Flexible Kinetic Model Determination of Reactions in Materials under Isothermal Conditions
Abstract
:1. Introduction
2. Experimental Section
3. Theoretical Foundation and Simulations
3.1. Simulation Using the Tridimensional Avrami–Erofeev Equation A3
3.2. Simulation Using the Contracting Volume Model R3 with Particle Size Distribution
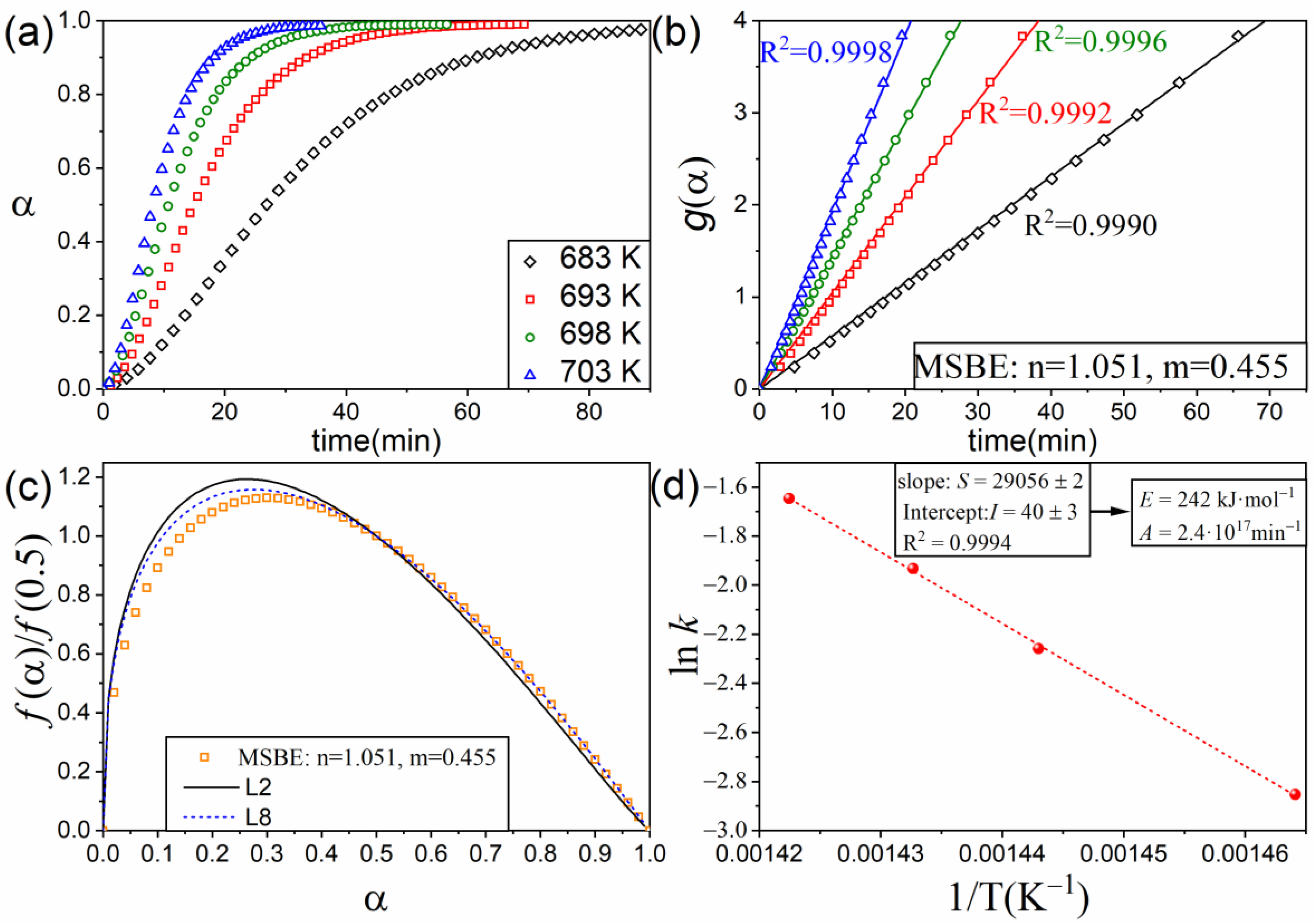
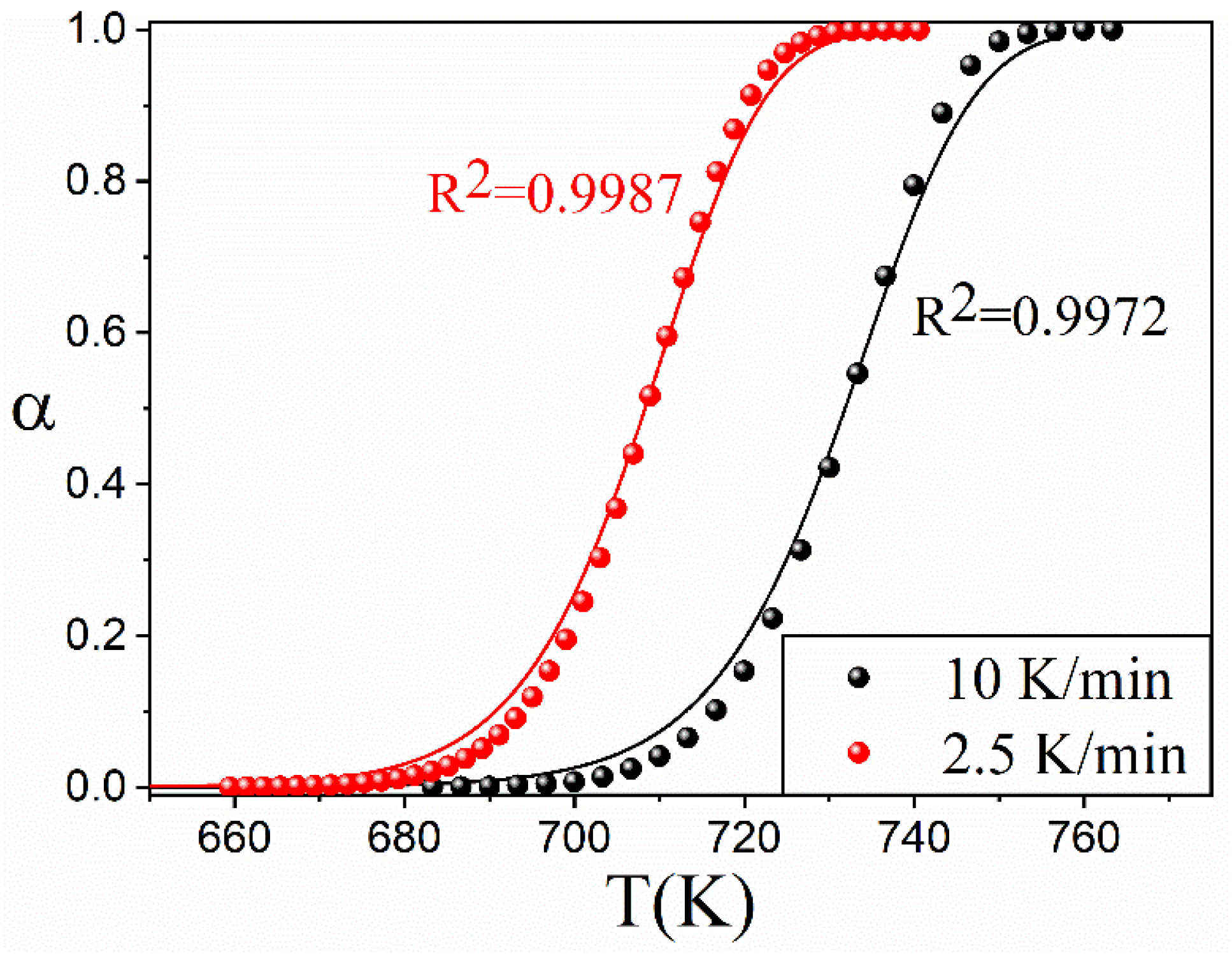
4. Application to an Experimental Case: The Thermal Decomposition of Ethylene-Propylene-Diene
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanchirico, R.; Santonocito, M.L.; Di Sarli, V.; Lisi, L. Shelf Life Prediction of Picric Acid via Model-Based Kinetic Analysis of Its Thermal Decomposition. Materials 2022, 15, 8899. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Sakai, Y.; Kikuchi, S.; Koga, N. Thermal decomposition of perlite concrete under different water vapor pressures. J. Therm. Anal. Calorim. 2021, 147, 6309–6322. [Google Scholar] [CrossRef]
- Málek, J.; Svoboda, R. Kinetic Processes in Amorphous Materials Revealed by Thermal Analysis: Application to Glassy Selenium. Molecules 2019, 24, 2725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manchón-Gordón, A.; Blázquez, J.; Conde, C.; Conde, A. Nanocrystallization kinetics understood as multiple microprocesses following the classical theory of crystallization. J. Alloys Compd. 2016, 675, 81–85. [Google Scholar] [CrossRef]
