Vanadium Nitride Nanoparticles Grown on Carbon Fiber Cloth as an Advanced Binder-Free Anode for the Storage of Sodium and Potassium Ions
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of VN/Carbon Fiber Cloth and VN Powder
2.2. Characterization Techniques
2.3. Electrochemical Measurements
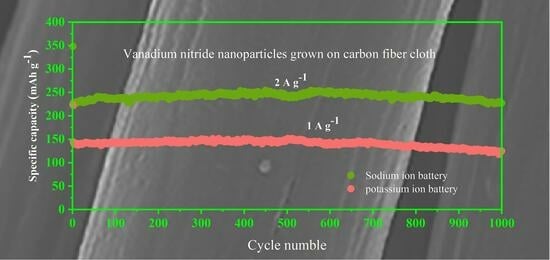
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ipadeola, A.K.; Eid, K.; Abdullah, A.M. Porous transition metal-based nanostructures as efficient cathodes for aluminium-air batteries. Curr. Opin. Electrochem. 2023, 37, 101198. [Google Scholar] [CrossRef]
- Bu, Y.; Wu, Y.; Li, X.; Pei, Y. Operational risk analysis of a containerized lithium-ion battery energy storage system based on STPA and fuzzy evaluation. Process Saf. Environ. Prot. 2023, 176, 627–640. [Google Scholar] [CrossRef]
- Abu, S.M.; Hannan, M.A.; Lipu, M.S.H.; Mannan, M.; Ker, P.J.; Hossain, M.J.; Mahlia, T.M.I. State of the art of lithium-ion battery material potentials: An analytical evaluations. issues and future research directions. J. Clean. Prod. 2023, 394, 136246. [Google Scholar] [CrossRef]
- Ji, L.; Lin, Z.; Alcoutlabi, M.; Zhang, X. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, H.; Kumar, G.; Sharma, S.; Aneja, R.; Sharma, A.K.; Kumar, R.; Kumar, P. Progress and challenges in electrochemical energy storage devices: Fabrication, electrode material, and economic aspects. Chem. Eng. J. 2023, 468, 143706. [Google Scholar] [CrossRef]
- Gao, P.; Yuan, P.; Yue, T.; Zhao, X.; Shen, B. Recycling metal resources from various spent batteries to prepare electrode materials for energy storage: A critical review. J. Energy Storage 2023, 68, 107652. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From lithium-ion to sodium-ion batteries: Advantages, challenges, and surprises. Angew. Chem. 2018, 57, 101–120. [Google Scholar] [CrossRef]
- Kim, S.W.; Seo, D.H.; Ma, X.H.; Ceder, G.; Kang, K. Electrode Materials for Rechargeable Sodium-Ion Batteries: Potential Alternatives to Current Lithium-Ion Batteries. Adv. Energy. Mater. 2012, 2, 710–721. [Google Scholar] [CrossRef]
- Myung, J.Y.; Myung, S.T.; Sun, Y.K. Recent progress in rechargeable potassium batteries. Adv. Funct. Mater. 2018, 28, 1802938. [Google Scholar]
- Lei, Y.; Zhang, J.; Chen, X.; Min, W.; Wang, R.; Yan, M.; Xu, J. From spent lithium-ion batteries to high performance sodium-ion batteries: A case study. Mater. Today Energy 2022, 26, 100997. [Google Scholar] [CrossRef]
- Huang, Z.; Gu, Z.; Heng, Y.; Ang, E.H.; Geng, H.; Wu, X. Advanced layered oxide cathodes for sodium/potassium-ion batteries: Development, challenges and prospects. Chem. Eng. J. 2023, 452, 139438. [Google Scholar] [CrossRef]
- Kei, K.; Mouad, D.; Tomooki, H.; Shinichi, K.; Shinichi, K. Towards K-ion and Na-ion batteries as “beyond Li-ion”. Chem. Rec. 2018, 18, 459–479. [Google Scholar]
