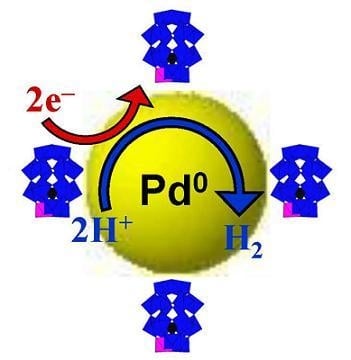
Pd0@Polyoxometalate Nanostructures as Green Electrocatalysts: Illustrative Example of Hydrogen Production
Abstract
:1. Introduction
2. Experimental
2.1. Electrochemistry Equipment, Apparatus and Procedures
2.2. Mother Solution Work-up
2.3. Electrode Preparation
3. Results and Discussion
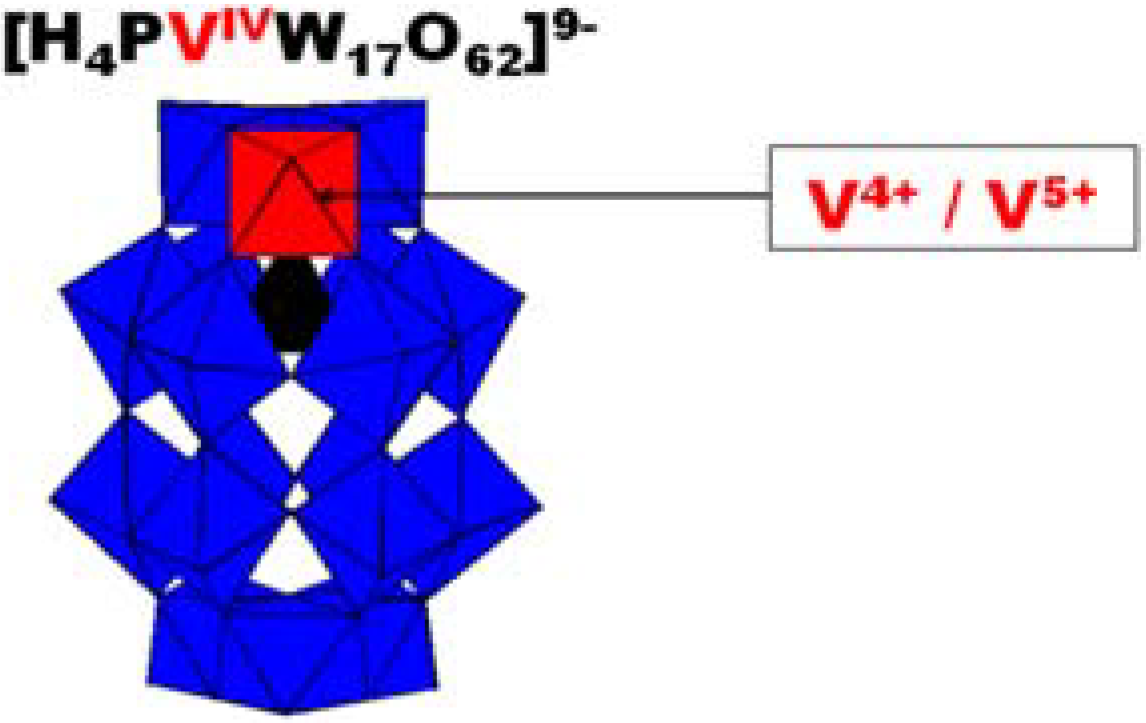
3.1. Synthesis of HPVIV
3.2. Pd0 @ POM Nanostructure Synthesis
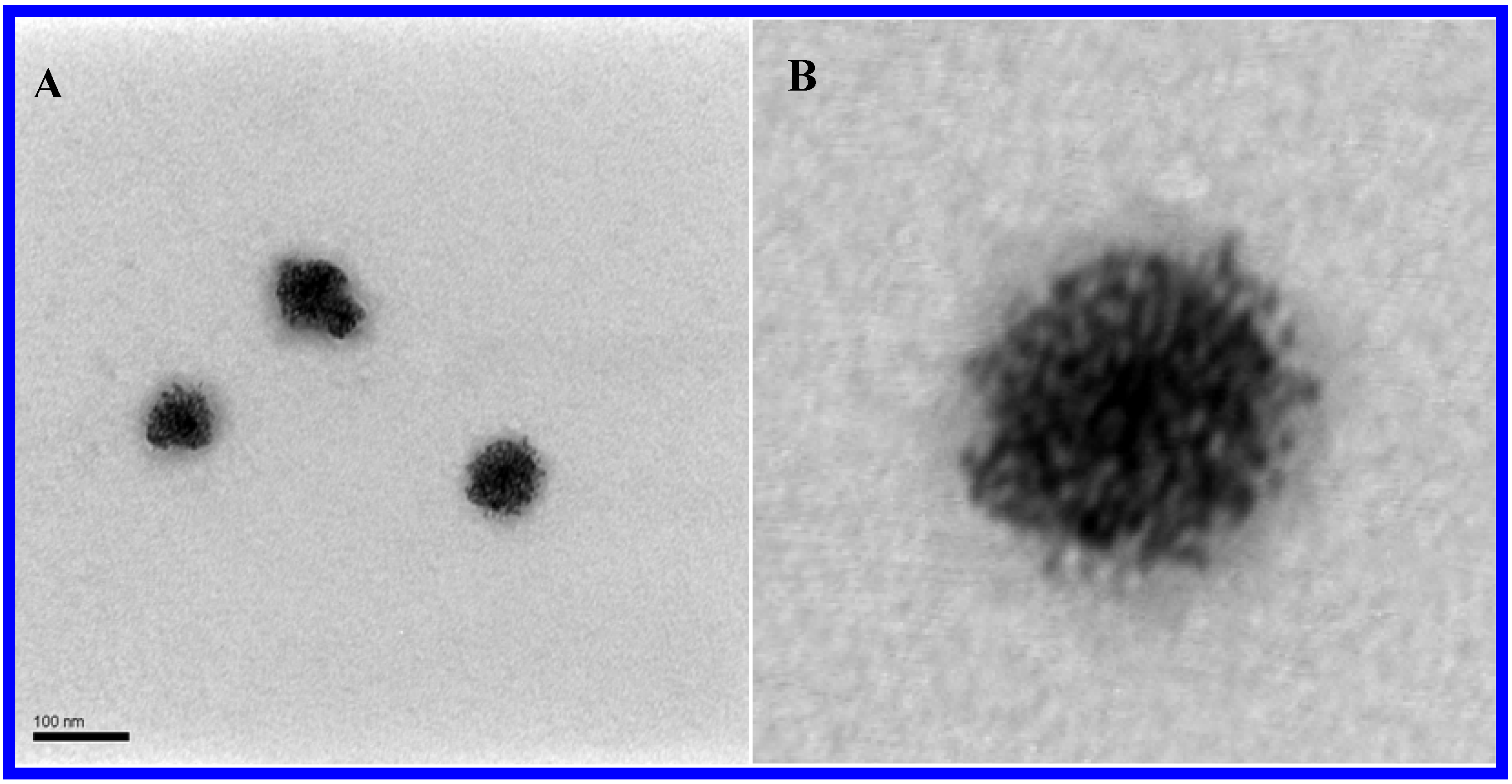
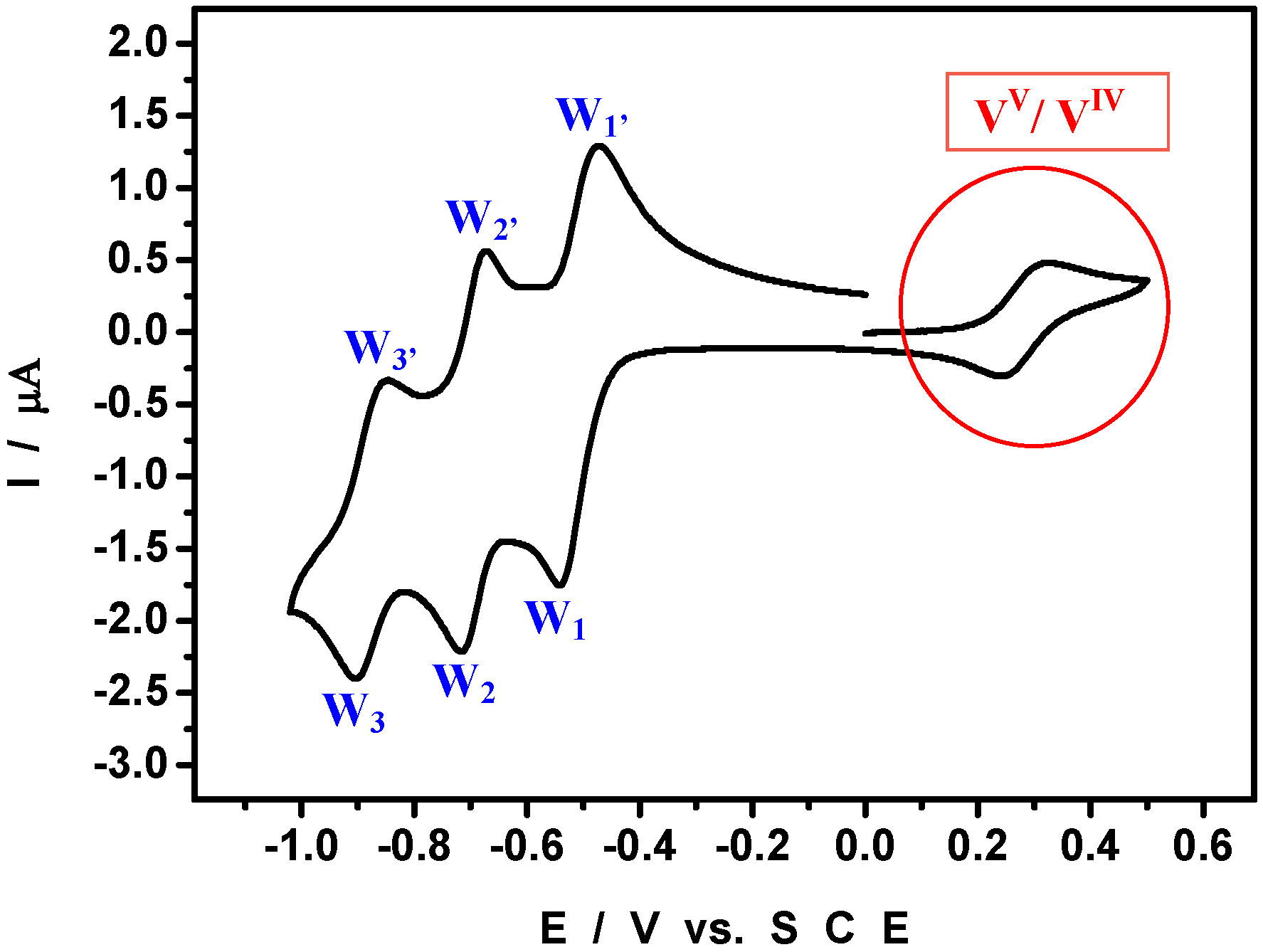
3.3. Electrochemistry
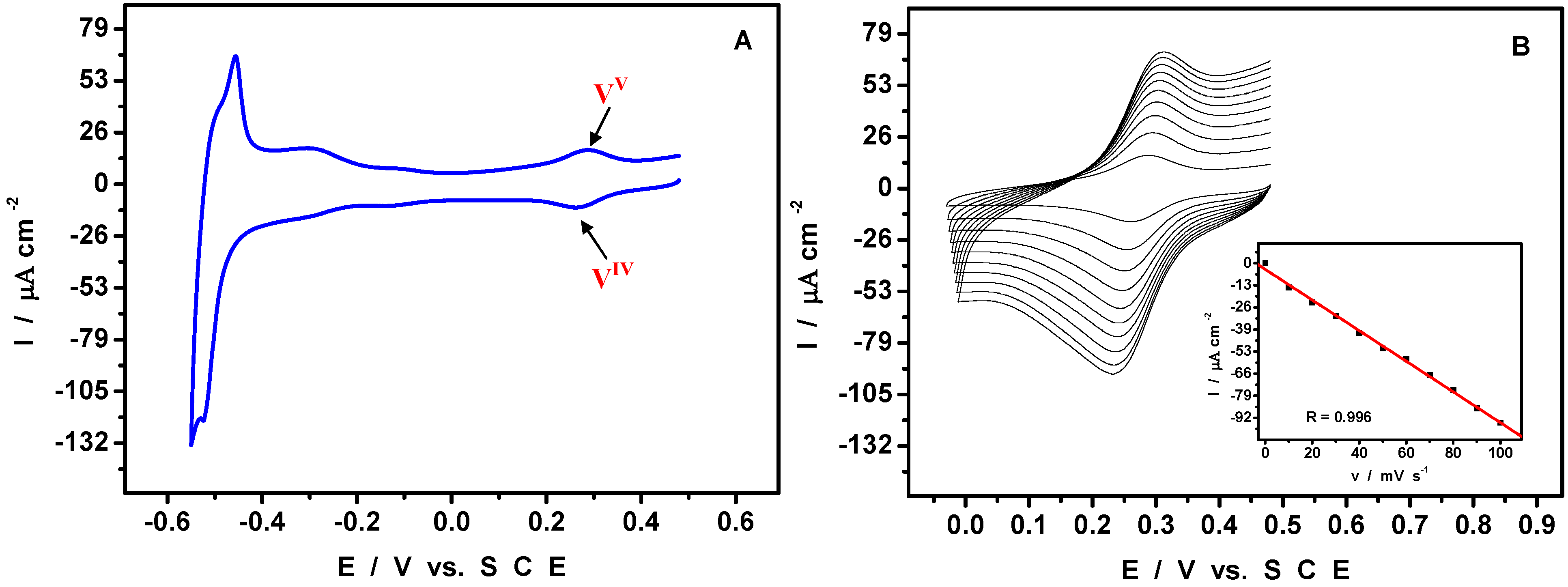
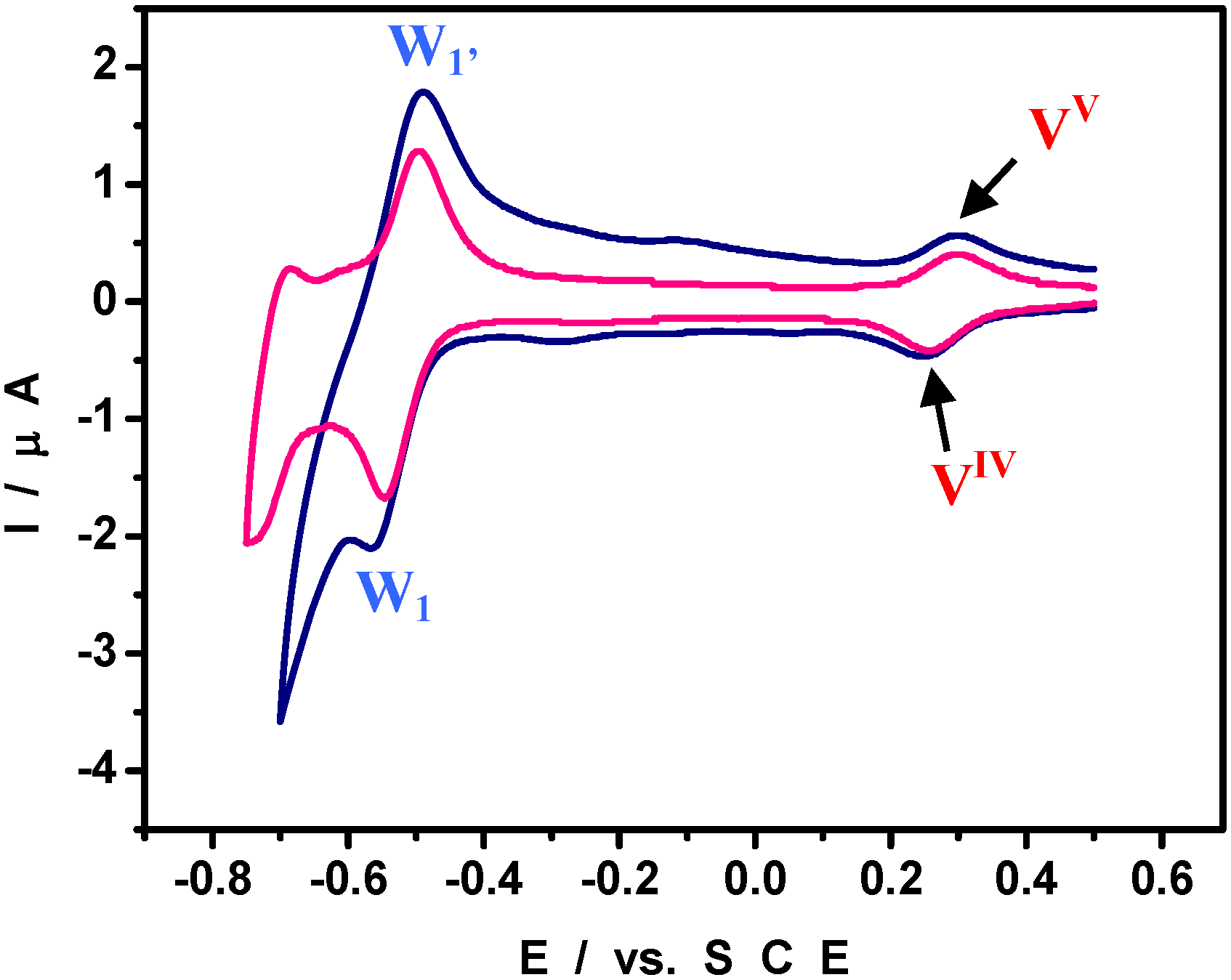
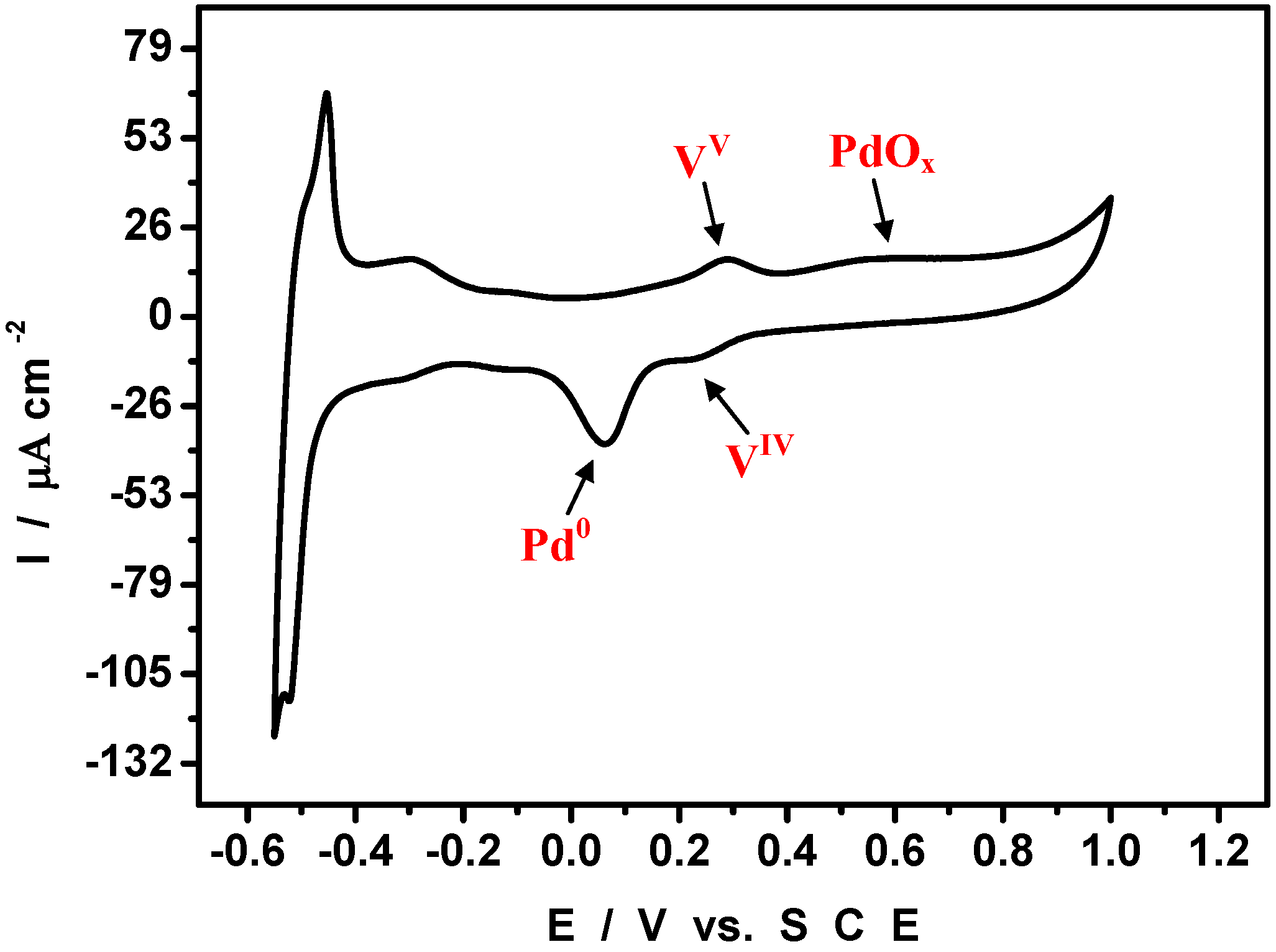
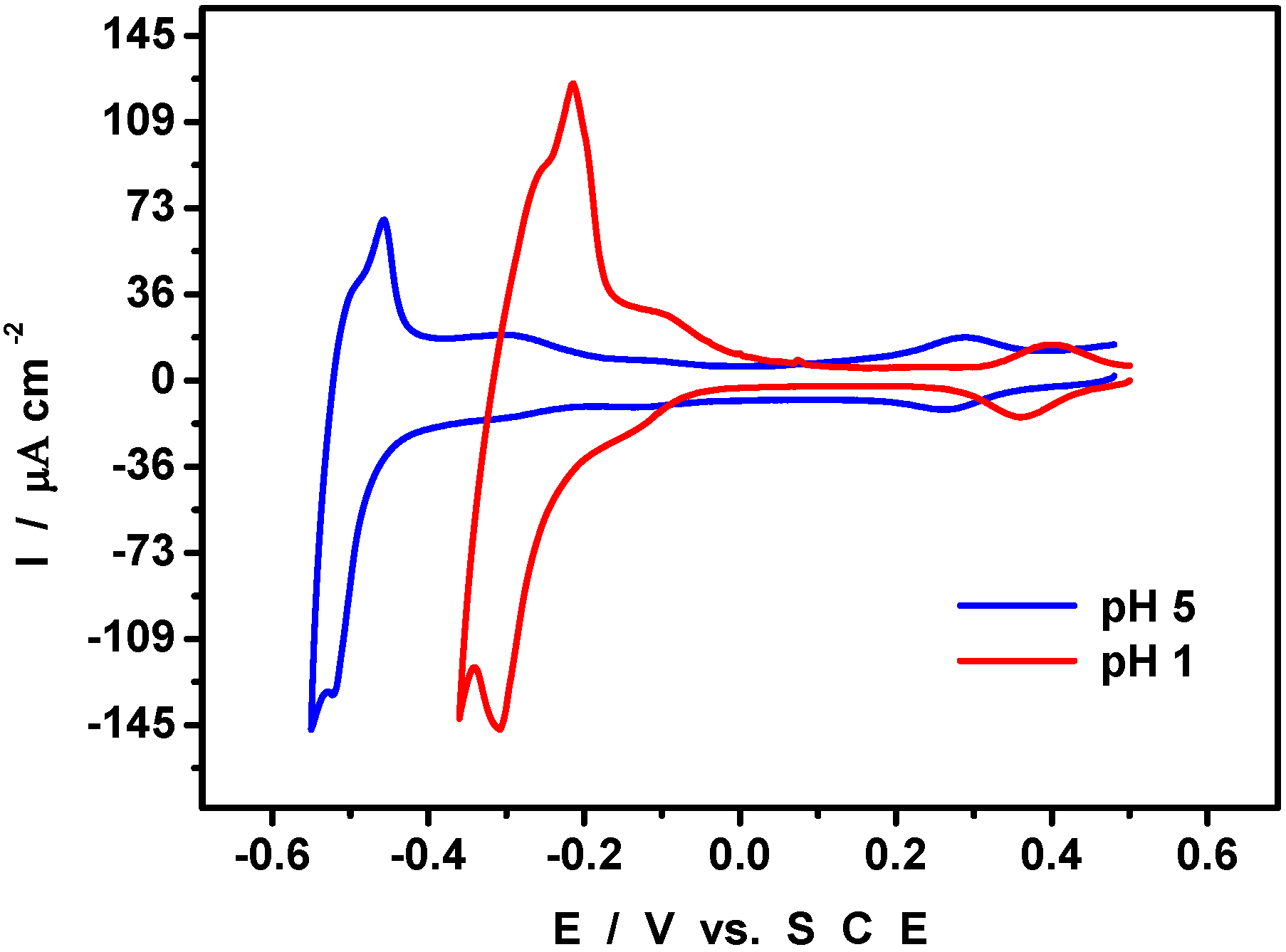
4. An Example of Electrocatalytic Behaviour based on Pd0@POM Nanostructures: Hydrogen Evolution Reaction
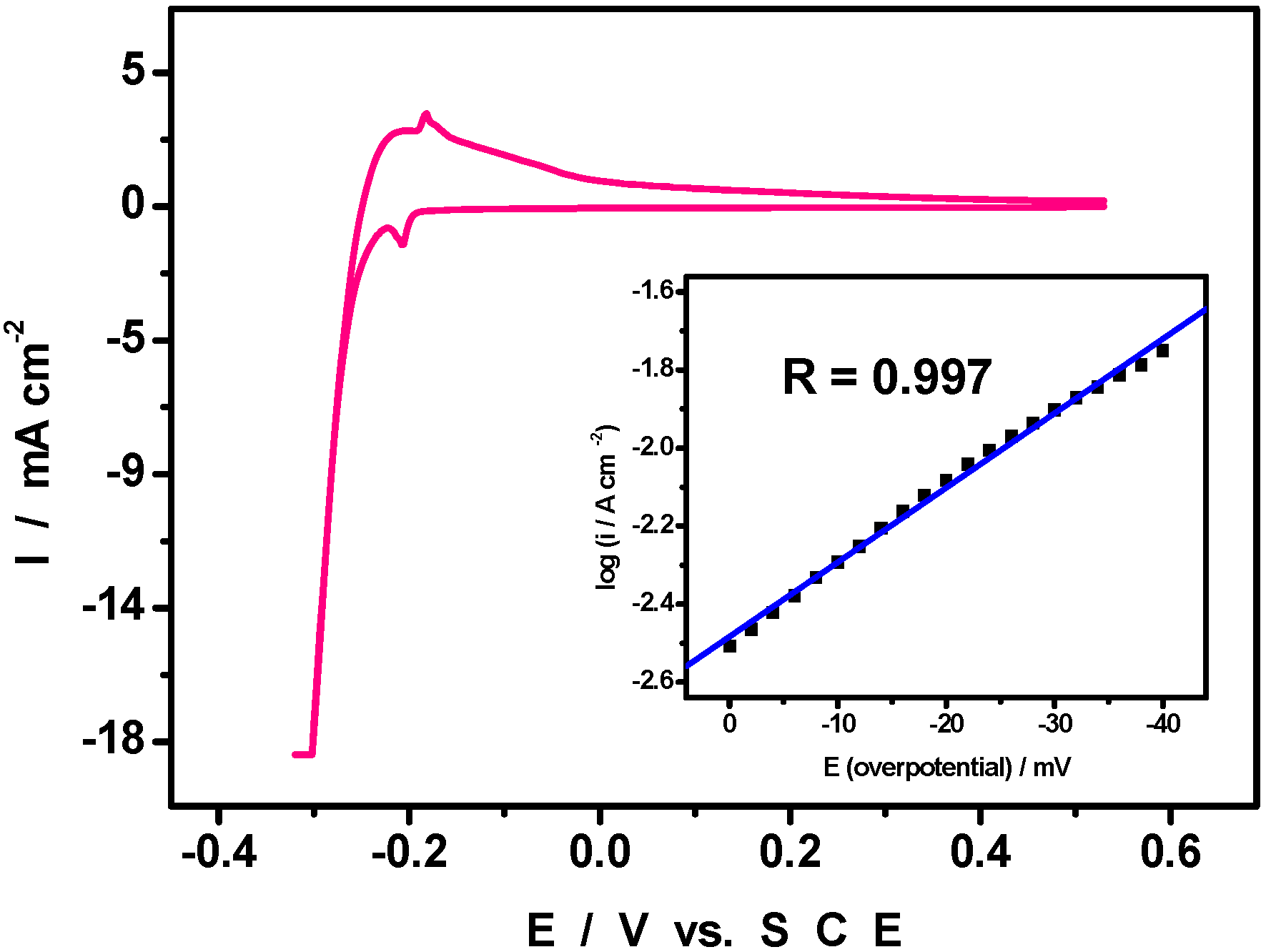
5. Conclusions
Acknowledgements
References
- Pope, M.T. Heteropoly and Isopoly Oxometalates; Springer-Verlag: New York, NY, USA, 1983. [Google Scholar]
- Polyoxometalates. Chem. Rev. 1998, 98, 1–390. [CrossRef] [PubMed]