- Ortiz, C.; Valverde, J.M.; Chacartegui, R.; Perez-Maqueda, L.A. Carbonation of Limestone Derived CaO for Thermochemical Energy Storage: From Kinetics to Process Integration in Concentrating Solar Plants. ACS Sustain. Chem. Eng. 2018, 6, 6404–6417. [Google Scholar] [CrossRef] [Green Version]
- Fedunik-Hofman, L.; Bayon, A.; Donne, S.W. Kinetics of Solid-Gas Reactions and Their Application to Carbonate Looping Systems. Energies 2019, 12, 2981. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Shan, J.; Zhao, J.; Tong, Z. Isothermal curing kinetics of a flame retardant epoxy resin containing DOPO investigated by DSC and rheology. Thermochim. Acta 2016, 632, 56–63. [Google Scholar] [CrossRef]
- Tziamtzi, C.K.; Chrissafis, K. Optimization of a commercial epoxy curing cycle via DSC data kinetics modelling and TTT plot construction. Polymer 2021, 230, 124091. [Google Scholar] [CrossRef]
- Bornosuz, N.; Gorbunova, I.; Petrakova, V.; Shutov, V.; Kireev, V.; Onuchin, D.; Sirotin, I. Isothermal Kinetics of Epoxyphosphazene Cure. Polymers 2021, 13, 297. [Google Scholar] [CrossRef]
- Koga, N.; Favergeon, L.; Kodani, S. Impact of atmospheric water vapor on the thermal decomposition of calcium hydroxide: A universal kinetic approach to a physico-geometrical consecutive reaction in solid–gas systems under different partial pressures of product gas. Phys. Chem. Chem. Phys. 2019, 21, 11615–11632. [Google Scholar] [CrossRef]
- Hara, M.; Okazaki, T.; Muravyev, N.V.; Koga, N. Physico-geometrical Kinetic Aspects of the Thermal Dehydration of Trehalose Dihydrate. J. Phys. Chem. C 2022, 126, 20423–20436. [Google Scholar] [CrossRef]
