Biosynthesis and Antimicrobial Evaluation of Zinc Oxide Nanoparticles Using Chlorella vulgaris Biomass against Multidrug-Resistant Pathogens
Abstract
:1. Introduction
2. Material and Methods
2.1. C. vulgaris Culturing Conditions
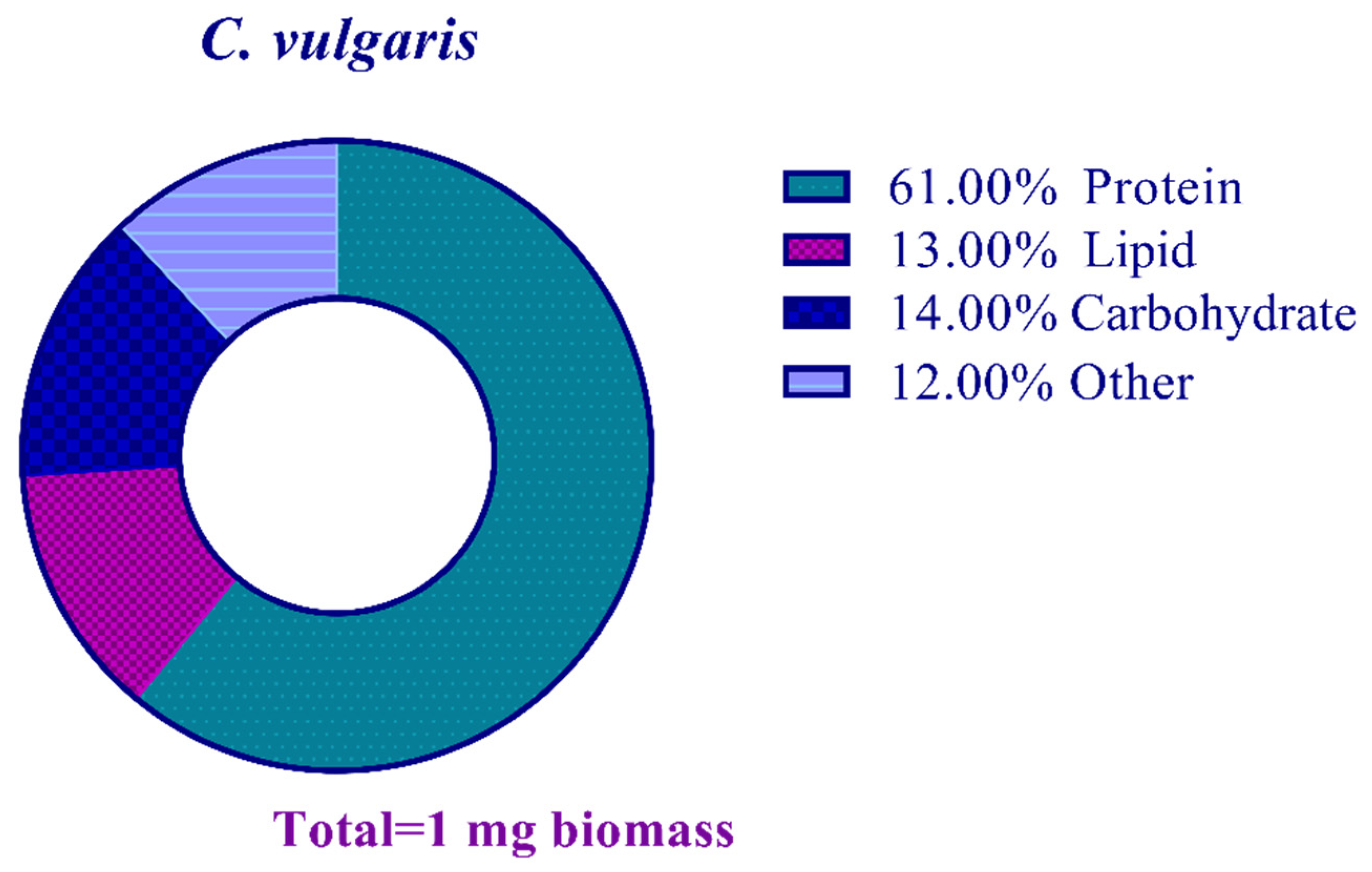
2.2. Biomass Composition of C. vulgaris
2.2.1. Protein Content
2.2.2. Lipid Content
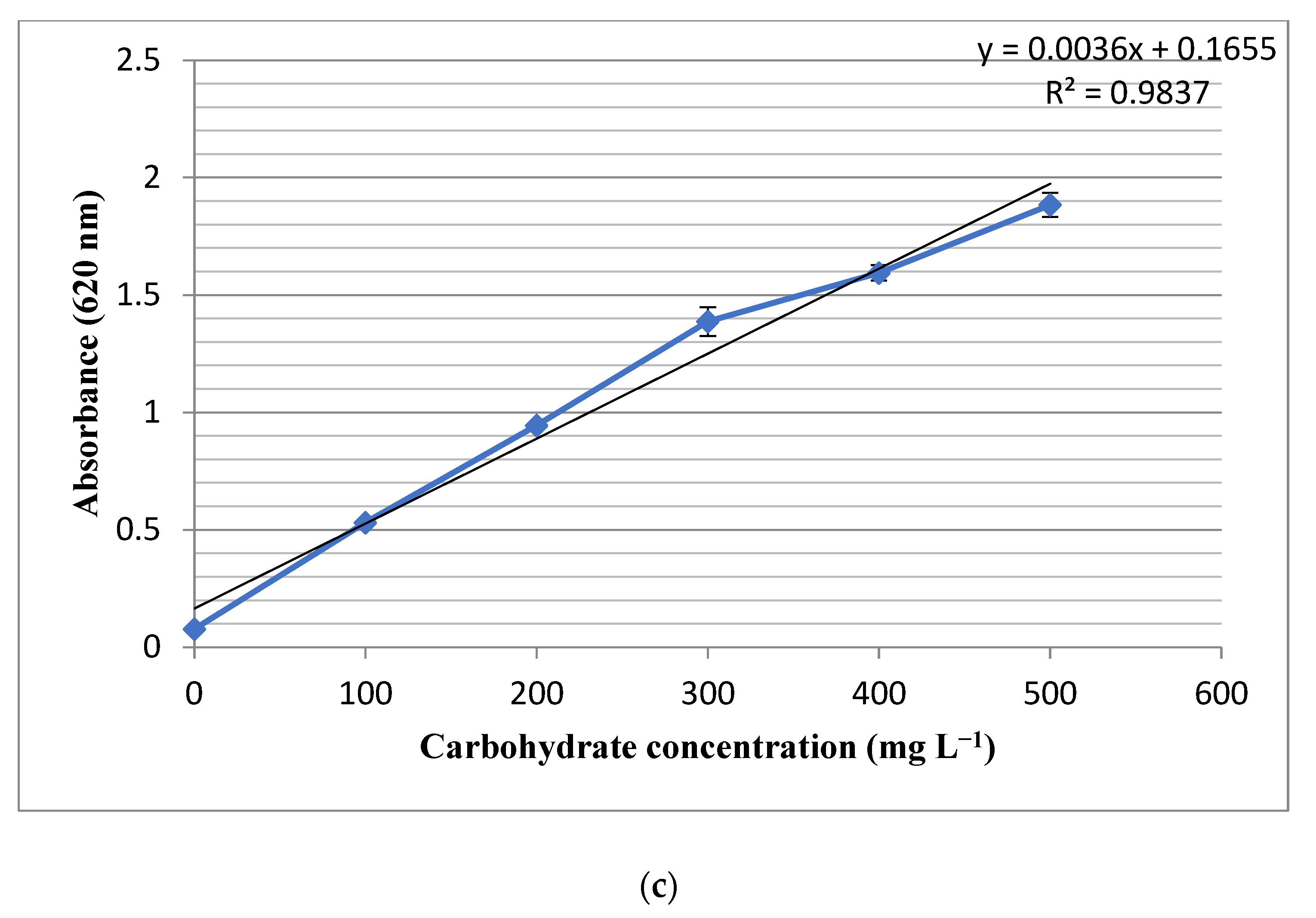
2.2.3. Carbohydrate Content
2.3. Synthesis of ZnO Nanorods
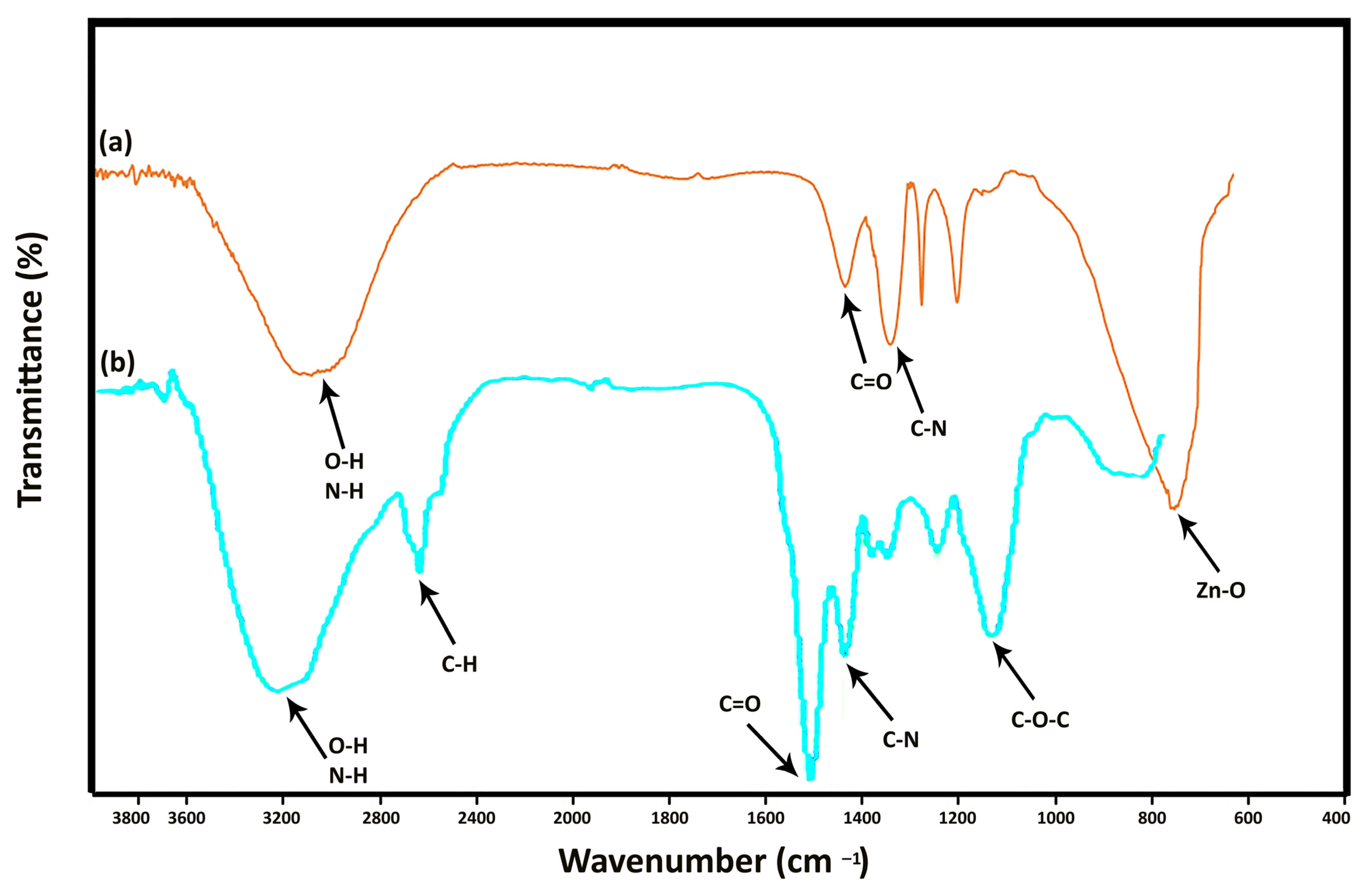
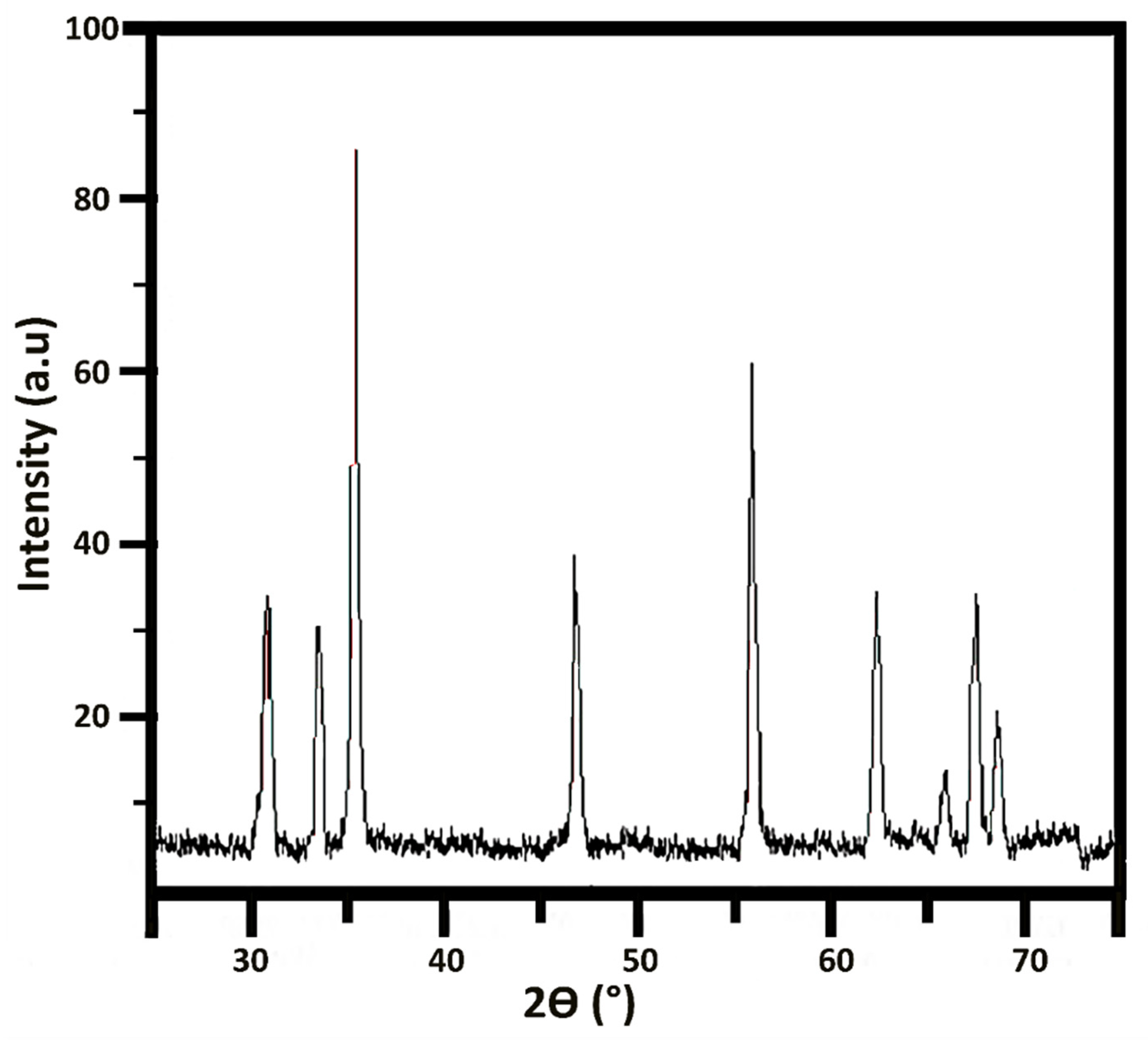
2.4. Characterization of ZnONP
2.5. Antimicrobial Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Growth Measure
3.2. Composition Analysis of Biomass
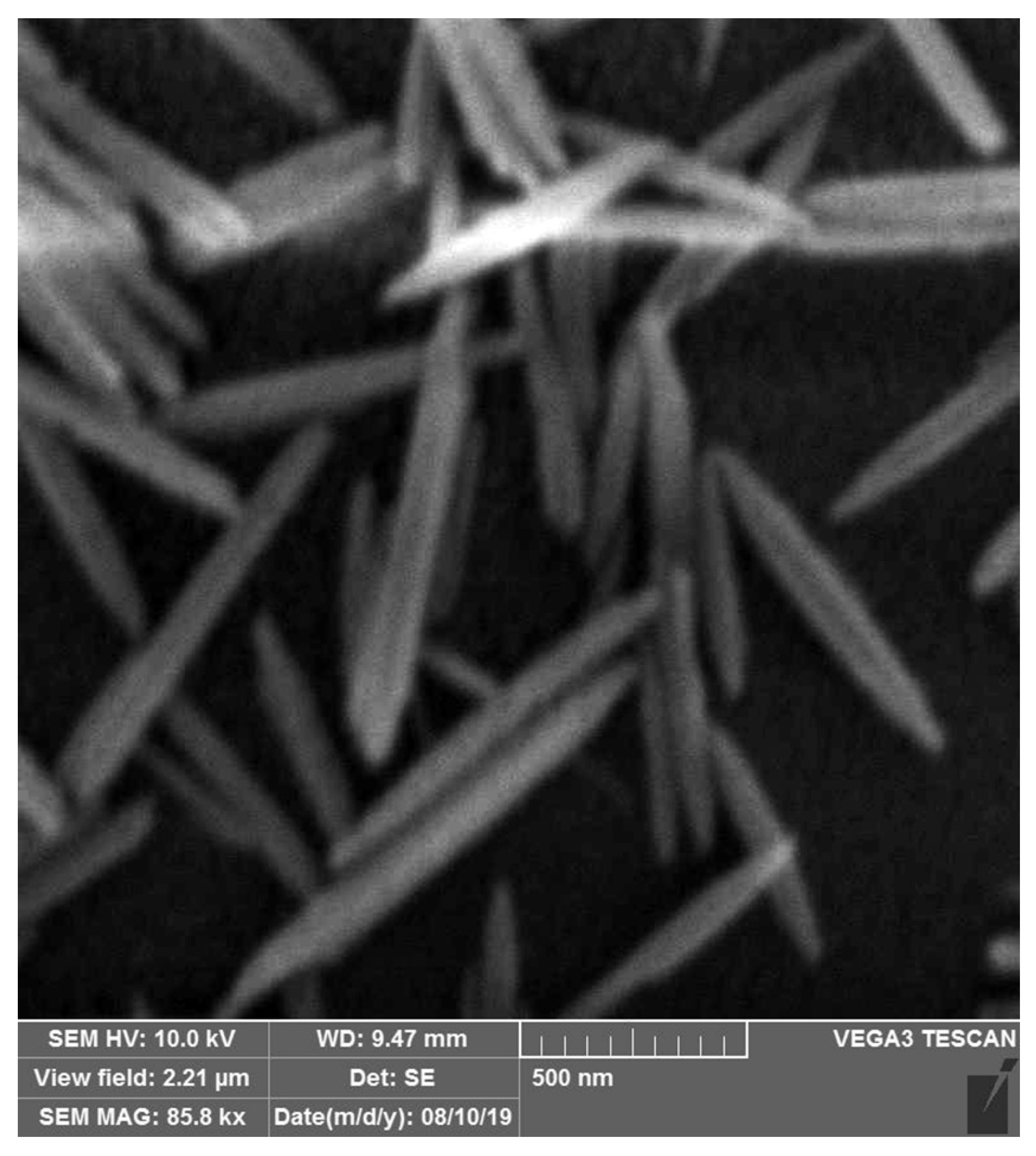
3.3. Characterization of ZnONP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Declaration of Competing Interest
Acknowledgments
Conflicts of Interest
Ethical Approval
Abbreviations
References
- Elsawy, S.; Elsherif, W.M.; Hamed, R. Effect of silver nanoparticles on vancomycin resistant Staphylococcus aureus infection in critically ill patients. Pathog. Glob. Health 2021, 115, 315–324. [Google Scholar] [CrossRef]
- Abootalebi, S.N.; Mousavi, S.M.; Hashemi, S.A.; Shorafa, E.; Omidifar, N.; Gholami, A. Antibacterial effects of green-synthesized silver nanoparticles using Ferula asafoetida against Acinetobacter baumannii isolated from the hospital environment and assessment of their cytotoxicity on the human cell lines. J. Nanomater. 2021, 2021, 6676555. [Google Scholar] [CrossRef]
