Hot-Injection Synthesis of Cesium Lead Halide Perovskite Nanowires with Tunable Optical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of CsOA Precursors
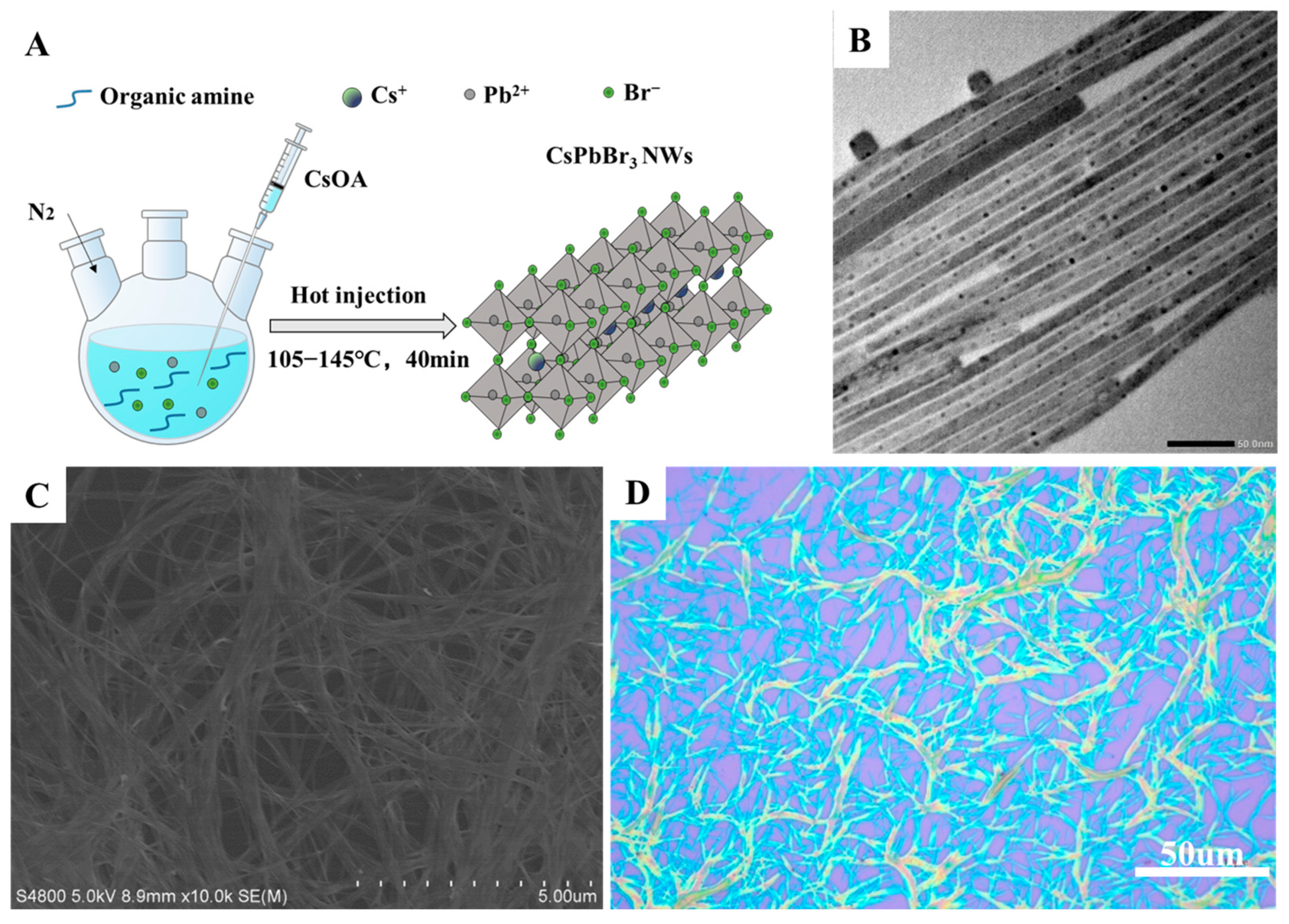
2.2.2. Synthesis of CsPbBr3 NWs
2.2.3. Preparation of PbX2 (X = Cl, I) Solution
2.2.4. Anion Exchange Reactions
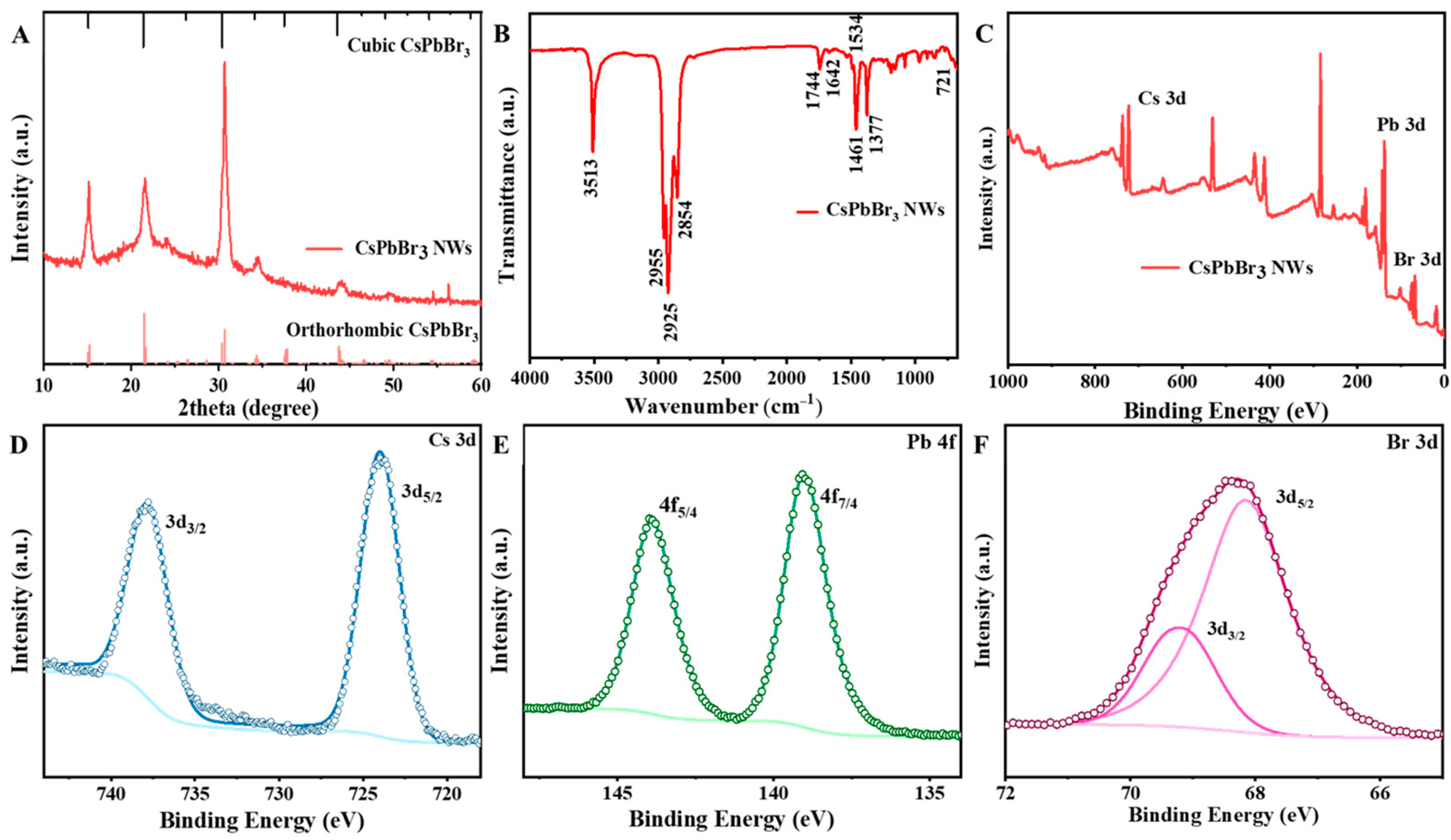
2.3. Characterization
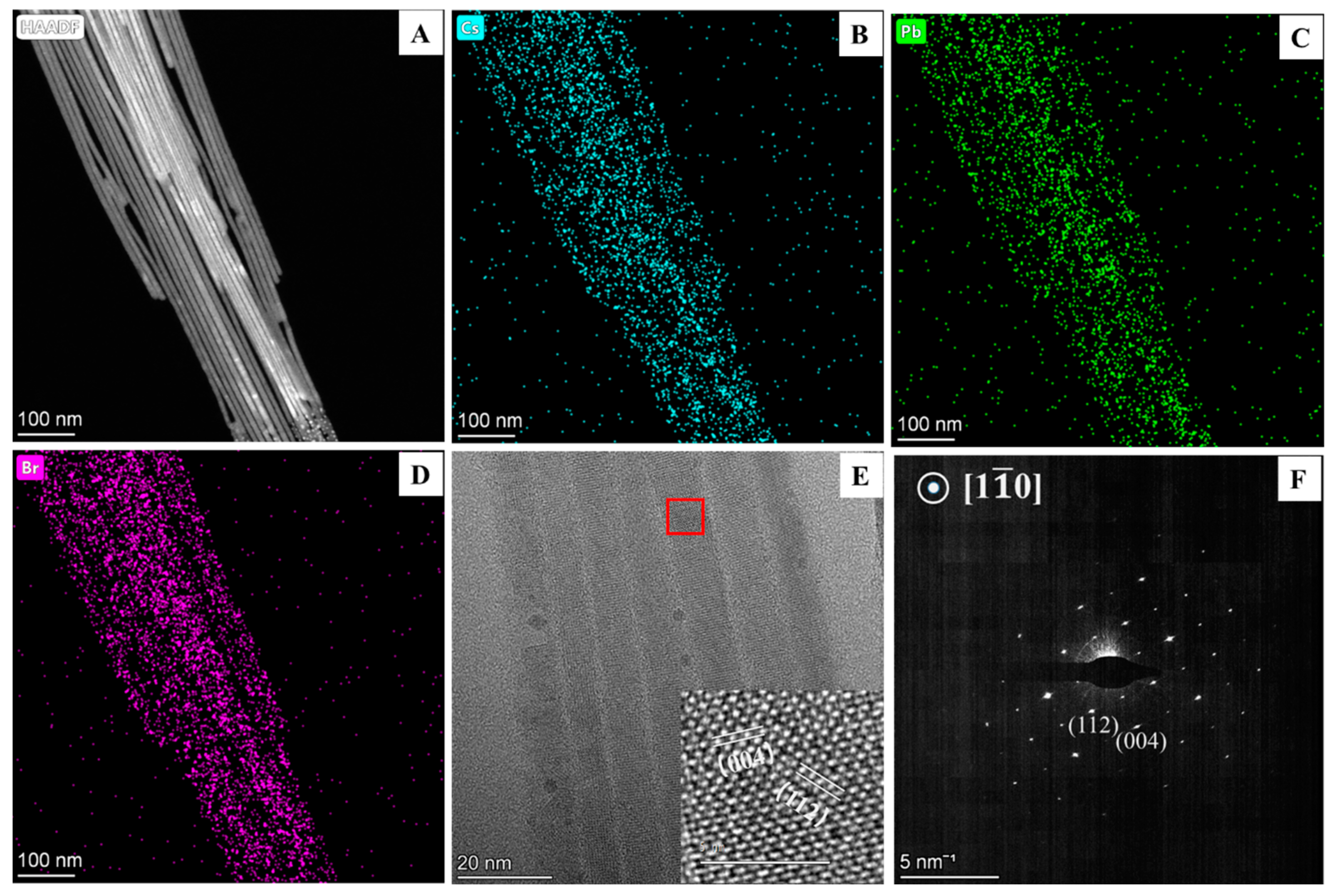
2.3.1. Characterization of Material Morphology
2.3.2. Characterization of Material Structure
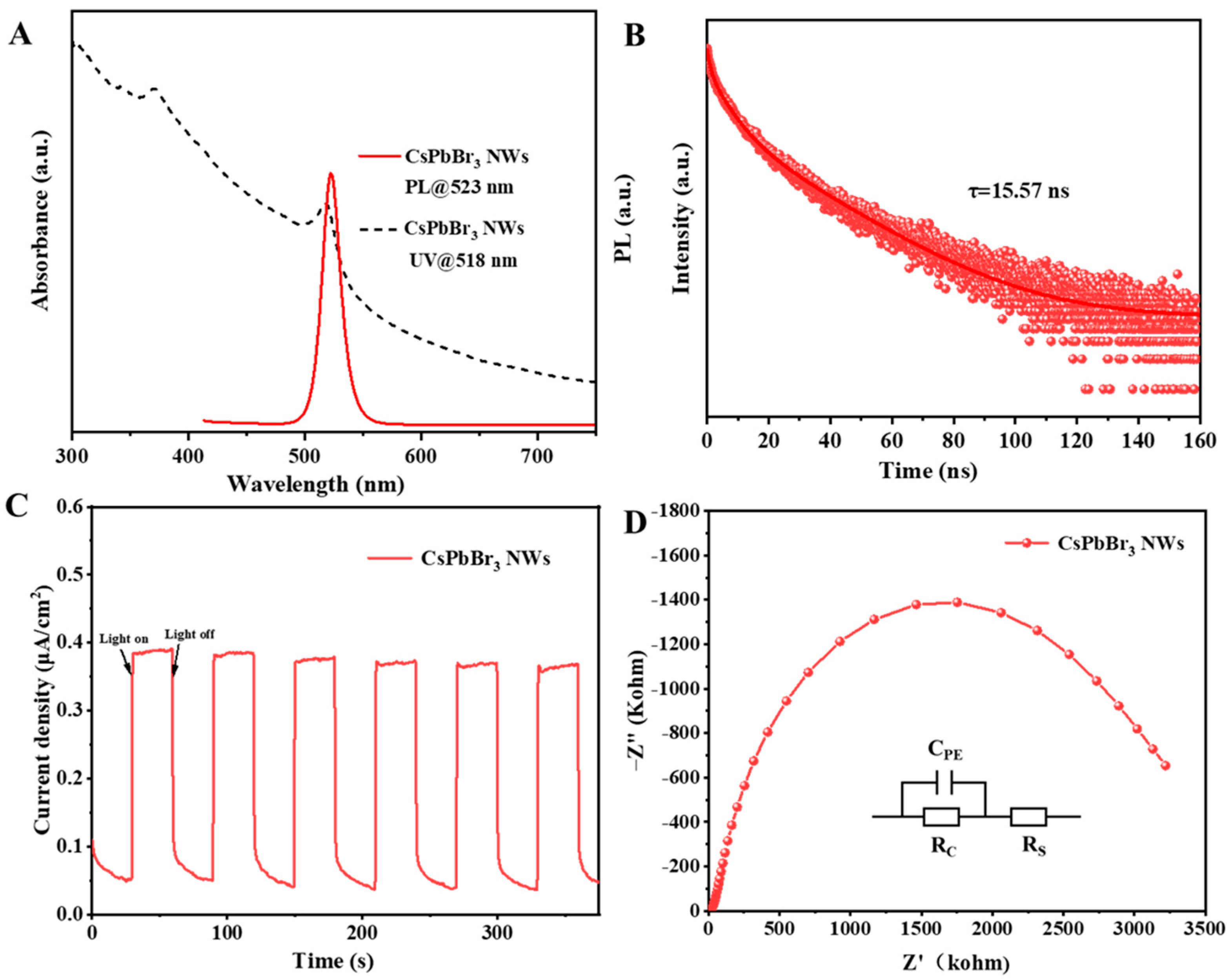
2.3.3. Optical Characterization of Materials
2.3.4. Electrochemical Characterization of Materials
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, N.; Xia, K.; He, Q.; Pan, J. Recent Progress in the Stability of Red-Emissive Perovskite Nanocrystals for Light-Emitting Diodes. ACS Mater. Lett. 2022, 4, 1233–1254. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Manna, L. What Defines a Halide Perovskite? ACS Energy Lett. 2020, 5, 604–610. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; D’Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the Optical Properties of Cesium Lead Halide Perovskite Nanocrystals by Anion Exchange Reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar] [CrossRef]
- He, Y.; Hadar, I.; Kanatzidis, M.G. Detecting Ionizing Radiation Using Halide Perovskite Semiconductors Processed through Solution and Alternative Methods. Nat. Photonics 2022, 16, 14–26. [Google Scholar] [CrossRef]