- Wang, G.; Min, X.; Peng, N.; Wang, Z. The isothermal kinetics of zinc ferrite reduction with carbon monoxide. J. Therm. Anal. Calorim. 2021, 146, 2253–2260. [Google Scholar] [CrossRef]
- Huo, J.; Zhao, S.; Pan, J.; Pu, W.; Varfolomeev, M.A.; Emelianov, D.A. Evolution of mass losses and evolved gases of crude oil and its SARA components during low-temperature oxidation by isothermal TG–FTIR analyses. J. Therm. Anal. Calorim. 2021, 147, 4099–4112. [Google Scholar] [CrossRef]
- Gil-González, E.; Perejón, A.; Sánchez-Jiménez, P.E.; Medina-Carrasco, S.; Kupčík, J.; Šubrt, J.; Criado, J.M.; Pérez-Maqueda, L.A. Crystallization Kinetics of Nanocrystalline Materials by Combined X-ray Diffraction and Differential Scanning Calorimetry Experiments. Cryst. Growth Des. 2018, 18, 3107–3116. [Google Scholar] [CrossRef] [Green Version]
- Koga, N.; Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Muravyev, N.V.; Pérez-Maqueda, L.A.; Saggese, C.; Sánchez-Jiménez, P.E. ICTAC Kinetics Committee recommendations for analysis of thermal decomposition kinetics. Thermochim. Acta 2023, 719, 179384. [Google Scholar] [CrossRef]
- Pérez-Maqueda, L.A.; Sánchez-Jiménez, P.E.; Perejón, A.; García-Garrido, C.; Criado, J.M.; Benítez-Guerrero, M. Scission kinetic model for the prediction of polymer pyrolysis curves from chain structure. Polym. Test. 2014, 37, 1–5. [Google Scholar] [CrossRef]