- Johnston, K.J.; Thorpe, K.E.; Jacob, J.T.; Murphy, D.J. The incremental cost of infections associated with multidrug-resistant organisms in the inpatient hospital setting-A national estimate. Health Serv. Res. 2019, 54, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Kim, K.-S. Nanomaterials as Delivery Vehicles and Components of New Strategies to Combat Bacterial Infections: Advantages and Limitations. Microorganisms 2019, 7, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi, S.M.; Behbudi, G.; Gholami, A.; Hashemi, S.A.; Nejad, Z.M.; Bahrani, S.; Chiang, W.-H.; Wei, L.C.; Omidifar, N. Shape-controlled synthesis of zinc nanostructures mediating macromolecules for biomedical applications. Biomater. Res. 2022, 26, 4. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Metal oxide nanoparticles as biomedical materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef]
- Roy, R.; Kumar, D.; Sharma, A.; Gupta, P.; Chaudhari, B.P.; Tripathi, A.; Das, M.; Dwivedi, P.D. ZnO nanoparticles induced adjuvant effect via toll-like receptors and Src signaling in Balb/c mice. Toxicol. Lett. 2014, 230, 421–433. [Google Scholar] [CrossRef]
- Labouta, H.I.; Schneider, M. Interaction of inorganic nanoparticles with the skin barrier: Current status and critical review. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 39–54. [Google Scholar] [CrossRef]
