Structural, Vibrational, and Magnetic Characterization of Orthoferrite LaFeO3 Ceramic Prepared by Reaction Flash Sintering
Abstract
:1. Introduction
2. Materials and Methods
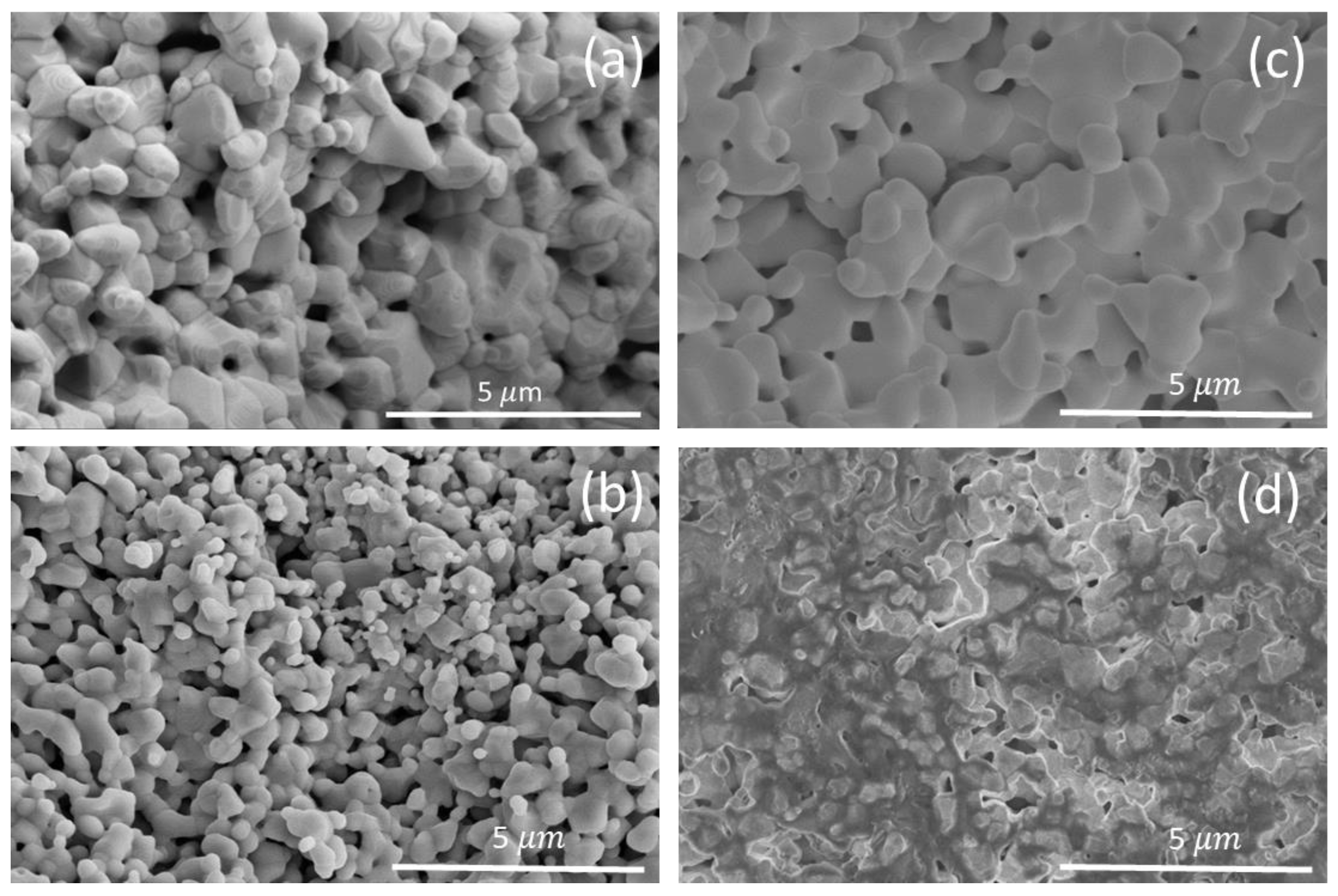
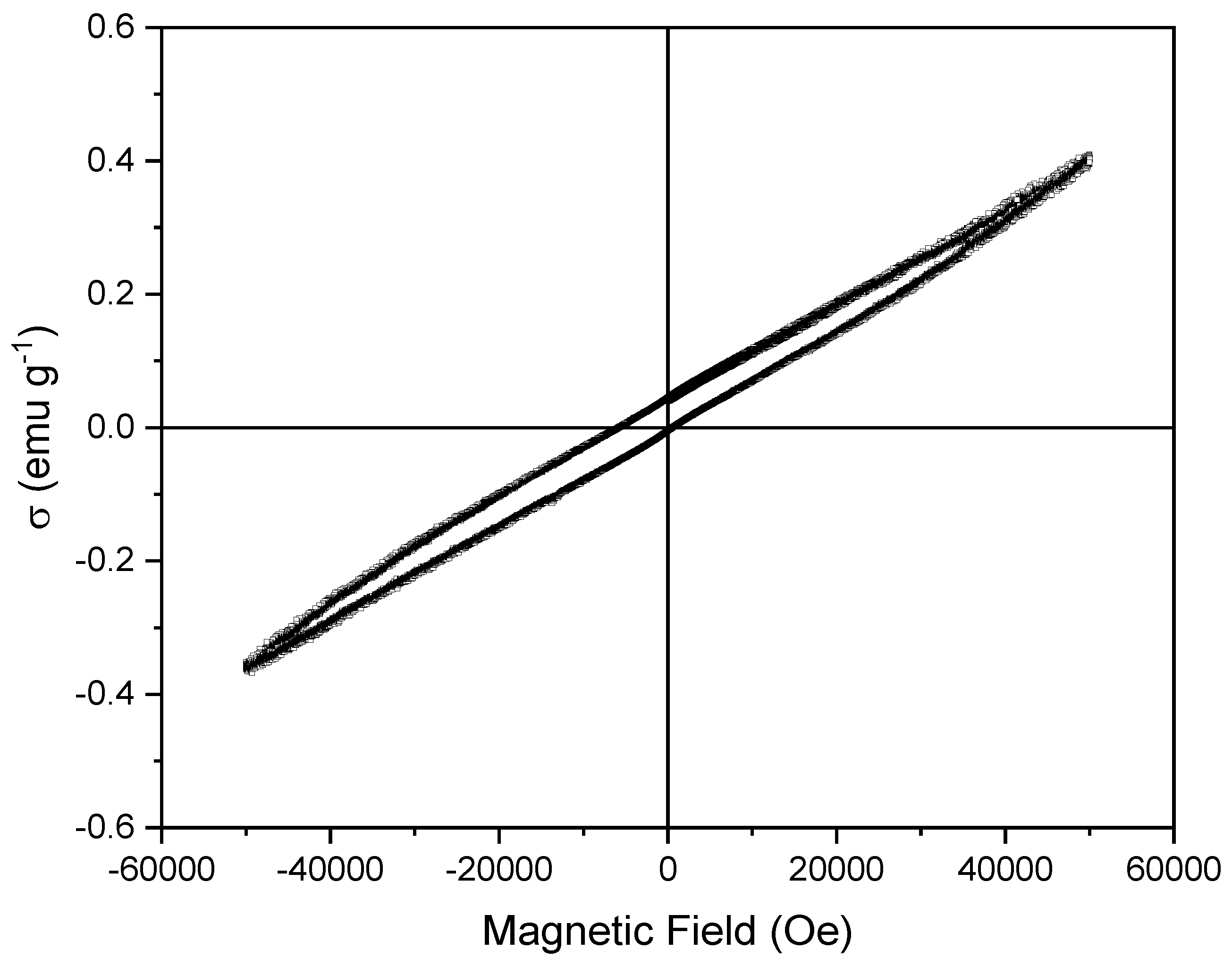
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Humayun, M.; Ullah, H.; Usman, M.; Habibi-Yangjeh, A.; Tahir, A.A.; Wang, C.; Luo, W. Perovskite-type lanthanum ferrite based photocatalysts: Preparation, properties, and applications. J. Energy Chem. 2022, 66, 314–338. [Google Scholar] [CrossRef]
- Rong, Q.; Zhang, Y.; Hu, J.; Li, K.; Wang, H.; Chen, M.; Lv, T.; Zhu, Z.; Zhang, J.; Liu, Q. Design of ultrasensitive Ag-LaFeO3 methanol gas sensor based on quasi molecular imprinting technology. Sci. Rep. 2018, 8, 14220. [Google Scholar] [CrossRef] [Green Version]
- Acharya, S.; Mondal, J.; Ghosh, S.; Roy, S.; Chakrabarti, P. Multiferroic behavior of lanthanum orthoferrite (LaFeO3). Mater. Lett. 2010, 64, 415–418. [Google Scholar] [CrossRef]
- Wu, J.; Fan, Z.; Xiao, D.; Zhu, J.; Wang, J. Multiferroic bismuth ferrite-based materials for multifunctional applications: Ceramic bulks, thin films and nanostructures. Prog. Mater. Sci. 2016, 84, 335–402. [Google Scholar] [CrossRef] [Green Version]
- Hazra, S.; Ghosh, N. Preparation of nanoferrites and their applications. J. Nanosci. Nanotechnol. 2014, 14, 1983–2000. [Google Scholar] [CrossRef]
- Cao, E.; Chu, Z.; Wang, H.; Hao, W.; Sun, L.; Zhang, Y. Effect of film thickness on the electrical and ethanol sensing characteristics of LaFeO3 nanoparticle-based thick film sensors. Ceram. Int. 2018, 44, 7180–7185. [Google Scholar] [CrossRef]
- Bidrawn, F.; Lee, S.; Vohs, J.M.; Gorte, R.J. The Effect of Ca, Sr, and Ba Doping on the Ionic Conductivity and Cathode Performance of LaFeO3. J. Electrochem. Soc. 2008, 155, B660. [Google Scholar] [CrossRef]
- Hao, X.; Zhang, Y. Low temperature gel-combustion synthesis of porous nanostructure LaFeO3 with enhanced visible-light photocatalytic activity in reduction of Cr (VI). Mater. Lett. 2017, 197, 120–122. [Google Scholar] [CrossRef]