- Hill, C.L. Polyoxometalates: Reactivity. In Comprehensive Coordination Chemistry II: Transition Metal Groups 3–6; Wedd, A.G., Ed.; Elsevier Science: New York, NY, USA, 2004; Volume 4, Chapter 4.11; pp. 679–759. [Google Scholar]
- Keita, B.; Nadjo, L. Electrochemistry of Polyoxometalates, Encyclopedia of Electrochemistry; Bard, A.J., Stratmann, M., Eds.; Wiley-VCH: Weinheim, Germany, 2006; Volume 7, pp. 607–700. [Google Scholar]
- Keita, B.; Nadjo, L. Polyoxometalate-based homogeneous catalysis of electrode reactions: Recent achievements. J. Mol. Catal. A: Chem. 2007, 262, 190–215. [Google Scholar] [CrossRef]
- Troupis, A.; Gkika, E.; Hiskia, A.; Papaconstantinou, E. Photocatalytic reduction of metals using polyoxometalates: recovery of metals or synthesis of metal nanoparticles. CR Chim. 2006, 9, 851–857. [Google Scholar] [CrossRef]
- Papaconstantinou, E. Relative electron-donating ability of simple alcohol radicals toward 12-heteropolytungstates: a pulse radiolysis study. J. Chem. Soc. Faraday Trans. 1982, 78, 2769–2772. [Google Scholar] [CrossRef]