- Zhan, X.; Zhang, X.; Liu, Z.; Chen, C.; Kong, L.; Jiang, S.; Xi, S.; Liao, G.; Liu, X. Boosting the Performance of Self-Powered CsPbCl3-Based UV Photodetectors by a Sequential Vapor-Deposition Strategy and Heterojunction Engineering. ACS Appl. Mater. Interfaces 2021, 13, 45744–45757. [Google Scholar] [CrossRef]
- He, X.; Hao, S.; Ouyang, D.; Liu, S.; Zhang, N.; Zeng, Z.; Zhang, Y.; Spanopoulos, I.; Wolverton, C.; Li, Y. Universal Vapor-Phase Synthesis of Large-Scale Ultrathin Perovskites with Superior Stability for Photodetectors and Image Sensors. Adv. Funct. Mater. 2024, 2313163. [Google Scholar] [CrossRef]
- Jeong, M.; Choi, I.W.; Go, E.M.; Cho, Y.; Kim, M.; Lee, B.; Jeong, S.; Jo, Y.; Choi, H.W.; Lee, J. Stable Perovskite Solar Cells with Efficiency Exceeding 24.8% and 0.3-V Voltage Loss. Science 2020, 369, 1615–1620. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G. Perovskite Solar Cells with Atomically Coherent Interlayers on SnO2 Electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef]
- Hu, H.; An, S.X.; Li, Y.; Orooji, S.; Singh, R.; Schackmar, F.; Laufer, F.; Jin, Q.; Feeney, T.; Diercks, A. Triple-Junction Perovskite-Perovskite-Silicon Solar Cells with Power Conversion Efficiency of 24.4%. Energy Environ. Sci. 2024, 17, 2800–2814. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, W.; Peng, X.; Sun, G.; Liu, X.; Liu, D.; Li, Z.; He, F.; Shen, C.; Gu, Q. Perovskite Light-Emitting Diodes with Eqe Exceeding 28% through a Synergetic Dual-Additive Strategy for Defect Passivation and Nanostructure Regulation. Adv. Mater. 2021, 33, 2103268. [Google Scholar] [CrossRef]
- Zhu, L.; Cao, H.; Xue, C.; Zhang, H.; Qin, M.; Wang, J.; Wen, K.; Fu, Z.; Jiang, T.; Xu, L. Unveiling the Additive-Assisted Oriented Growth of Perovskite Crystallite for High Performance Light-Emitting Diodes. Nat. Commun. 2021, 12, 5081. [Google Scholar] [CrossRef]
