.; Li, X.-Y.; Zgierski, M. Vibrational assignment and definite harmonic force field for porphine. J. Phys. Chem. 1996, 100, 13985–13992. [Google Scholar] [CrossRef]
- Gouterman, M. The porphyrins. Vol III; Dolphin, D., Ed.; Acad. Press: New York, NY, USA, 1978. [Google Scholar]
- Sundholm, D. Interpretation of the electronic absorption spectrum of free-base porphin using time-dependent density-functional theory. Phys. Chem. Chem. Phys. 2000, 2, 2275–2281. [Google Scholar] [CrossRef]
- Santoro, F.; Lami, A.; Improda, R.; bloino, J.; Barone, V. Effective method for the computation of optical spectra of large molecules at finite temperature including the Duschinsky and Herzberg-Teller effect: The Qx band of porphyrin as a case study. J. Chem. Phys. 2008, 128, 224311. [Google Scholar] [CrossRef] [PubMed]
- Boens, N.; Qin, W.; Basarić, N.; Hofkens, J.; Ameloot, M.; Pouget, J.; Lefиvre, J.-P.; Valeur, B.; Gratton, E.; vandeVen, M.; Silva, N.D.J.; Engelborghs, Y.; Willaert, K.; Sillen, A.; Visser, A.J.W.G.; van Hoek, A.; Lakowicz, J.R.; Malak, H.; Gryczynski, I.; Szabo, A.G.; Krajcarski, D.T.; Tamai, N.; Miura, A. Fluorescence lifetime standards for time and frequency domain fluorescence spectroscopy. Anal. Chem. 2007, 79, 2137–2149. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Şen, P.; Hirel, C.; Andraud, C.; Aronica, C.; Bretonnière, Y.; Mohammed, A.; Ågren, H.; Minaev, B.; Minaeva, V.; Baryshnikov, G.; et al. Fluorescence and FTIR Spectra Analysis of Trans-A2B2-Substituted Di- and Tetra-Phenyl Porphyrins. Materials 2010, 3, 4446-4475. https://doi.org/10.3390/ma3084446
Şen P, Hirel C, Andraud C, Aronica C, Bretonnière Y, Mohammed A, Ågren H, Minaev B, Minaeva V, Baryshnikov G, et al. Fluorescence and FTIR Spectra Analysis of Trans-A2B2-Substituted Di- and Tetra-Phenyl Porphyrins. Materials. 2010; 3(8):4446-4475. https://doi.org/10.3390/ma3084446
Chicago/Turabian StyleŞen, Pınar, Catherine Hirel, Chantal Andraud, Christophe Aronica, Yann Bretonnière, Abdelsalam Mohammed, Hans Ågren, Boris Minaev, Valentina Minaeva, Gleb Baryshnikov, and et al. 2010. "Fluorescence and FTIR Spectra Analysis of Trans-A2B2-Substituted Di- and Tetra-Phenyl Porphyrins" Materials 3, no. 8: 4446-4475. https://doi.org/10.3390/ma3084446