- Keita, B.; Liu, T.; Nadjo, L. Synthesis of remarkably stabilized metal nanostructures using polyoxometalates. J. Mater. Chem. 2009, 19, 19–33. [Google Scholar] [CrossRef]
- Keita, B.; Mbomekalle, I.M.; Nadjo, L.; Haut, C. Tuning the formal potentials of new VIV-substituted Dawson-type polyoxometalates for facile synthesis of metal nanoparticles. Electrochem. Commun. 2004, 6, 978–983. [Google Scholar] [CrossRef]
- Keita, B.; Zhang, G.; Dolbecq, A.; Mialane, P.; Secheresse, F.; Miserque, F.; Nadjo, L. MoV-MoVI Mixed valence polyoxometalates for facile synthesis of stabilized metal nanoparticles: Electrocatalytic oxidation of alcohols. J. Phys. Chem. C. 2007, 111, 8145–8148. [Google Scholar] [CrossRef]
- Zhang, G.; Keita, B.; Dolbecq, A.; Mialane, P.; Secheresse, F.; Miserque, F.; Nadjo, L. Green chemistry-type one-step synthesis of silver nanostructures based on MoV-MoVI mixed-valence polyoxometalates. Chem. Mater. 2007, 19, 5821–5823. [Google Scholar] [CrossRef]