- Hearne, G.; Pasternak, M.; Taylor, R.; Lacorre, P. Electronic structure and magnetic properties of LaFeO3 at high pressure. Phys. Rev. B 1995, 51, 11495. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, H.; Tan, W.; Huo, D. Magnetization reversal, critical behavior, and magnetocaloric effect in NdMnO3: The role of magnetic ordering of Nd and Mn moments. J. Appl. Phys. 2022, 132, 183907. [Google Scholar] [CrossRef]
- Pelosato, R.; Sora, I.N.; Leonelli, C.; Russo, P.; Cimino, F.; Mortalò, C.; Varvaro, G.; Marchetti, E.; Agostinelli, E. Micro-extruded LaSrCuFeO-based polystyrene magnetic composites: Morphological and magnetic characterization. J. Alloy. Compd. 2022, 917, 165499. [Google Scholar] [CrossRef]
- Peng, K.; Fu, L.; Yang, H.; Ouyang, J. Perovskite LaFeO3/montmorillonite nanocomposites: Synthesis, interface characteristics and enhanced photocatalytic activity. Sci. Rep. 2016, 6, 19723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köferstein, R.; Ebbinghaus, S.G. Synthesis and characterization of nano-LaFeO3 powders by a soft-chemistry method and corresponding ceramics. Solid State Ion. 2013, 231, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Thuy, N.T.; Minh, D.L. Size effect on the structural and magnetic properties of nanosized perovskite LaFeO3 prepared by different methods. Adv. Mater. Sci. Eng. 2012, 2012, 380306. [Google Scholar] [CrossRef] [Green Version]
- Gosavi, P.V.; Biniwale, R.B. Pure phase LaFeO3 perovskite with improved surface area synthesized using different routes and its characterization. Mater. Chem. Phys. 2010, 119, 324–329. [Google Scholar] [CrossRef]
- Mitra, A.; Mahapatra, A.S.; Mallick, A.; Shaw, A.; Ghosh, M.; Chakrabarti, P.K. Simultaneous enhancement of magnetic and ferroelectric properties of LaFeO3 by co-doping with Dy3+ and Ti4+. J. Alloy. Compd. 2017, 726, 1195–1204. [Google Scholar] [CrossRef]
- Palai, R.; Katiyar, R.S.; Schmid, H.; Tissot, P.; Clark, S.J.; Robertson, J.; Redfern, S.A.T.; Catalan, G.; Scott, J.F. β phase and γ−β metal-insulator transition in multiferroic BiFeO3. Phys. Rev. B 2008, 77, 014110. [Google Scholar] [CrossRef] [Green Version]