- Ramadan, A.J.; Jeong, W.H.; Oliver, R.D.; Jiang, J.; Dasgupta, A.; Yuan, Z.; Smith, J.; Lee, J.E.; Motti, S.G.; Gough, O. The Role of the Organic Cation in Developing Efficient Green Perovskite Leds Based on Quasi-2d Perovskite Heterostructures. Adv. Funct. Mater. 2024, 34, 2309653. [Google Scholar] [CrossRef]
- Qin, C.; Sandanayaka, A.S.; Zhao, C.; Matsushima, T.; Zhang, D.; Fujihara, T.; Adachi, C. Stable Room-Temperature Continuous-Wave Lasing in Quasi-2D Perovskite Films. Nature 2020, 585, 53–57. [Google Scholar] [CrossRef]
- Zhuge, M.; Yin, J.; Liu, Y.; Wang, Y.; Li, Z.; Xiao, S.; Yu, S.; Song, Q. Precise Control of Single-Crystal Perovskite Nanolasers. Adv. Mater. 2023, 35, 2300344. [Google Scholar] [CrossRef]
- Tian, J.; Tan, Q.Y.; Wang, Y.; Yang, Y.; Yuan, G.; Adamo, G.; Soci, C. Perovskite Quantum Dot One-Dimensional Topological Laser. Nat. Commun. 2023, 14, 1433. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, Y.; Xu, H.; Chen, W.; Liu, B.; Zhang, J.; Zhang, H.; Wang, Z.; Kang, D.-H.; Zeng, J. Homogenizing out-of-Plane Cation Composition in Perovskite Solar Cells. Nature 2023, 624, 557–563. [Google Scholar] [CrossRef]
- Kumar, G.S.; Sumukam, R.R.; Rajaboina, R.K.; Savu, R.N.; Srinivas, M.; Banavoth, M. Perovskite Nanowires for Next-Generation Optoelectronic Devices: Lab to Fab. ACS Appl. Energy Mater. 2022, 5, 1342–1377. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, X.; Li, L.; Yao, Y.; Ye, H.; Shang, X.; Chen, X.; Luo, J. Realization of Vis-Nir Dual-Modal Circularly Polarized Light Detection in Chiral Perovskite Bulk Crystals. J. Am. Chem. Soc. 2021, 143, 14077–14082. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zhao, K.; Yang, Z.; Feng, J.; Zhang, X.; Wang, K.; Meng, L.; Ye, H.; Liu, M. A 1300 mm2 Ultrahigh-Performance Digital Imaging Assembly Using High-Quality Perovskite Single Crystals. Adv. Mater. 2018, 30, 1707314. [Google Scholar] [CrossRef]
- Liu, P.; Han, N.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. High-Quality Ruddlesden–Popper Perovskite Film Formation for High-Performance Perovskite Solar Cells. Adv. Mater. 2021, 33, 2002582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Nazeeruddin, M.K.; Choy, W.C. Perovskite Photovoltaics: The Significant Role of Ligands in Film Formation, Passivation, and Stability. Adv. Mater. 2019, 31, 1805702. [Google Scholar] [CrossRef]
- Haque, A.; Ravi, V.K.; Shanker, G.S.; Sarkar, I.; Nag, A.; Santra, P.K. Internal Heterostructure of Anion-Exchanged Cesium Lead Halide Nanocubes. J. Phys. Chem. C 2017, 122, 13399–13406. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Z.; Hu, Z.; Zeng, F.; Zhang, Z.; Yao, Y.; Yao, Z.; Tang, X.; Du, J.; Zang, Z. Enhanced Single-Mode Lasers of All-Inorganic Perovskite Nanocube by Localized Surface Plasmonic Effect from Au Nanoparticles. J. Lumin. 2019, 208, 402–407. [Google Scholar] [CrossRef]
- Bohn, B.J.; Tong, Y.; Gramlich, M.; Lai, M.L.; Döblinger, M.; Wang, K.; Hoye, R.L.; Müller-Buschbaum, P.; Stranks, S.D.; Urban, A.S. Boosting Tunable Blue Luminescence of Halide Perovskite Nanoplatelets through Postsynthetic Surface Trap Repair. Nano Lett. 2018, 18, 5231–5238. [Google Scholar] [CrossRef]