- Keita, B.; Biboum, R.N.; Mbomekalle, I.M.; Floquet, S.; Simonnet-Jegat, C.; Cadot, E.; Miserque, F.; Berthet, P.; Nadjo, L. One-step synthesis and stabilization of gold nanoparticles in water with the simple oxothiometalate Na2[Mo3(μ3-S)(μ -S)3(Hnta)3]. J. Mater. Chem. 2008, 18, 3196–3199. [Google Scholar] [CrossRef]
- Zhang, J.; Keita, B.; Nadjo, L.; Mbomekalle, I.M.; Liu, T. Self-Assembly of Polyoxometalate Macroanion-Capped Pd Nanoparticles in Aqueous Solution. Langmuir 2008, 24, 5277–5283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Keita, B.; Biboum, R.N.; Miserque, F.; Berthet, P.; Dolbecq, A.; Mialane, P.; Catala, L.; Nadjo, L. Synthesis of various crystalline gold nanostructures in water: The polyoxometalate β-[H4PMo12O40]3- as the reducing and stabilizing agent. J. Mater. Chem. 2009, 19, 8639–8644. [Google Scholar] [CrossRef]
- Maksimov, G.M.; Zaikovskii, V.I.; Matveev, K.I.; Likholobov, V.A. Preparation of polyoxometalate-stabilized colloidal solutions of palladium metal and catalysts supported on them. Kinet. Catal. 2000, 41, 844–852. [Google Scholar] [CrossRef]