- Wang, C.; Hu, X.; Gao, Y.; Ji, Y. ZnO Nanoparticles Treatment Induces Apoptosis by Increasing Intracellular ROS Levels in LTEP-a-2 Cells. BioMed Res. Int. 2015, 2015, 423287. [Google Scholar] [CrossRef] [Green Version]
- Devi, G.K.; Suruthi, P.; Veerakumar, R.; Vinoth, S.; Subbaiya, R.; Chozhavendhan, S. A Review on Metallic Gold and Silver Nanoparticles. Res. J. Pharm. Technol. 2019, 12, 935–943. [Google Scholar] [CrossRef]
- Taghizadeh, S.-M.; Lal, N.; Ebrahiminezhad, A.; Moeini, F.; Seifan, M.; Ghasemi, Y.; Berenjian, A. Green and Economic Fabrication of Zinc Oxide (ZnO) Nanorods as a Broadband UV Blocker and Antimicrobial Agent. Nanomaterials 2020, 10, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Lee, J.Y.; Wang, D.I.C.; Ting, Y.P. Silver Nanoplates: From Biological to Biomimetic Synthesis. ACS Nano 2007, 1, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Chen, M.; Gui, J.; Huang, S.; Liu, Y.; Shentu, H.; He, J.; Fang, Z.; Wang, W.; Zhang, Y. Preparation of Chlorella vulgaris polysaccharides and their antioxidant activity in vitro and in vivo. Int. J. Biol. Macromol. 2019, 137, 139–150. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Hussein, M.H.; Shaaban-Dessuuki, S.A.; Dalal, S.R. Production, extraction and characterization of Chlorella vulgaris soluble polysaccharides and their applications in AgNPs biosynthesis and biostimulation of plant growth. Sci. Rep. 2020, 10, 3011. [Google Scholar] [CrossRef] [Green Version]