- Yang, D.; Zou, Y.; Li, P.; Liu, Q.; Wu, L.; Hu, H.; Xu, Y.; Sun, B.; Zhang, Q.; Lee, S.-T. Large-Scale Synthesis of Ultrathin Cesium Lead Bromide Perovskite Nanoplates with Precisely Tunable Dimensions and Their Application in Blue Light-Emitting Diodes. Nano Energy 2018, 47, 235–242. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, H.; Stoumpos, C.C.; Ding, Q.; Wang, J.; Kanatzidis, M.G.; Zhu, X.; Jin, S. Broad Wavelength Tunable Robust Lasing from Single-Crystal Nanowires of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). ACS Nano 2016, 10, 7963–7972. [Google Scholar] [CrossRef]
- Zhang, D.; Eaton, S.W.; Yu, Y.; Dou, L.; Yang, P. Solution-Phase Synthesis of Cesium Lead Halide Perovskite Nanowires. J. Am. Chem. Soc. 2015, 137, 9230–9233. [Google Scholar] [CrossRef]
- Xu, Y.-F.; Yang, M.-Z.; Chen, B.-X.; Wang, X.-D.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. A CsPbBr3 Perovskite Quantum Dot/Graphene Oxide Composite for Photocatalytic CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 5660–5663. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, H.; Song, Z.; Zheng, M.; Liu, R.; Pan, X.; Wan, H.; Zhang, J.; Wang, H.; Li, X. Welding Perovskite Nanowires for Stable, Sensitive, Flexible Photodetectors. ACS Nano 2020, 14, 2777–2787. [Google Scholar] [CrossRef]
- Quan, L.N.; Kang, J.; Ning, C.-Z.; Yang, P. Nanowires for Photonics. Chem. Rev. 2019, 119, 9153–9169. [Google Scholar] [CrossRef]
- Shamsi, J.; Urban, A.S.; Imran, M.; De Trizio, L.; Manna, L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef] [PubMed]