- Maksimova, G.M.; Chuvilin, A.L.; Moroz, E.M.; Likholobov, V.A.; Matveev, K.I. Preparation of colloidal solutions of noble metals stabilized by polyoxometalates and supported catalysts based on these solutions. Kinet. Catal. 2004, 45, 870–878. [Google Scholar] [CrossRef]
- Kulesza, P.J.; Chojak, M.; Karnicka, K.; Miecznikowski, K.; Palys, B.; Lewera, A.; Wieckowski, A. Network films composed of conducting polymer-linked and polyoxometalate-stabilized platinum nanoparticles. Chem. Mater. 2004, 16, 4128–4134. [Google Scholar] [CrossRef]
- Garrigue, P.; Delville, M.-H.; Labrugere, C.; Cloutet, E.; Kulesza, P.J.; Morand, J.-P.; Kuhn, A. Top-down approach for the preparation of colloidal carbon nanoparticles. Chem. Mater. 2004, 16, 2984–2986. [Google Scholar] [CrossRef]
- Jiang, S.P.; Ye, Y.; He, T.; Ho, S.B. Nanostructured palladium-La0.75Sr0.25Cr0.5Mn0.5O3/Y2O3-ZrO2 composite anodes for direct methane and ethanol solid oxide fuel cells. J. Power Sources 2008, 185, 179–182. [Google Scholar] [CrossRef]