- Nishikawa, K.; Ueta, Y.; Hara, D.; Yamada, S.; Koga, N. Kinetic characterization of multistep thermal oxidation of carbon/carbon composite in flowing air. J. Therm. Anal. Calorim. 2016, 128, 891–906. [Google Scholar] [CrossRef]
- Lorenzo, A.T.; Arnal, M.L.; Albuerne, J.; Müller, A.J. DSC isothermal polymer crystallization kinetics measurements and the use of the Avrami equation to fit the data: Guidelines to avoid common problems. Polym. Test. 2007, 26, 222–231. [Google Scholar] [CrossRef]
- Biasin, A.; Segre, C.; Salviulo, G.; Zorzi, F.; Strumendo, M. Investigation of CaO–CO2 reaction kinetics by in-situ XRD using synchrotron radiation. Chem. Eng. Sci. 2015, 127, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Grimm, S.; Hemberger, P.; Kasper, T.; Atakan, B. Mechanism and Kinetics of the Thermal Decomposition of Fe(C5H5)2 in Inert and Reductive Atmosphere: A Synchrotron-Assisted Investigation in A Microreactor. Adv. Mater. Interfaces 2022, 9, 2200192. [Google Scholar] [CrossRef]
- Ben Hafsia, K.; Ponçot, M.; Chapron, D.; Royaud, I.; Dahoun, A.; Bourson, P. A novel approach to study the isothermal and non-isothermal crystallization kinetics of Poly(Ethylene Terephthalate) by Raman spectroscopy. J. Polym. Res. 2016, 23, 1–14. [Google Scholar] [CrossRef]
- Arcenegui-Troya, J.; Sánchez-Jiménez, P.E.; Perejón, A.; Moreno, V.; Valverde, J.M.; Pérez-Maqueda, L.A. Kinetics and cyclability of limestone (CaCO3) in presence of steam during calcination in the CaL scheme for thermochemical energy storage. Chem. Eng. J. 2021, 417, 129194. [Google Scholar] [CrossRef]