- Vighnesh, K.; Wang, S.; Liu, H.; Rogach, A.L. Hot-Injection Synthesis Protocol for Green-Emitting Cesium Lead Bromide Perovskite Nanocrystals. ACS Nano 2022, 16, 19618–19625. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, Y.; Wang, A.; Wang, Q.; Tang, H.; Shen, W.; Li, Z.; Deng, Z. Controlled Synthesis of Composition Tunable Formamidinium Cesium Double Cation Lead Halide Perovskite Nanowires and Nanosheets with Improved Stability. Chem. Mater. 2017, 29, 2157–2166. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; He, W.; Peng, H.; Dai, Q. CsPbBr3 Perovskite Nanowires and Their Optical Properties. Opt. Mater. 2020, 109, 110399. [Google Scholar] [CrossRef]
- Bierman, M.J.; Lau, Y.A.; Kvit, A.V.; Schmitt, A.L.; Jin, S. Dislocation-Driven Nanowire Growth and Eshelby Twist. Science 2008, 320, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zou, Y.; Wu, L.; Pan, Q.; Yang, D.; Hu, H.; Tan, Y.; Zhong, Q.; Xu, Y.; Liu, H. Solvothermal Synthesis of High-Quality All-Inorganic Cesium Lead Halide Perovskite Nanocrystals: From Nanocube to Ultrathin Nanowire. Adv. Funct. Mater. 2017, 27, 1701121. [Google Scholar] [CrossRef]
- Kazes, M.; Udayabhaskararao, T.; Dey, S.; Oron, D. Effect of Surface Ligands in Perovskite Nanocrystals: Extending in and Reaching Out. Acc. Chem. Res. 2021, 54, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Lin, S.Y.; Tang, A.C.; Singh, B.P.; Tong, H.C.; Chen, C.Y.; Lee, Y.C.; Tsai, T.L.; Liu, R.S. Mesoporous Silica Particles Integrated with All-Inorganic CsPbBr3 Perovskite Quantum-Dot Nanocomposites (MP-PQDs) with High Stability and Wide Color Gamut Used for Backlight Display. Angew. Chem. Int. Ed. 2016, 55, 7924–7929. [Google Scholar] [CrossRef]