- Liu, Q.; Hu, Z.; Li, W.; Zou, C.; Jin, H.; Wang, S.; Chou, S.; Dou, S.X. Sodium transition metal oxides: The preferred cathode choice for future sodium-ion batteries? Energy Environ. Sci. 2021, 14, 157–179. [Google Scholar] [CrossRef]
- Liu, S. Na2Ru0.8Mn0.2O3: A novel cathode material for ultrafast sodium ion battery with large capacity and superlong cycle life. J. Power Sources 2019, 1, 14–22. [Google Scholar] [CrossRef]
- Cao, Y.; He, Y.; Gang, H.; Wu, B.; Yan, L.; Wei, D.; Wang, H. Stability study of transition metal oxide electrode materials. J. Power Sources 2023, 560, 232710. [Google Scholar] [CrossRef]
- Pan, J.; Li, C.; Peng, Y.; Wang, L.; Li, B.; Zheng, G.; Song, M. Application of transition metal (Ni, Co and Zn) oxides based electrode materials for ion-batteries and supercapacitors. Int. J. Electrochem. Sci. 2023, 18, 100233. [Google Scholar] [CrossRef]
- Dong, S.; Chen, X.; Gu, L.; Zhou, X.; Xu, H.; Wang, H.; Liu, Z.; Han, P.; Yao, J.; Wang, L.; et al. Facile preparation of mesoporous titanium nitride microspheres for electrochemical energy storage. ACS Appl. Mater. Interfaces 2011, 3, 93–98. [Google Scholar] [CrossRef]
- Yang, H.; Xu, R.; Yao, Y.; Zhou, X.F.; Yu, Y. Multicore–Shell Bi@N-doped Carbon Nanospheres for High Power Density and Long Cycle Life Sodium- and Potassium-Ion Anodes. Adv. Funct. Mater. 2019, 29, 1809195. [Google Scholar] [CrossRef]
- Wu, H.; Yu, Q.; Lao, C.Y.; Qin, M.; Wang, W.A.; Liu, Z.; Man, C.; Wang, L.; Jia, B.; Qu, X. Scalable synthesis of VN quantum dots encapsulated in ultralarge pillared N-doped mesoporous carbon microsheets for superior potassium storage. Energy Storage Mater. 2019, 18, 43–50. [Google Scholar] [CrossRef]
- Wang, J.; Yue, X.; Xie, Z.; Abudula, A.; Guan, G. MOFs-derived transition metal sulfide composites for advanced sodium ion batteries. Energy Storage Mater. 2021, 41, 404–426. [Google Scholar] [CrossRef]
- Lim, Y.V.; Li, X.L.; Yang, H.Y. Recent Tactics and Advances in the application of metal sulfides as high-performance anode materials for rechargeable sodium-ion batteries. Adv. Funct. Mater. 2021, 31, 2006761. [Google Scholar] [CrossRef]
- Xie, J.M.; Zhuang, R.; Du, Y.X.; Pei, Y.W.; Tan, D.M.; Xu, F. Advances in sulfur-doped carbon materials for use as anodes in sodium-ion batteries. New Carbon Mater. 2023, 38, 305–316. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Y.; Huang, H.; Wang, W.; Zhang, J.; Yu, Q. Development of electrode materials for flexible potassium-ion batteries. Compos. Part B Eng. 2023, 258, 110712. [Google Scholar] [CrossRef]
- Huang, Y.; Haider, R.; Xu, S.; Liu, K.; Ma, Z.-F.; Yuan, X. Recent Progress of Novel Non-Carbon Anode Materials for Potassium-Ion Battery. Energy Storage Mater. 2022, 51, 327–360. [Google Scholar] [CrossRef]