- Farhadi, S.; Momeni, Z.; Taherimehr, M. Rapid synthesis of perovskite-type LaFeO3 nanoparticles by microwave-assisted decomposition of bimetallic La[Fe(CN)6]·5H2O compound. J. Alloy. Compd. 2009, 471, L5–L8. [Google Scholar] [CrossRef]
- Ravindranathan, P.; Komarneni, S.; Roy, R. Synthesis of lithium aluminate, mullite and coloured zirconia by a combustion process. J. Mater. Sci. Lett. 1993, 12, 369–371. [Google Scholar] [CrossRef]
- Zahi, S.; Daud, A.; Hashim, M. A comparative study of nickel–zinc ferrites by sol–gel route and solid-state reaction. Mater. Chem. Phys. 2007, 106, 452–456. [Google Scholar] [CrossRef]
- Coutinho, P.V.; Cunha, F.; Barrozo, P. Structural, vibrational and magnetic properties of the orthoferrites LaFeO3 and YFeO3: A comparative study. Solid State Commun. 2017, 252, 59–63. [Google Scholar] [CrossRef]
- Perejón, A.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A.; Criado, J.M.; Romero de Paz, J.; Sáez-Puche, R.; Masó, N.; West, A.R. Single phase, electrically insulating, multiferroic La-substituted BiFeO3 prepared by mechanosynthesis. J. Mater. Chem. C 2014, 2, 8398–8411. [Google Scholar] [CrossRef]
- Köferstein, R.; Jäger, L.; Ebbinghaus, S.G. Magnetic and optical investigations on LaFeO3 powders with different particle sizes and corresponding ceramics. Solid State Ion. 2013, 249–250, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Perejón, A.; Sánchez-Jiménez, P.E.; Poyato, R.; Masó, N.; West, A.R.; Criado, J.M.; Pérez-Maqueda, L.A. Preparation of phase pure, dense fine grained ceramics by conventional and spark plasma sintering of La-substituted BiFeO3 nanoparticles. J. Eur. Ceram. Soc. 2015, 35, 2283–2293. [Google Scholar] [CrossRef] [Green Version]
- Cologna, M.; Rashkova, B.; Raj, R. Flash Sintering of Nanograin Zirconia in <5 s at 850 °C. J. Am. Ceram. Soc. 2010, 93, 3556–3559. [Google Scholar]
- Naik, K.; Jha, S.K.; Raj, R. Correlations between conductivity, electroluminescence and flash sintering. Scr. Mater. 2016, 118, 1–4. [Google Scholar] [CrossRef]