- Imran, M.; Di Stasio, F.; Dang, Z.; Canale, C.; Khan, A.H.; Shamsi, J.; Brescia, R.; Prato, M.; Manna, L. Colloidal Synthesis of Strongly Fluorescent CsPbBr3 Nanowires with Width Tunable Down to the Quantum Confinement Regime. Chem. Mater. 2016, 28, 6450–6454. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Y.; Bekenstein, Y.; Yu, Y.; Gibson, N.A.; Wong, A.B.; Eaton, S.W.; Kornienko, N.; Kong, Q.; Lai, M. Synthesis of Composition Tunable and Highly Luminescent Cesium Lead Halide Nanowires through Anion-Exchange Reactions. J. Am. Chem. Soc. 2016, 138, 7236–7239. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Lin, J.; Li, C.; Hu, S.; Huang, Y.; Yu, C.; Wen, Z.; Liu, Z.; Fang, Y.; Tang, C. Solvothermal Synthesis of Cesium Lead Halide Perovskite Nanowires with Ultra-High Aspect Ratios for High-Performance Photodetectors. Nanoscale 2018, 10, 21451–21458. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Schünemann, S.; Song, S.; Tüysüz, H. Structural Effects on Optoelectronic Properties of Halide Perovskites. Chem. Soc. Rev. 2018, 47, 7045–7077. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Huang, S.; Du, J.; Tang, X.; Zeng, F.; Liu, Z.; Zhang, Z.; Shi, T.; Yang, J.; Wu, D. Surface Ligand Engineering for CsPbBr3 Quantum Dots Aiming at Aggregation Suppression and Amplified Spontaneous Emission Improvement. Adv. Opt. Mater. 2020, 8, 2000977. [Google Scholar] [CrossRef]