- Barbarino, E.; Lourenço, S.O. An evaluation of methods for extraction and quantification of protein from marine macro- and microalgae. J. Appl. Phycol. 2005, 17, 447–460. [Google Scholar] [CrossRef]
- Van Wychen, S.; Long, W.; Black, S.K.; Laurens, L.M.L. MBTH: A novel approach to rapid, spectrophotometric quantitation of total algal carbohydrates. Anal. Biochem. 2017, 518, 90–93. [Google Scholar] [CrossRef] [Green Version]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Kahzad, N.; Salehzadeh, A. Green synthesis of CuFe2O4@ Ag nanocomposite using the Chlorella vulgaris and evaluation of its effect on the expression of norA efflux pump gene among Staphylococcus aureus strains. Biol. Trace Elem. Res. 2020, 198, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, J.; Nallamuthu, T. Characterization of biosynthesized gold nanoparticles from aqueous extract of Chlorella vulgaris and their anti-pathogenic properties. Appl. Nanosci. 2015, 5, 603–607. [Google Scholar] [CrossRef] [Green Version]
- Borzouyan Dastjerdi, M.; Amini, A.; Nazari, M.; Cheng, C.; Benson, V.; Gholami, A.; Ghasemi, Y. Novel versatile 3D bio-scaffold made of natural biocompatible hagfish exudate for tissue growth and organoid modeling. Int. J. Biol. Macromol. 