- Molina-Molina, S.; Gil-González, E.; Durán-Olivencia, F.J.; Valverde, J.M.; Perejón, A.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A. A novel Multi-Phase Flash Sintering (MPFS) technique for 3D complex-shaped ceramics. Appl. Mater. Today 2022, 26, 101274. [Google Scholar] [CrossRef]
- Gil-González, E.; Perejón, A.; Sánchez-Jiménez, P.E.; Sayagués, M.J.; Raj, R.; Pérez-Maqueda, L.A. Phase-pure BiFeO3 produced by reaction flash-sintering of Bi2O3 and Fe2O3. J. Mater. Chem. A 2018, 6, 5356–5366. [Google Scholar] [CrossRef] [Green Version]
- Gil-González, E.; Perejón, A.; Sánchez-Jiménez, P.E.; Román-González, D.; Pérez-Maqueda, L.A. Control of experimental conditions in reaction flash-sintering of complex stoichiometry ceramics. Ceram. Int. 2020, 46, 29413–29420. [Google Scholar] [CrossRef]
- Manchón-Gordón, A.F.; Sánchez-Jiménez, P.E.; Blázquez, J.S.; Perejón, A.; Pérez-Maqueda, L.A. Reactive flash sintering of SrFe12O19 ceramic permanent magnets. J. Alloy. Compd. 2022, 922, 166203. [Google Scholar] [CrossRef]
- Wu, Y.; Su, X.; An, G.; Hong, W. Dense Na0.5K0.5NbO3 ceramics produced by reactive flash sintering of NaNbO3-KNbO3 mixed powders. Scr. Mater. 2020, 174, 49–52. [Google Scholar] [CrossRef]
- Su, X.; Jiao, Z.; Fu, M.; An, G.; Wu, Y.; Tian, Q.; Xu, P.; Wu, W.; Chang, X.; Liu, J. Ultrafast synthesis and densification of ZrO2 doped KNN ceramics by reactive flash sintering. Int. J. Appl. Ceram. Technol. 2021, 18, 1999–2009. [Google Scholar] [CrossRef]
- Bhandari, S.; Mishra, T.P.; Bram, M.; Guillon, O.; Yadav, D. Flash sintering behaviour of 8YSZ-NiO composites. Ceram. Int. 2022, 48, 33236–33244. [Google Scholar] [CrossRef]
- Kok, D.; Yadav, D.; Sortino, E.; McCormack, S.J.; Tseng, K.-P.; Kriven, W.M.; Raj, R.; Mecartney, M.L. α-Alumina and spinel react into single-phase high-alumina spinel in <3 seconds during flash sintering. J. Am. Ceram. Soc. 2019, 102, 644–653. [Google Scholar]