- Muhamad, E.N.; Takeguchi, T.; Wang, G.; Anzai, Y.; Ueda, W. Electrochemical characteristics of Pd anode catalyst modified with TiO2 nanoparticles in polymer electrolyte fuel cell. Inorg. Chem. 2009, 56, B32–B37. [Google Scholar]
- Chen, J.; Liang, F.; Chi, B.; Pu, J.; Jiang, S.P.; Jian, L. Palladium and ceria infiltrated La0.8Sr0.2Co0.5Fe0.5O3-δ cathodes of solid oxide fuel cells. J. Power Sources 2009, 194, 275–280. [Google Scholar] [CrossRef]
- Ohara, S.; Hatakeyama, Y.; Umetsu, M.; Sato, K.; Naka, T.; Adschiri, T. Palladium-polyelectrolyte hybrid nanoparticles for hydrogen sensor in fuel cells. J. Power Sources 2009, 193, 367–370. [Google Scholar] [CrossRef]
- Bi, L.H.; Kortz, U.; Keita, B.; Nadjo, L.; Daniels, L. The palladium(II)-substituted, lone pair containing tungstoarsenates(III) [Na2(H2O)2PdWO(H2O)(α-AsW9O33)2]10- and [Cs2Na(H2O)8Pd3(α -AsW9O33)2]9-. Eur. J. Inorg. Chem. 2005, 15, 3034–3041. [Google Scholar] [CrossRef]