- Cheng, S.; Jiu, S.; Li, H. Kinetics of Dehydroxylation and Decarburization of Coal Series Kaolinite during Calcination: A Novel Kinetic Method Based on Gaseous Products. Materials 2021, 14, 1493. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, W.; Fu, M.; Li, C.; Xue, Z. Discussion on Criterion of Determination of the Kinetic Parameters of the Linear Heating Reactions. Minerals 2022, 12, 81. [Google Scholar] [CrossRef]
- Pérez-Maqueda, L.; Sánchez-Jiménez, P.; Criado, J. Evaluation of the integral methods for the kinetic study of thermally stimulated processes in polymer science. Polymer 2005, 46, 2950–2954. [Google Scholar] [CrossRef] [Green Version]
- Arcenegui-Troya, J.; Sánchez-Jiménez, P.E.; Perejón, A.; Pérez-Maqueda, L.A. Determination of the activation energy under isothermal conditions: Revisited. J. Therm. Anal. Calorim. 2022, 1–8. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, P.E.; Perejón, A.; Pérez-Maqueda, L.A.; Criado, J.M. New Insights on the Kinetic Analysis of Isothermal Data: The Independence of the Activation Energy from the Assumed Kinetic Model. Energy Fuels 2014, 29, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Jin, B.; Xiao, Y.; Zhang, Q.; Chai, Z.; Huang, Q.; Chu, S.; Peng, R. Study on the isothermal decomposition kinetics and mechanism of nitrocellulose. Polym. Test. 2019, 75, 337–343. [Google Scholar] [CrossRef]