- Ma, B.; Zhu, Y.; Wang, K.; Sun, Z.; Ren, K.; Wang, Y. Reactive flash sintering and electrical transport properties of high-entropy (MgCoNiCuZn) 1-xLixO oxides. J. Am. Ceram. Soc. 2022, 105, 3765–3773. [Google Scholar] [CrossRef]
- Mao, H.-R.; Guo, R.-F.; Cao, Y.; Jin, S.-B.; Qiu, X.-M.; Shen, P. Ultrafast densification of high-entropy oxide (La0.2Nd0.2Sm0.2Eu0.2Gd0.2)2Zr2O7 by reactive flash sintering. J. Eur. Ceram. Soc. 2021, 41, 2855–2860. [Google Scholar] [CrossRef]
- Li, W.; Chen, L.; Liu, D.; Liu, J.; An, L. Ultra-low temperature reactive flash sintering synthesis of high-enthalpy and high-entropy Ca0.2Co0.2Ni0.2Cu0.2Zn0.2O oxide ceramics. Mater. Lett. 2021, 304, 130679. [Google Scholar] [CrossRef]
- Avila, V.; Yoon, B.; Ingraci Neto, R.R.; Silva, R.S.; Ghose, S.; Raj, R.; Jesus, L.M. Reactive flash sintering of the complex oxide Li0.5La0.5TiO3 starting from an amorphous precursor powder. Scr. Mater. 2020, 176, 78–82. [Google Scholar] [CrossRef]
- Avila, V.; Yoon, B.; Ghose, S.; Raj, R.; Jesus, L.M. Phase evolution during reactive flash sintering of Li6.25Al0.25La3Zr2O12 starting from a chemically prepared powder. J. Eur. Ceram. Soc. 2021, 41, 4552–4557. [Google Scholar] [CrossRef]
- Gaur, A.; Sglavo, V.M. Densification of La0.6Sr0.4Co0.2Fe0.8O3 ceramic by flash sintering at temperature less than 100 °C. J. Mater. Sci. 2014, 49, 6321–6332. [Google Scholar] [CrossRef]
- Ni, N.; Xiao, W.; Zheng, C.; Jiang, J.; Yu, Y.; Hao, W.; Tang, M.; Shen, H.; Peng, D. Flash cosintering of a lanthanum strontium cobalt ferrite nanofibre/Gd-doped ceria bilayer structure. J. Eur. Ceram. Soc. 2022, 42, 2870–2878. [Google Scholar] [CrossRef]
- Lim, Y.; Park, J.; Lee, H.; Ku, M.; Kim, Y.-B. Rapid fabrication of lanthanum strontium cobalt ferrite (LSCF) with suppression of LSCF/YSZ chemical side reaction via flash light sintering for SOFCs. Nano Energy 2021, 90, 106524. [Google Scholar] [CrossRef]
- Brand, R.A.; Lauer, J.; Herlach, D.M. The evaluation of hyperfine field distributions in overlapping and asymmetric mossbauer-spectra: A study of the amorphous alloy Pd77.5-XCu6Si16.5FeX. J. Phys. F-Met. Phys. 1983, 13, 675–683. [Google Scholar] [CrossRef]