- Izarova, N.V.; Biboum, R.N.; Keita, B.; Mifsud, M.; Arends, I.W.C.E.; Jameson, G.B.; Kortz, U. Self-assembly of star-shaped heteropoly-15-palladate(II). Dalton Trans. 2009, 43, 9385–9387. [Google Scholar] [CrossRef] [PubMed]
- Chubarova, E.V.; Dickman, M.H.; Keita, B.; Nadjo, L.; Miserque, F.; Mifsud, M.; Arends, I.W.C.E.; Kortz, U. Self-assembly of a heteropolyoxopalladate nanocube: [PdII3AsV8O34(OH)6]8-. Angew. Chem. Int. Ed. 2008, 47, 9542–9546. [Google Scholar] [CrossRef]
- Keita, B.; Nadjo, L. Surface modifications with heteropoly and isopoly oxometalates. 1. qualitative aspects of the activation of electrode surfaces towards the hydrogen evolution reaction. J. Electroanal. Chem. 1988, 243, 87–103. [Google Scholar] [CrossRef]
- Mbomekalle, I.M.; Keita, B.; Lu, Y.W.; Nadjo, L.; Contant, R.; Belai, N.; Pope, M.T. Synthesis, characterization and electrochemistry of the novel Dawson-type tungstophosphate [H4PW18O62]7- and first transition metal ions derivatives. Eur. J. Inorg. Chem. 2004, 2, 276–285. [Google Scholar] [CrossRef]
- Handbook of Chemistry and Physics, 66th ed.; CRC Press Inc.: Boca Raton, FL, USA, 1985–1986; p. D-154.