- Chew, J.J.; Soh, M.; Sunarso, J.; Yong, S.-T.; Doshi, V.; Bhattacharya, S. Isothermal kinetic study of CO2 gasification of torrefied oil palm biomass. Biomass-Bioenergy 2020, 134, 105487. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, P.E.; Perejón, A.; Arcenegui-Troya, J.; Pérez-Maqueda, L.A. Predictions of polymer thermal degradation: Relevance of selecting the proper kinetic model. J. Therm. Anal. Calorim. 2021, 147, 2335–2341. [Google Scholar] [CrossRef]
- Arcenegui-Troya, J.; Sánchez-Jiménez, P.E.; Perejón, A.; Pérez-Maqueda, L.A. Relevance of Particle Size Distribution to Kinetic Analysis: The Case of Thermal Dehydroxylation of Kaolinite. Processes 2021, 9, 1852. [Google Scholar] [CrossRef]
- Hotta, M.; Tone, T.; Koga, N. Effects of Particle Size on the Kinetics of Physico-geometrical Consecutive Reactions in Solid–Gas Systems: Thermal Decomposition of Potassium Hydrogen Carbonate. J. Phys. Chem. C 2021, 125, 22023–22035. [Google Scholar] [CrossRef]
- Blázquez, J.S.; Manchón-Gordón, A.F.; Ipus, J.J.; Conde, C.F.; Conde, A. On the Use of JMAK Theory to Describe Mechanical Amorphization: A Comparison between Experiments, Numerical Solutions and Simulations. Metals 2018, 8, 450. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Maqueda, L.A.; Criado, J.M.; Sánchez-Jiménez, P.E. Combined Kinetic Analysis of Solid-State Reactions: A Powerful Tool for the Simultaneous Determination of Kinetic Parameters and the Kinetic Model without Previous Assumptions on the Reaction Mechanism. J. Phys. Chem. A 2006, 110, 12456–12462. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.L.