- Jones, G.M.; Biesuz, M.; Ji, W.; John, S.F.; Grimley, C.; Manière, C.; Dancer, C.E.J. Promoting microstructural homogeneity during flash sintering of ceramics through thermal management. MRS Bull. 2021, 46, 59–66. [Google Scholar] [CrossRef]
- Charalambous, H.; Jha, S.K.; Christian, K.H.; Lay, R.T.; Tsakalakos, T. Flash Sintering using Controlled Current Ramp. J. Eur. Ceram. Soc. 2018, 38, 3689–3693. [Google Scholar] [CrossRef]
- Acharya, S.; Deb, A.K.; Das, D.; Chakrabarti, P.K. Enhanced magnetic behavior of Al substituted LaFeO3 (La(1−x)AlxFeO3, x=0.10 and 0.30). Mater. Lett. 2011, 65, 1280–1282. [Google Scholar] [CrossRef]
- Geller, S.; Raccah, P.M. Phase Transitions in Perovskitelike Compounds of the Rare Earths. Phys. Rev. B 1970, 2, 1167–1172. [Google Scholar] [CrossRef]
- Perez-Maqueda, L.A.; Gil-Gonzalez, E.; Perejon, A.; Lebrun, J.-M.; Sanchez-Jimenez, P.E.; Raj, R. Flash sintering of highly insulating nanostructured phase-pure BiFeO3. J. Am. Ceram. Soc. 2017, 100, 3365–3369. [Google Scholar] [CrossRef]
- Taibi, A.; Chaguetmi, S.; Sánchez-Jiménez, P.E.; Perejón, A.; García, J.E.; Satha, H.; Pérez-Maqueda, L.A. Pure perovskite BiFeO3–BaTiO3 ceramics prepared by reaction flash sintering of Bi2O3–Fe2O3–BaTiO3 mixed powders. Ceram. Int. 2021, 47, 26947–26954. [Google Scholar] [CrossRef]
- Smirnova, I.S. Normal modes of the LaMnO3 Pnma phase: Comparison with La2CuO4 Cmca phase. Phys. B Condens. Matter 1999, 262, 247–261. [Google Scholar] [CrossRef]
- Todorov, N.; Abrashev, M.; Ivanov, V.; Tsutsumanova, G.; Marinova, V.; Wang, Y.-Q.; Iliev, M. Comparative Raman study of isostructural YCrO3 and YMnO3: Effects of structural distortions and twinning. Phys. Rev. B 2011, 83, 224303. [Google Scholar] [CrossRef] [Green Version]
- Weber, M.C.; Guennou, M.; Zhao, H.J.; Íñiguez, J.; Vilarinho, R.; Almeida, A.; Moreira, J.A.; Kreisel, J. Raman spectroscopy of rare-earth orthoferrites R FeO3 (R= La, Sm, Eu, Gd, Tb, Dy). Phys. Rev. B 2016, 94, 214103. [Google Scholar] [CrossRef]