- Liu, T. Supramolecular structures of polyoxomolybdate-based giant molecules in aqueous solution. J. Am. Chem. Soc. 2002, 124, 10942–10943. [Google Scholar] [CrossRef] [PubMed]
- Kistler, M.L.; Bhatt, A.; Liu, G.; Casa, D.; Liu, T. A Complete Macroion-"Blackberry" assembly-macroion transition with continuously adjustable assembly sizes in {Mo132} water/acetone systems. J. Am. Chem. Soc. 2007, 129, 6453–6460. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liu, T.; Mal, S.S.; Kortz, U. Wheel-shaped polyoxotungstate [Cu20Cl(OH)24(H2O)12(P8W48O184)]25- macroanions form supramolecular "Blackberry" structure in aqueous solution. J. Am. Chem. Soc. 2006, 128, 10103–10110. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.C.; Wen, T.C. Voltammetric investigation of palladium oxides III: effects of hydration and pH on the electrocatalytic properties of Pd(IV)/Pd(II) and the reduction behavior of palladous oxide. Electrochim. Acta 1996, 41, 1505–1514. [Google Scholar] [CrossRef]
- Izarova, N.V.; Dickman, M.H.; Biboum, R.N.; Keita, B.; Nadjo, L.; Ramachandran, V.; Dalal, N.S.; Kortz, U. Heteropoly-13-palladates(II) [Pd(II)(13)(As(V)Ph)(8)O(32)](6-) and [Pd(II)(13)Se(IV)(8)O(32)](6-). Inorg. Chem. 2009, 48, 7504–7506. [Google Scholar] [CrossRef] [PubMed]
- Rand, D.A.J.; Woods, R. A study of the dissolution of platinum, palladium, rhodium and gold electrodes in 1 M sulphuric acid by cyclic voltammetry. J. Electroanal. Chem. 1972, 35, 209–218. [Google Scholar] [CrossRef]
- Enyo, M.; Conway, B.E.; Bockris, J.O’.M. Comprehensive Treatise of Electrochemistry; Yeager, E., Khan, S.V.M., White, R.E., Eds.; Plenum Press: New York, NY, USA, 1983; Vol. 7, p. 241. [Google Scholar]
- Trasatti, S. Work function, electronegativity, and electrochemical behavior of metals. III. Electrolytic hydrogen evolution in acid solutions. J. Electroanal. Chem. 1972, 39, 163–184. [Google Scholar] [CrossRef]
- Norskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, J23–J26. [Google Scholar] [CrossRef]
- Li, F.; Bertoncello, P.; Ciani, I.; Mantovani, G.; Unwin, P.R. Incorporation of functionalized palladium nanoparticles within ultrathin Nafion films: a nanostructured composite for electrolytic and redox-mediated hydrogen evolution. Adv. Funct. Mater. 2008, 18, 1685–1693. [Google Scholar] [CrossRef]
- Keita, B.; Kortz, U.; Brudna Holzle, L.R.; Brown, S.; Nadjo, L. Efficient hydrogen-evolving cathodes based on proton and electron reservoir behaviors of the phosphotungstate [H7P8W48O184]33- and the Co(II)-containing silicotungstates [Co6(H2O)30{Co9Cl2(OH)3(H2O)9(β-SiW8O31)3}]5- and [{Co3(B-β-SiW9O33(OH))(B-β-SiW8O29OH)2}2]22-. Langmuir 2007, 23, 9531–9534. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Biboum, R.N.; Keita, B.; Franger, S.; Nanseu Njiki, C.P.; Zhang, G.; Zhang, J.; Liu, T.; Mbomekalle, I.-M.; Nadjo, L. Pd0@Polyoxometalate Nanostructures as Green Electrocatalysts: Illustrative Example of Hydrogen Production. Materials 2010, 3, 741-754. https://doi.org/10.3390/ma3010741
Biboum RN, Keita B, Franger S, Nanseu Njiki CP, Zhang G, Zhang J, Liu T, Mbomekalle I-M, Nadjo L. Pd0@Polyoxometalate Nanostructures as Green Electrocatalysts: Illustrative Example of Hydrogen Production. Materials. 2010; 3(1):741-754. https://doi.org/10.3390/ma3010741
Chicago/Turabian StyleBiboum, Rosa N., Bineta Keita, Sylvain Franger, Charles P. Nanseu Njiki, Guangjin Zhang, Jie Zhang, Tianbo Liu, Israel-Martyr Mbomekalle, and Louis Nadjo. 2010. "Pd0@Polyoxometalate Nanostructures as Green Electrocatalysts: Illustrative Example of Hydrogen Production" Materials 3, no. 1: 741-754. https://doi.org/10.3390/ma3010741