2020, 158, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, W.; Su, K.; Zhang, R.; Han, H.; Deng, Y.; Luo, S. Green synthesis of ZnO nano particles using chlorella vulgaris extract as additives. In IOP Conference Series: Materials Science and Engineering; Zhang, X., Li, W., Su, K., Zhang, R., Han, H., Deng, Y., Luo, S., Eds.; IOP Publishing: Bristol, UK, 2019. [Google Scholar]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The Effect of Charge at the Surface of Silver Nanoparticles on Antimicrobial Activity against Gram-Positive and Gram-Negative Bacteria: A Preliminary Study. J. Nanomater. 2015, 2015, 53. [Google Scholar] [CrossRef]
- Nabavizadeh, M.; Abbaszadegan, A.; Gholami, A.; Kadkhoda, Z.; Mirhadi, H.; Ghasemi, Y.; Safari, A.; Hemmateenejad, B.; Dorostkar, S.; Sharghi, H. Antibiofilm Efficacy of Positively Charged Imidazolium-Based Silver Nanoparticles in Enterococcus faecalis Using Quantitative Real-Time PCR. Jundishapur J. Microbiol. 2017, 10, e55616. [Google Scholar] [CrossRef] [Green Version]
- Gholami, A.; Ebrahiminezhad, A.; Abootalebi, N.; Ghasemi, Y. Synergistic Evaluation of Functionalized Magnetic Nanoparticles and Antibiotics against Staphylococcus aureus and Escherichia coli. Pharm. Nanotechnol. 