- Gao, M.; Liu, H.; Yu, S.; Louisia, S.; Zhang, Y.; Nenon, D.P.; Alivisatos, A.P.; Yang, P. Scaling Laws of Exciton Recombination Kinetics in Low Dimensional Halide Perovskite Nanostructures. J. Am. Chem. Soc. 2020, 142, 8871–8879. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zhang, M.; Biesold, G.M.; Choi, W.; He, Y.; Li, Z.; Shen, D.; Lin, Z. Recent Advances in Synthesis, Properties, and Applications of Metal Halide Perovskite Nanocrystals/Polymer Nanocomposites. Adv. Mater. 2021, 33, 2005888. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Obliger, A.; Lu, D.; Kley, C.S.; Bischak, C.G.; Kong, Q.; Lei, T.; Dou, L.; Ginsberg, N.S.; Limmer, D.T. Intrinsic Anion Diffusivity in Lead Halide Perovskites Is Facilitated by a Soft Lattice. Proc. Natl. Acad. Sci. USA 2018, 115, 11929–11934. [Google Scholar] [CrossRef]
- Pan, D.; Fu, Y.; Chen, J.; Czech, K.J.; Wright, J.C.; Jin, S. Visualization and Studies of Ion-Diffusion Kinetics in Cesium Lead Bromide Perovskite Nanowires. Nano Lett. 2018, 18, 1807–1813. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Li, H.; Liu, C.; Wang, X.; Zhang, Q.; Liu, J.; Wang, M.; Liu, Y. Hot-Injection Synthesis of Cesium Lead Halide Perovskite Nanowires with Tunable Optical Properties. Materials 2024, 17, 2173. https://doi.org/10.3390/ma17102173
He J, Li H, Liu C, Wang X, Zhang Q, Liu J, Wang M, Liu Y. Hot-Injection Synthesis of Cesium Lead Halide Perovskite Nanowires with Tunable Optical Properties. Materials. 2024; 17(10):2173. https://doi.org/10.3390/ma17102173
Chicago/Turabian StyleHe, Jiazhen, Hang Li, Chengqi Liu, Xiaoqian Wang, Qi Zhang, Jinfeng Liu, Mingwei Wang, and Yong Liu. 2024. "Hot-Injection Synthesis of Cesium Lead Halide Perovskite Nanowires with Tunable Optical Properties" Materials 17, no. 10: 2173. https://doi.org/10.3390/ma17102173