- Lakshmi, R.V.; Bera, P.; Hiremath, M.; Dubey, V.; Kundu, A.K.; Barshilia, H.C. Structural, magnetic, and dielectric properties of solution combustion synthesized LaFeO3, LaFe0.9Mn0.1O3, and LaMnO3 perovskites. Phys. Chem. Chem. Phys. 2022, 24, 5462–5478. [Google Scholar] [CrossRef] [PubMed]
- Iliev, M.N.; Abrashev, M.V.; Laverdière, J.; Jandl, S.; Gospodinov, M.M.; Wang, Y.Q.; Sun, Y.Y. Distortion-dependent Raman spectra and mode mixing in RMnO3 perovskites (R=La,Pr,Nd,Sm,Eu,Gd,Tb,Dy,Ho,Y). Phys. Rev. B 2006, 73, 064302. [Google Scholar] [CrossRef]
- Iliev, M.; Abrashev, M.; Lee, H.-G.; Popov, V.; Sun, Y.; Thomsen, C.; Meng, R.; Chu, C. Raman spectroscopy of orthorhombic perovskitelike YMnO3 and LaMnO3. Phys. Rev. B 1998, 57, 2872. [Google Scholar] [CrossRef]
- Saha, J.; Jana, Y.M.; Mukherjee, G.D.; Mondal, R.; Kumar, S.; Gupta, H.C. Structure, Mössbauer spectroscopy and vibration phonon spectra in valence-bond force-field model approach for distorted perovskites AFeO3 (A = La, Y). Mater. Chem. Phys. 2020, 240, 122286. [Google Scholar] [CrossRef]
- Gong, S.; Xie, Z.; Li, W.; Wu, X.; Han, N.; Chen, Y. Highly active and humidity resistive perovskite LaFeO3 based catalysts for efficient ozone decomposition. Appl. Catal. B Environ. 2019, 241, 578–587. [Google Scholar] [CrossRef]
- Auwal, I.A.; Baykal, A.; Güngüneş, H.; Shirsath, S.E. Structural investigation and hyperfine interactions of BaBixLaxFe12−2xO19 (0.0≤x≤0.5) hexaferrites. Ceram. Int. 2016, 42, 3380–3387. [Google Scholar] [CrossRef]
- Xia, P.; Mo, J.; Chen, J.; Liu, M.; Xia, Y. Magnetic Properties and Mössbauer Study of Perovskite LaFeO3 and LaFe0.5Cr0.5O3. physica status solidi (RRL) Rapid Res. Lett. 2022, 16, 2200023. [Google Scholar] [CrossRef]
- Sharma, P.; Masrour, R.; Jabar, A.; Fan, J.; Kumar, A.; Ling, L.; Ma, C.; Wang, C.; Yang, H. Structural and magnetocaloric properties of rare-earth orthoferrite perovskite: TmFeO3. Chem. Phys. Lett. 2020, 740, 137057. [Google Scholar] [CrossRef]