2018, 6, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Zargarnezhad, S.; Gholami, A.; Khoshneviszadeh, M.; Abootalebi, S.N.; Ghasemi, Y. Antimicrobial Activity of Isoniazid in Conjugation with Surface-Modified Magnetic Nanoparticles against Mycobacterium tuberculosis and Nonmycobacterial Microorganisms. J. Nanomater. 2020, 2020, 7372531. [Google Scholar] [CrossRef]
- Gholami, A.; Mohammadi, F.; Ghasemi, Y.; Omidifar, N.; Ebrahiminezhad, A. Antibacterial activity of SPIONs versus ferrous and ferric ions under aerobic and anaerobic conditions: A preliminary mechanism study. IET Nanobiotechnol. 2020, 14, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Gholami, A.; Rasoul-Amini, S.; Ebrahiminezhad, A.; Seradj, S.H.; Ghasemi, Y. Lipoamino Acid Coated Superparamagnetic Iron Oxide Nanoparticles Concentration and Time Dependently Enhanced Growth of Human Hepatocarcinoma Cell Line (Hep-G2). J. Nanomater. 2015, 2015, 150. [Google Scholar] [CrossRef] [Green Version]
- Emami-Karvani, Z.; Chehrazi, P. Antibacterial activity of ZnO nanoparticle on gram-positive and gram-negative bacteria. Afr. J. Microbiol. Res. 2011, 5, 1368–1373. [Google Scholar]
- Umamageswari, S.; Manipriya, B.; Kalyani, M. Evaluation of antibacterial activity of zinc oxide nanoparticles against biofilm producing methicillin resistant Staphylococcus aureus (MRSA). Res. J. Pharm. Technol. 2018, 11, 1884–1888. [Google Scholar] [CrossRef]