- Xu, L.; Chen, X.; Guo, W.; Zeng, L.; Yang, T.; Xiong, P.; Chen, Q.; Zhang, J.; Wei, M.; Qian, Q. Co-construction of sulfur vacancies and carbon confinement in V5S8/CNFs to induce an ultra-stable performance for half/full sodium-ion and potassium-ion batteries. Nanoscale 2021, 13, 5033–5044. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Guo, W.; Zeng, L.; Xia, X.; Wang, Y.; Xiong, P.; Chen, Q.; Zhang, J.; Wei, M.; Qian, Q. V3Se4 embedded within N/P co-doped carbon fibers for sodium/potassium ion batteries. Chem. Eng. J. 2021, 419, 129607. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.C.; Bianchini, M.; Seo, D.H.; Rodriguez-Garcia, J.; Ceder, G. Recent progress and perspective in electrode materials for K-ion batteries. Adv. Energy Mater. 2017, 8, 1702384. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X.; Wu, D.; Zhao, X.; Zhou, Z. S-doped N-rich carbon nanosheets with expanded interlayer distance as anode materials for sodium-ion batteries. Adv. Mater. 2017, 29, 1604108. [Google Scholar] [CrossRef]
- Wang, W.; Bao, J.Z.; Sun, C.F. Liquid-phase exfoliated WS2-graphene composite anodes for potassium-ion batteries. Chin. J. Struct. Chem. 2020, 39, 493–499. [Google Scholar]
- Liu, R.; Yang, L.; Wang, W.; Zhao, E.; Wang, B.; Zhang, X.; Liu, H.; Zeng, C. Surface redox pseudocapacitance-based vanadium nitride nanoparticles toward a long-cycling sodium-ion battery. Mater. Today Energy 2023, 34, 101300. [Google Scholar] [CrossRef]
- Peng, Q.; Rehman, J.; Eid, K.; Alofi, A.S.; Laref, A.; Albaqami, M.D.; Alotabi, R.G.; Shibl, M.F. Vanadium Carbide (V4C3) MXene as an Efficient Anode for Li-Ion and Na-Ion Batteries. Nanomaterials 2022, 12, 2825. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Hu, X.; Chen, J.X.; Liu, Y.J.; Huang, T.Z.; Wen, Z.H. In situ formation of vanadium nitride quantum dots on N- doped carbon hollow spheres for superior lithium and sodium storage. J. Mater. Chem. A 2019, 7, 9289–9296. [Google Scholar] [CrossRef]
- Cheng, H.; García-Aráez, N.; Hector, A.L. Synthesis of vanadium nitride–hard carbon composites from cellulose and their performance for sodium-ion batteries. ACS Appl. Energy Mater. 2020, 3, 4286–4294. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.A.; Lu, S.; Xiang, Y. Carbon anode materials: A detailed comparison between Na-ion and K-ion batteries. Adv. Energy Mater. 2021, 11, 2003640. [Google Scholar] [CrossRef]
- Chao, D.; Zhu, C.; Yang, P.; Xia, X.; Liu, J.; Wang, J.; Fan, X.; Savilov, S.V.; Lin, J.; Fan, H.J.; et al. Array of nanosheets render ultrafast and high-capacity Na-ion storage by tunable pseudocapacitance. Nat. Commun. 2016, 7, 12122. [Google Scholar] [CrossRef]
- Cao, D.; Kang, W.; Wang, W.; Sun, K.; Wang, Y.; Ma, P.; Sun, D. Okra-Like Fe7S8/C@ZnS/N-C@C with Core–Double-Shelled Structures as Robust and High-Rate Sodium Anode. Small 2020, 16, 1907641. [Google Scholar] [CrossRef]
- Xu, L.; Xiong, P.; Zeng, L.; Liu, R.; Liu, J.; Luo, F.; Li, X.; Chen, Q.; Wei, M.; Qian, Q. Facile fabrication of a vanadium nitride/carbon fiber composite for half/full sodium-ion and potassium-ion batteries with long-term cycling performance. Nanoscale 2020, 12, 10693–10702. [Google Scholar] [CrossRef]