- Karakuscu, A.; Cologna, M.; Yarotski, D.; Won, J.; Francis, J.S.; Raj, R.; Uberuaga, B.P. Defect structure of flash-sintered strontium titanate. J. Am. Ceram. Soc. 2012, 95, 2531–2536. [Google Scholar] [CrossRef]
- Manchón-Gordón, A.F.; Perejón, A.; Gil-González, E.; Kowalczyk, M.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A. Low Temperature Magnetic Transition of BiFeO3 Ceramics Sintered by Electric Field-Assisted Methods: Flash and Spark Plasma Sintering. Materials 2023, 16, 189. [Google Scholar] [CrossRef] [PubMed]
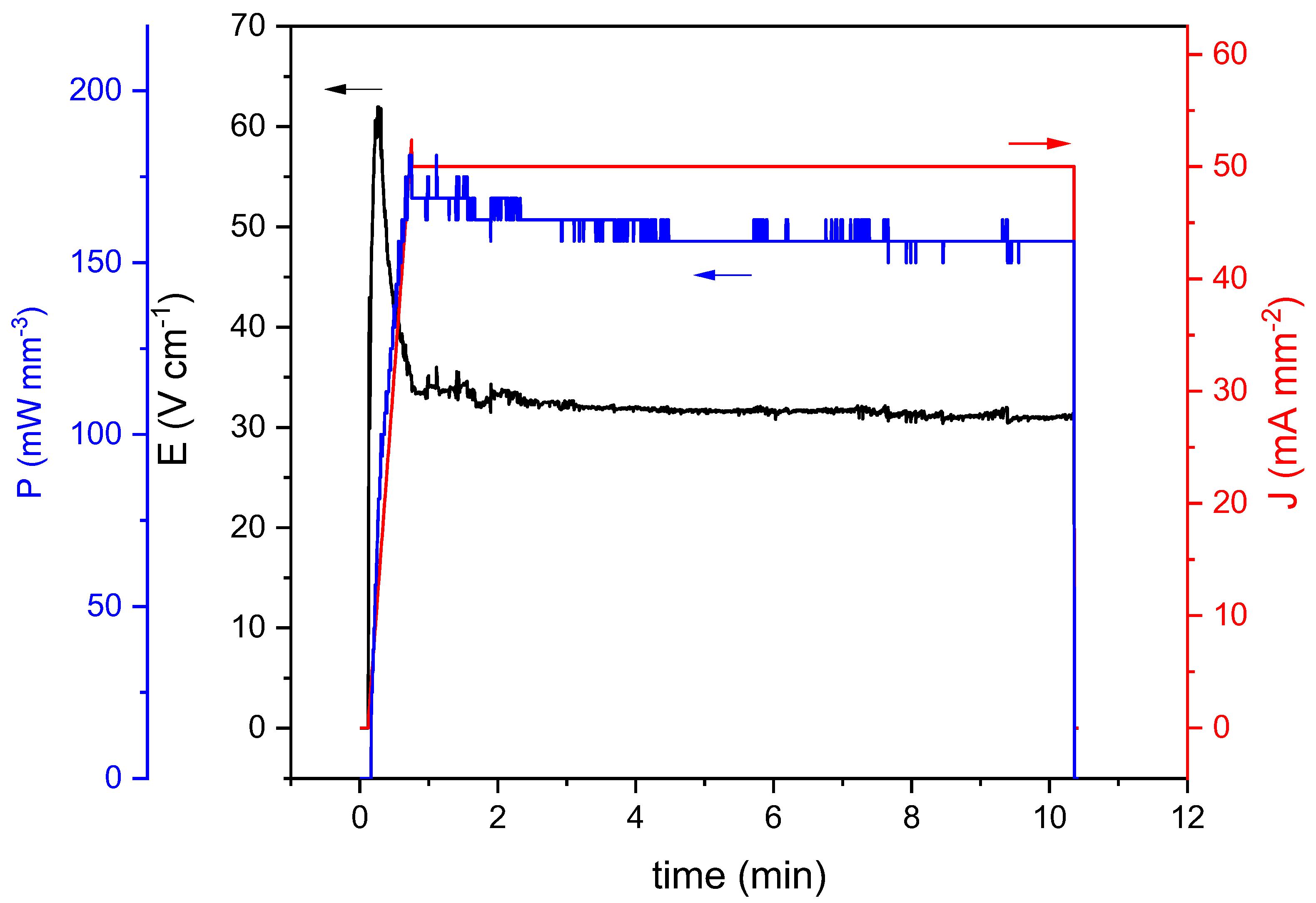
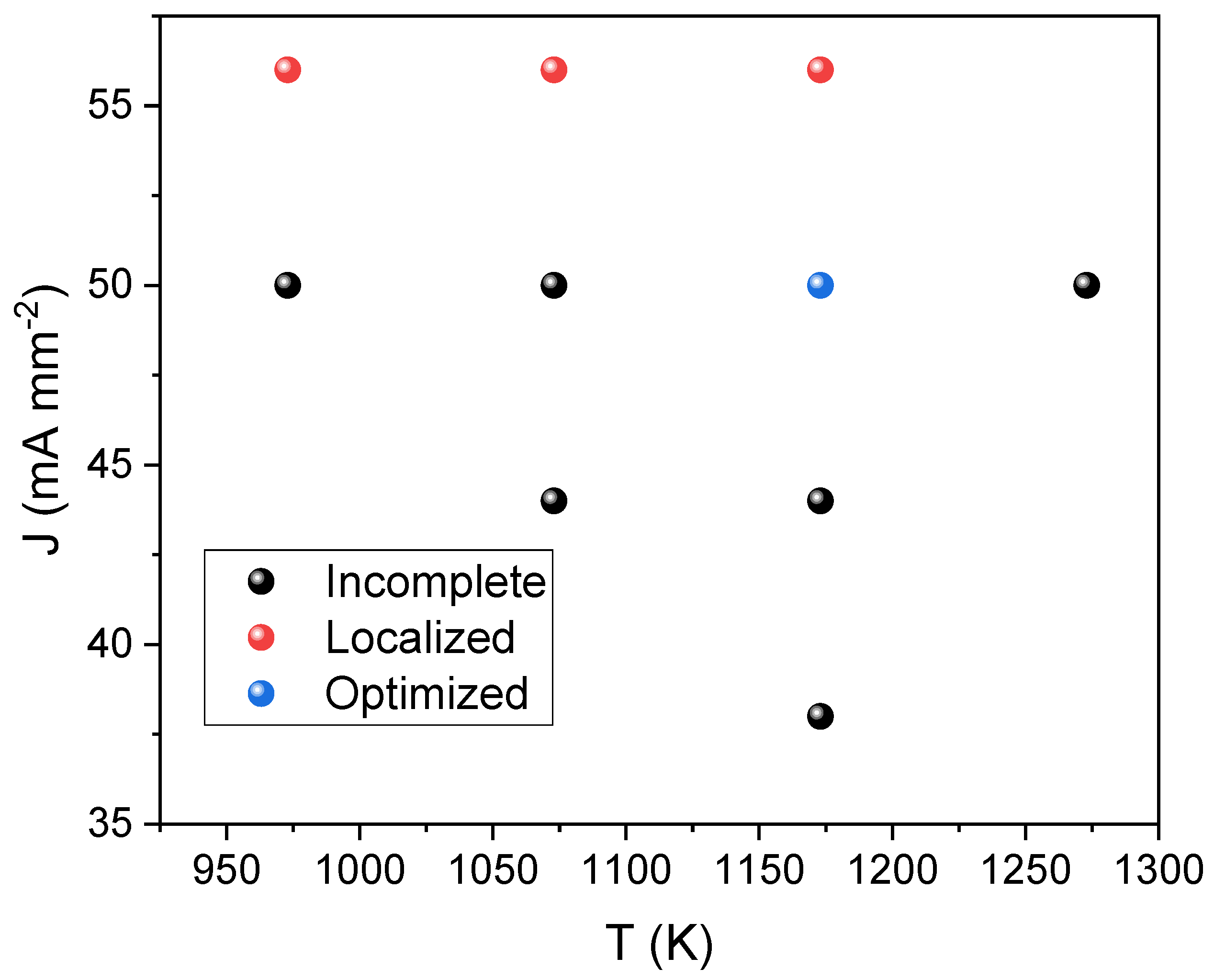
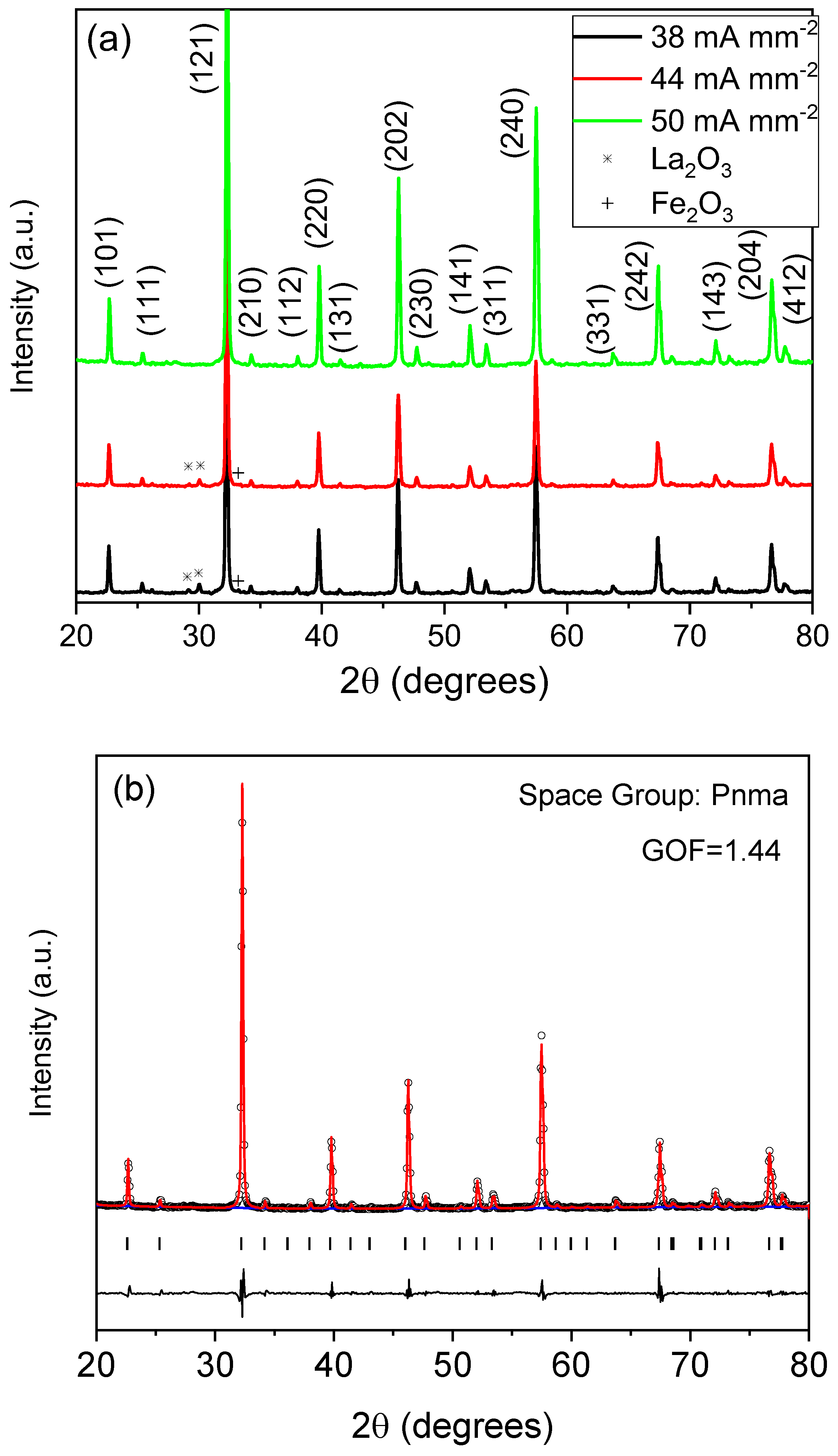
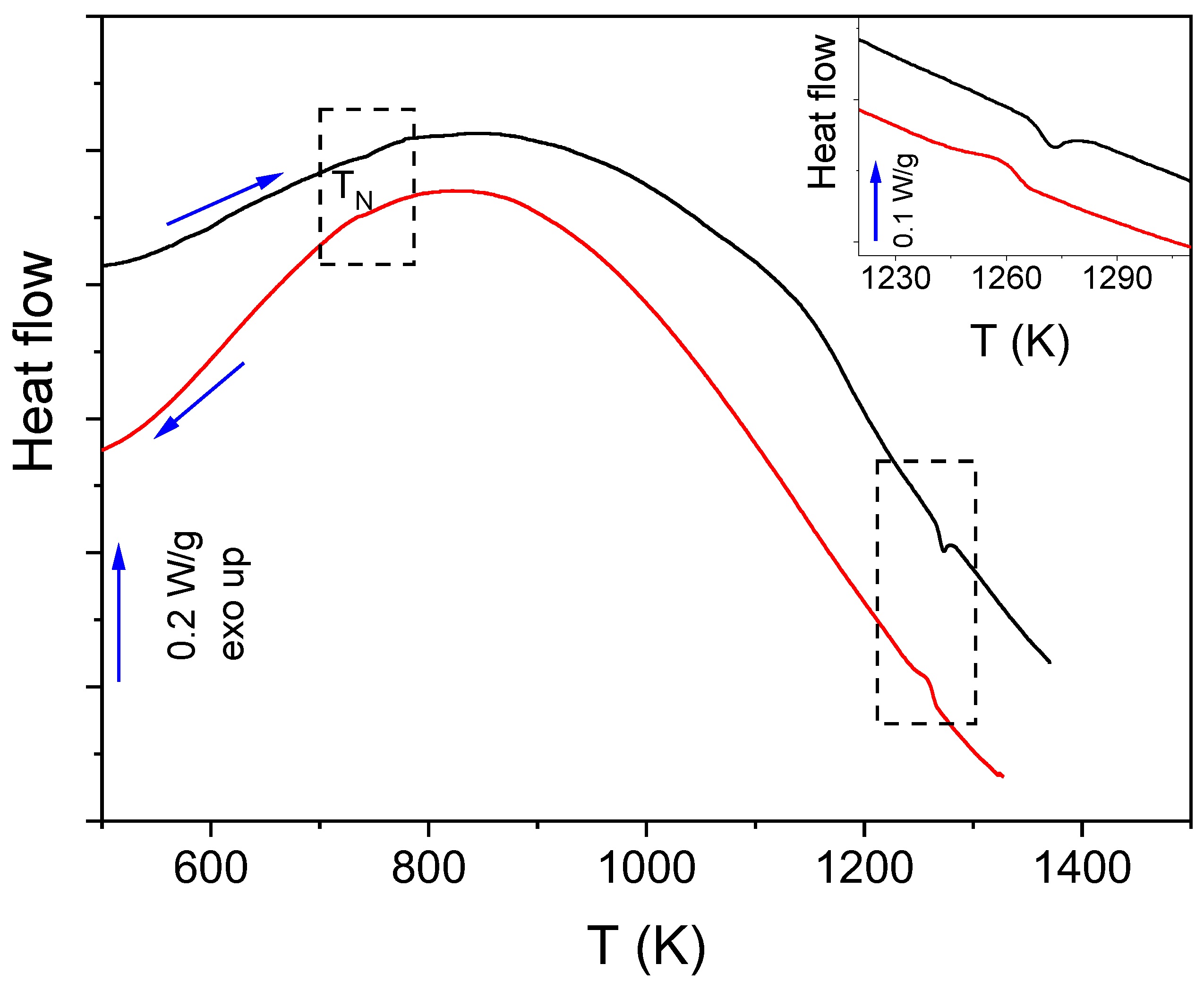


| Raman Modes | Raman Shift (cm−1) |
|---|---|
| B1g(5) | 166 |
| Ag(5) | 188 |
| Ag(4) | 274 |
| 301 | |
| 334 | |
| B1g(3) | 438 |
| B3g(3) | 486 |
| Ag(3) | 515 |
| B1g | 631 |
| Second order scattering | 1133 |
| 1305 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manchón-Gordón, A.F.; Sánchez-Jiménez, P.E.; Blázquez, J.S.; Perejón, A.; Pérez-Maqueda, L.A. Structural, Vibrational, and Magnetic Characterization of Orthoferrite LaFeO3 Ceramic Prepared by Reaction Flash Sintering. Materials 2023, 16, 1019. https://doi.org/10.3390/ma16031019
Manchón-Gordón AF, Sánchez-Jiménez PE, Blázquez JS, Perejón A, Pérez-Maqueda LA. Structural, Vibrational, and Magnetic Characterization of Orthoferrite LaFeO3 Ceramic Prepared by Reaction Flash Sintering. Materials. 2023; 16(3):1019. https://doi.org/10.3390/ma16031019
Chicago/Turabian StyleManchón-Gordón, Alejandro F., Pedro E. Sánchez-Jiménez, Javier S. Blázquez, Antonio Perejón, and Luis A. Pérez-Maqueda. 2023. "Structural, Vibrational, and Magnetic Characterization of Orthoferrite LaFeO3 Ceramic Prepared by Reaction Flash Sintering" Materials 16, no. 3: 1019. https://doi.org/10.3390/ma16031019
APA StyleManchón-Gordón, A. F., Sánchez-Jiménez, P. E., Blázquez, J. S., Perejón, A., & Pérez-Maqueda, L. A. (2023). Structural, Vibrational, and Magnetic Characterization of Orthoferrite LaFeO3 Ceramic Prepared by Reaction Flash Sintering. Materials, 16(3), 1019. https://doi.org/10.3390/ma16031019