- Aswathanarayan, B.J.; Vittal, R.R. Antimicrobial, biofilm inhibitory and anti-infective activity of metallic nanoparticles against pathogens MRSA and Pseudomonas aeruginosa PA01. Pharm. Nanotechnol. 2017, 5, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Bahadur, D. Defect-mediated reactive oxygen species generation in Mg-substituted ZnO nanoparticles: Efficient nanomaterials for bacterial inhibition and cancer therapy. ACS Omega 2018, 3, 2956–2965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Ebrahiminezhad, A.; Moeeni, F.; Taghizadeh, S.-M.; Seifan, M.; Bautista, C.; Novin, D.; Ghasemi, Y.; Berenjian, A. Xanthan gum capped ZnO microstars as a promising dietary zinc supplementation. Foods 2019, 8, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Kadiyala, U.; Turali-Emre, E.S.; Bahng, J.H.; Kotov, N.A.; VanEpps, J.S. Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus (MRSA). Nanoscale 2018, 10, 4927–4939. [Google Scholar] [CrossRef]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamo, G.; Fierli, D.; Romancino, D.P.; Picciotto, S.; Barone, M.E.; Aranyos, A.; Božič, D.; Morsbach, S.; Raccosta, S.; Stanly, C.; et al. Nanoalgosomes: Introducing extracellular vesicles produced by microalgae. J. Extracell. Vesicles 2021, 10, e12081. [Google Scholar] [CrossRef] [PubMed]
- Picciotto, S.; Barone, M.E.; Fierli, D.; Aranyos, A.; Adamo, G.; Božič, D.; Romancino, D.P.; Stanly, C.; Parkes, R.; Morsbach, S.; et al. Isolation of extracellular vesicles from microalgae: Towards the production of sustainable and natural nanocarriers of bioactive compounds. Biomater. Sci. 2021, 9, 2917–2930. [Google Scholar] [CrossRef]