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Suñol, J.J. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim. Acta 2014, 590, 1–23. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, S.; Bell, D.A. Steam gasification of powder river basin coal: Surface reaction kinetics and intraparticle mass transfer restrictions. J. Therm. Anal. Calorim. 2021, 146, 2209–2222. [Google Scholar] [CrossRef]
- Arcenegui-Troya, J.; Durán-Martín, J.D.; Perejón, A.; Valverde, J.M.; Maqueda, L.A.P.; Jiménez, P.E.S. Overlooked pitfalls in CaO carbonation kinetics studies nearby equilibrium: Instrumental effects on calculated kinetic rate constants. Alex. Eng. J. 2021, 61, 6129–6138. [Google Scholar] [CrossRef]
- Soustelle, M. Handbook of Heterogenous Kinetics; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A.; Perejón, A.; Criado, J.M. A new model for the kinetic analysis of thermal degradation of polymers driven by random scission. Polym. Degrad. Stab. 2010, 95, 733–739. [Google Scholar] [CrossRef]
- Perejón, A.; Sánchez-Jiménez, P.E.; Gil-González, E.; Pérez-Maqueda, L.A.; Criado, J.M. Pyrolysis kinetics of ethylene–propylene (EPM) and ethylene–propylene–diene (EPDM). Polym. Degrad. Stab. 2013, 98, 1571–1577. [Google Scholar] [CrossRef] [Green Version]

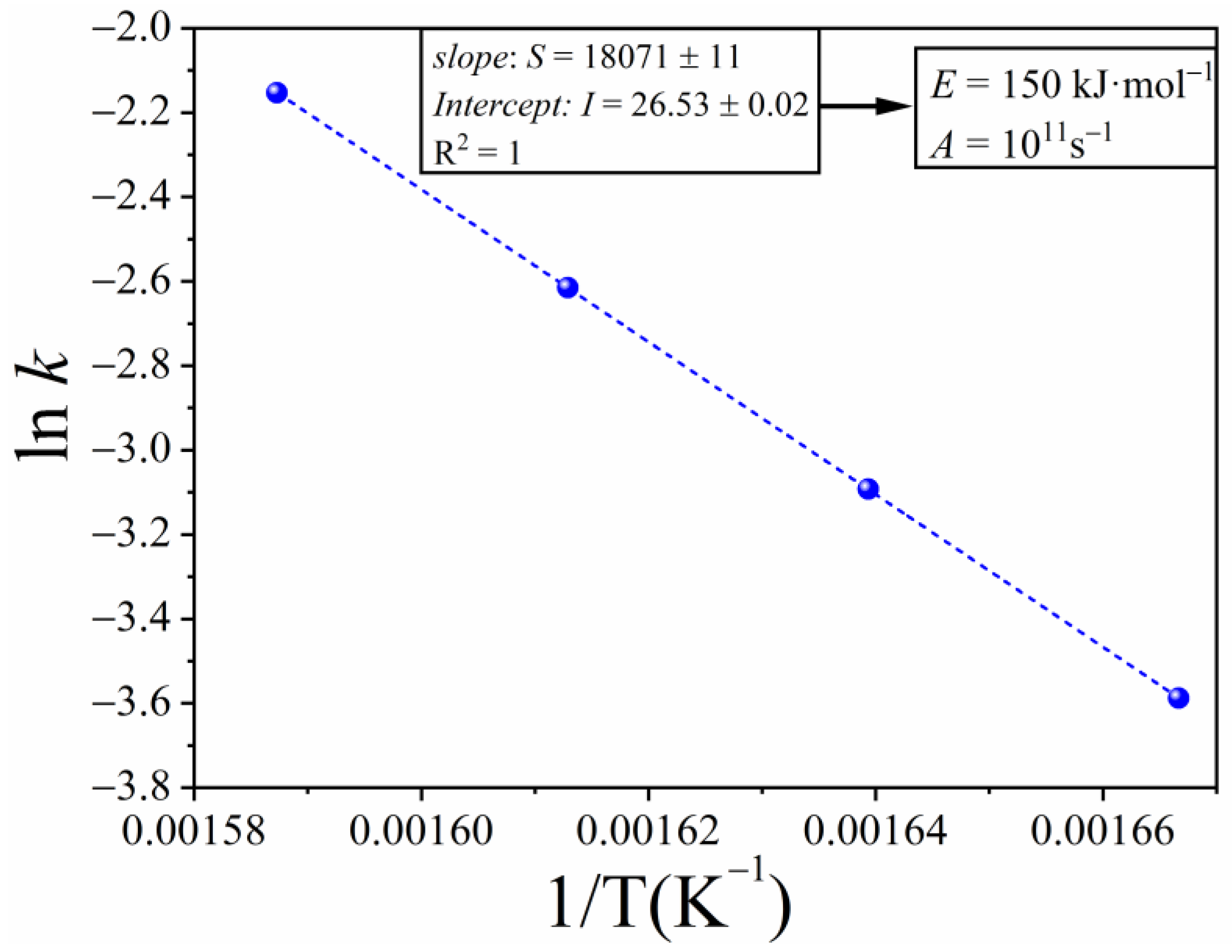
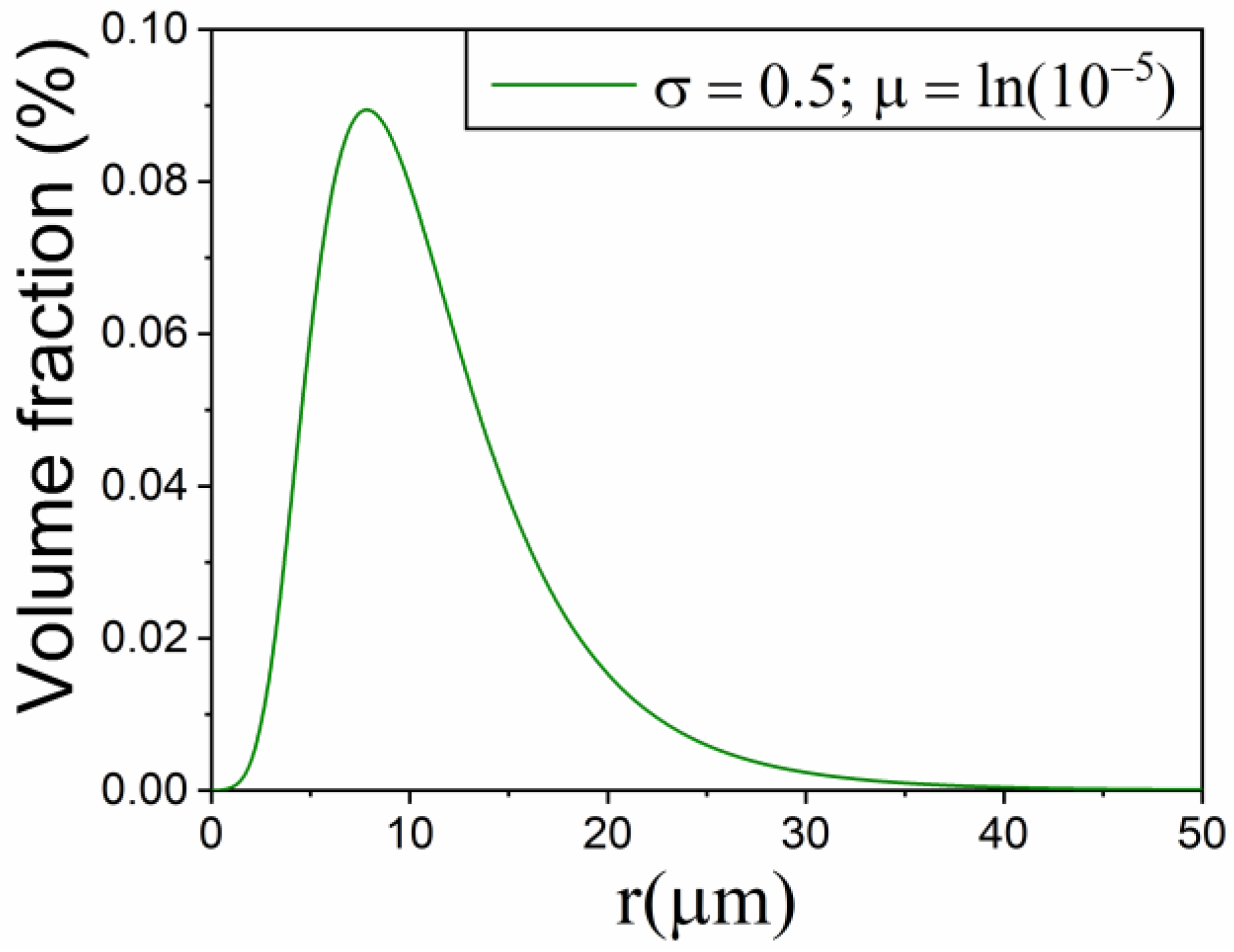

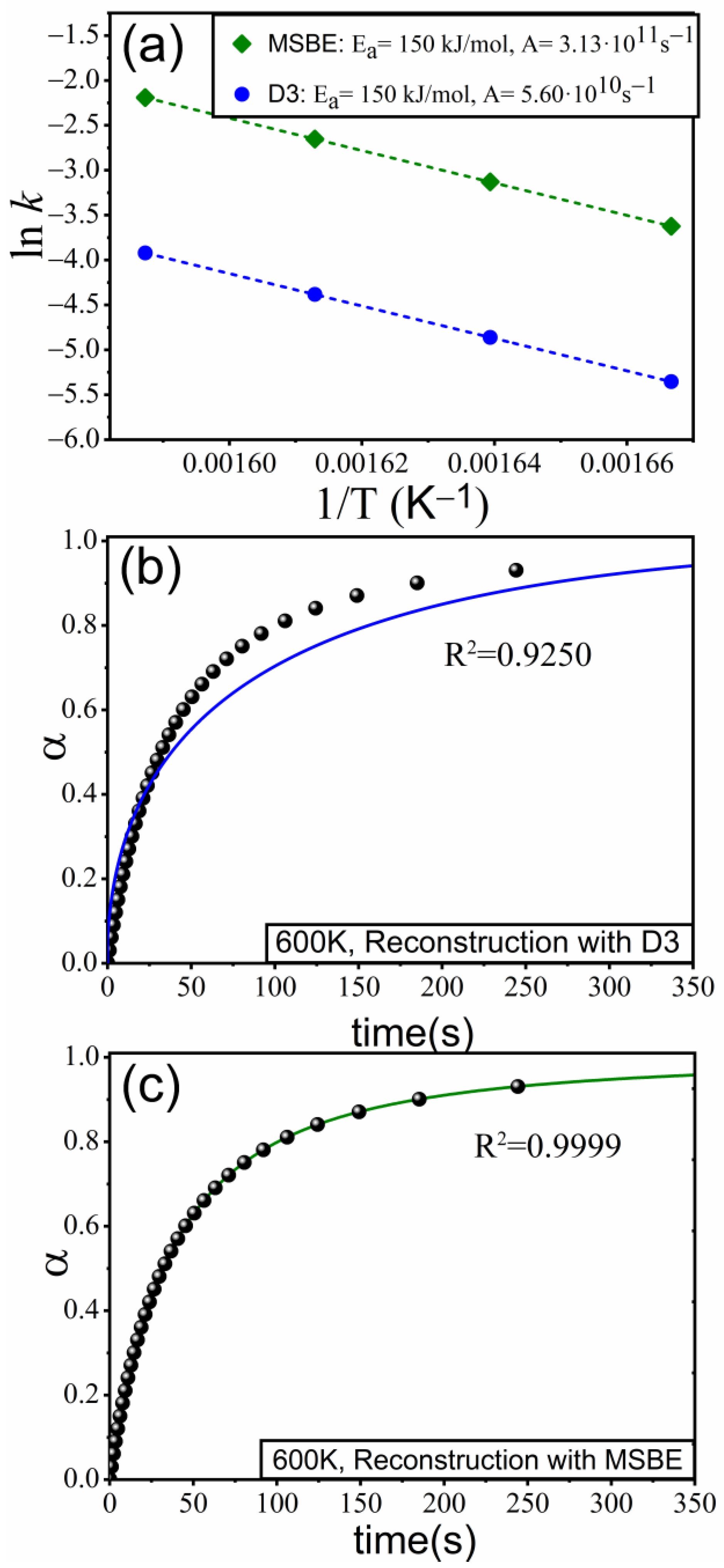
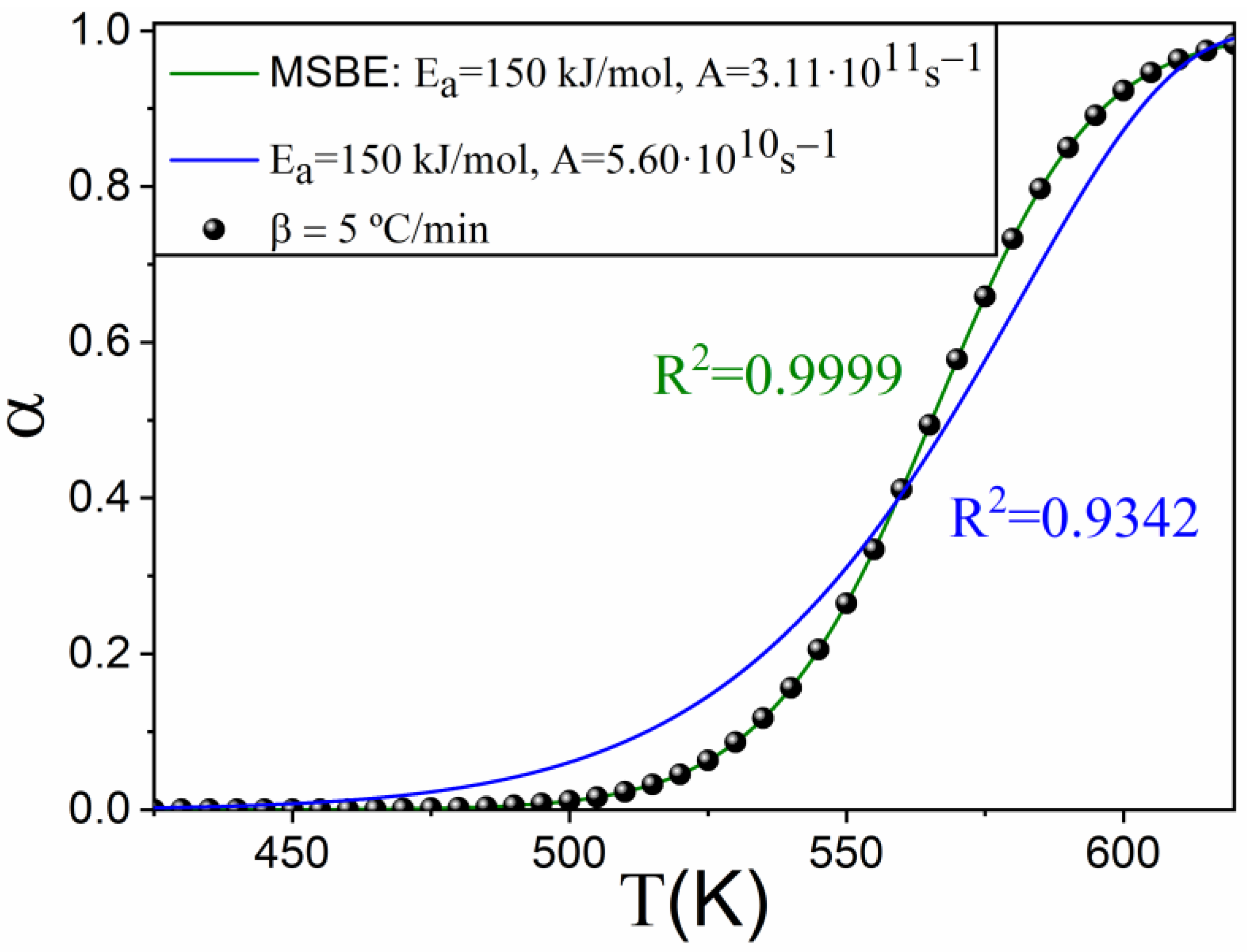
| Kinetic Model | m | ||
|---|---|---|---|
| Contracting area: R2 | 1/2 | 0 | |