- Asadi, K.; Gholami, A. Virosome-based nanovaccines; a promising bioinspiration and biomimetic approach for preventing viral diseases: A review. Int. J. Biol. Macromol. 2021, 182, 648–658. [Google Scholar] [CrossRef]





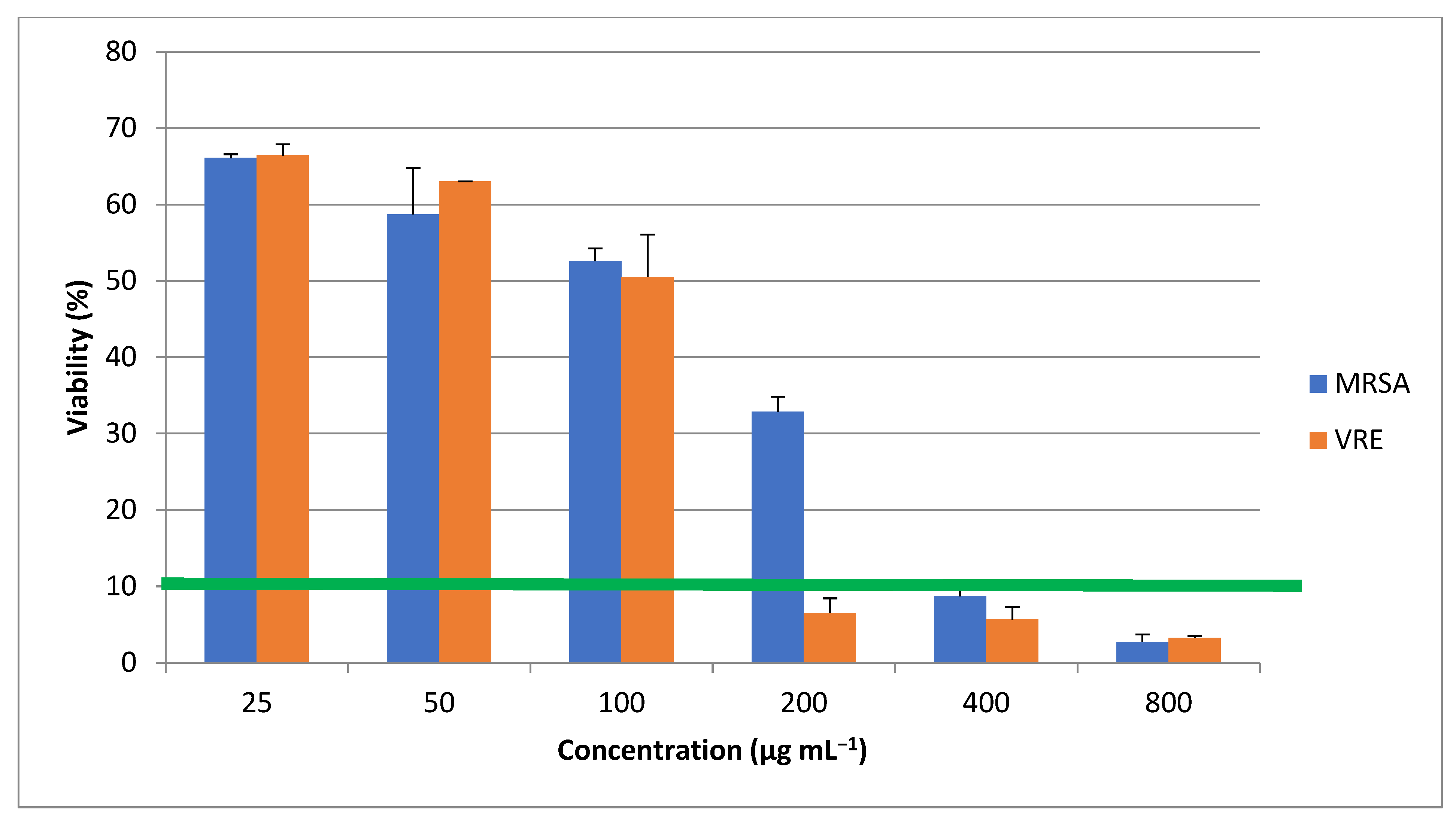
| MRSA | VRE | |
|---|---|---|
| ZnONP (µg mL−1) | 400 | 200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morowvat, M.H.; Kazemi, K.; Jaberi, M.A.; Amini, A.; Gholami, A. Biosynthesis and Antimicrobial Evaluation of Zinc Oxide Nanoparticles Using Chlorella vulgaris Biomass against Multidrug-Resistant Pathogens. Materials 2023, 16, 842. https://doi.org/10.3390/ma16020842
Morowvat MH, Kazemi K, Jaberi MA, Amini A, Gholami A. Biosynthesis and Antimicrobial Evaluation of Zinc Oxide Nanoparticles Using Chlorella vulgaris Biomass against Multidrug-Resistant Pathogens. Materials. 2023; 16(2):842. https://doi.org/10.3390/ma16020842
Chicago/Turabian StyleMorowvat, Mohammad Hossein, Kimia Kazemi, Maral Ansari Jaberi, Abbas Amini, and Ahmad Gholami. 2023. "Biosynthesis and Antimicrobial Evaluation of Zinc Oxide Nanoparticles Using Chlorella vulgaris Biomass against Multidrug-Resistant Pathogens" Materials 16, no. 2: 842. https://doi.org/10.3390/ma16020842