| Contracting volume: R3 | 2/3 | 0 | |
| 1D Avrami–Erofeev equation: F1 | 1 | 0 | |
| 2D Avrami–Erofeev equation: A2 | 0.806 | 0.515 | |
| 3D Avrami–Erofeev equation: A3 | 0.748 | 0.693 | |
| 2D diffusion: D2 | 0.425 | −1.008 | |
| 3D diffusion, Jander equation: D3 | 0.951 | −1.004 |
| Kinetic Model | |
|---|---|
| MSBE: n = 1.584, m = −0.019 | 0.9999 |
| R3 | 0.6844 |
| R2 | 0.6283 |
| A2 | 0.5823 |
| A3 | 0.4934 |
| D2 | 0.7642 |
| D3 | 0.9893 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arcenegui-Troya, J.; Perejón, A.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A. Flexible Kinetic Model Determination of Reactions in Materials under Isothermal Conditions. Materials 2023, 16, 1851. https://doi.org/10.3390/ma16051851
Arcenegui-Troya J, Perejón A, Sánchez-Jiménez PE, Pérez-Maqueda LA. Flexible Kinetic Model Determination of Reactions in Materials under Isothermal Conditions. Materials. 2023; 16(5):1851. https://doi.org/10.3390/ma16051851
Chicago/Turabian StyleArcenegui-Troya, Juan, Antonio Perejón, Pedro E. Sánchez-Jiménez, and Luis A. Pérez-Maqueda. 2023. "Flexible Kinetic Model Determination of Reactions in Materials under Isothermal Conditions" Materials 16, no. 5: 1851. https://doi.org/10.3390/ma16051851
APA StyleArcenegui-Troya, J., Perejón, A., Sánchez-Jiménez, P. E., & Pérez-Maqueda, L. A. (2023). Flexible Kinetic Model Determination of Reactions in Materials under Isothermal Conditions. Materials, 16(5), 1851. https://doi.org/10.3390/ma16051851