- Zeng, F.; Lu, T.; He, W.; Chu, S.; Qu, Y.; Pan, Y. In situ carbon encapsulation of ultrafine VN in yolk-shell nanospheres for highly reversible sodium storage. Carbon 2021, 175, 289–298. [Google Scholar] [CrossRef]
- Cheng, Q.; Deng, Q.; Zhong, W.; Tan, T.; Liu, X.; Chen, C.; Hu, J.; Lin, Z.; Huang, K.; Yang, C. Criticality of solid electrolyte interphase in achieving high performance of sodium-ion batteries. Chem. Eng. J. 2023, 457, 141097. [Google Scholar] [CrossRef]
- Liang, Y.; Song, N.; Zhang, Z.; Chen, W.; Feng, J.; Xi, B.; Xiong, S. Integrating Bi@C Nanospheres in Porous Hard Carbon Frameworks for Ultrafast Sodium Storage. Adv. Mater. 2022, 34, 2202673. [Google Scholar] [CrossRef]
- Chen, H.; Sun, N.; Zhu, Q.; Soomro, R.A.; Xu, B. Microcrystalline Hybridization Enhanced Coal-Based Carbon Anode for Advanced Sodium-Ion Batteries. Adv. Sci. 2022, 9, 2200023. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ding, S.; Xu, S.; Ma, Z.-F.; Wang, J.; Yuan, X. Highly effective solid electrolyte interface on SnO2@C enabling stable potassium storage performance. Chem. Eng. J. 2022, 446, 137265. [Google Scholar] [CrossRef]
- Wei, S.; Wang, C.; Chen, S.; Zhang, P.; Zhu, K.; Wu, C.; Song, P.; Wen, W.; Song, L. Dial the Mechanism Switch of VN from Conversion to Intercalation toward Long Cycling Sodium-Ion Battery. Adv. Energy Mater. 2020, 10, 1903712. [Google Scholar] [CrossRef]
- Jin, T.; Li, H.; Li, Y.; Jiao, L.; Chen, J. Intercalation pseudocapacitance in flexible and self-standing V2O3 porous nanofibers for high-rate and ultra-stable K ion storage. Nano Energy 2018, 50, 462–467. [Google Scholar] [CrossRef]
- Cao, L.; Luo, B.; Xu, B.; Zhang, J.; Wang, C.; Xiao, Z.; Li, S.; Li, Y.; Zhang, B.; Zou, G.; et al. Stabilizing Intermediate Phases via Efficient Entrapment Effects of Layered VS4/SnS@C Heterostructure for Ultralong Lifespan Potassium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2103802. [Google Scholar] [CrossRef]
- Niu, X.; Zhang, Y.; Tan, L.; Yang, Z.; Yang, J.; Liu, T.; Zeng, L.; Zhu, Y.; Guo, L. Amorphous FeVO4 as a promising anode material for potassium-ion batteries. Energy Storage Mater. 2019, 22, 160–167. [Google Scholar] [CrossRef]
- Hu, T.; Yang, W.; Wang, C.; Bu, Y.; Jin, F.; Zhang, D.; Gu, M.; Liu, W.; Liang, Q.; Liu, R.; et al. Multilayer Porous Vanadium Nitride Microsheets Anodes for Highly Stable Na-ion Batteries. Chem. Res. Chin. Univ. 2021, 37, 286–292. [Google Scholar] [CrossRef]






| Sample | Fields | Current Density (A g−1) | Cycle Number | Capacity Retention (mAh g−1) |
|---|---|---|---|---|
| VN@CF [30] | SIBs | 0.1 | 500 | 204 |
| VNQD@NC HSs [32] | SIBs | 1 | 1400 | 306 |
| VN/CNFs [37] | SIBs | 2 | 4000 | 237 |
| VN@rGO [43] | SIBs | 1 | 10,000 | 155 |
| VN-QDs/CM [19] | PIBs | 0.1 | 100 | 228 |
| V2O3@PNCNFs [44] | PIBs | 0.05 | 500 | 230 |
| VS4/SnS@C [45] | PIBs | 1 | 6000 | 168.4 |
| FeVO4/C composite [46] | PIBs | 0.3 | 2000 | 250 |
| VN [47] | SIBs | 0.2 | 100 | 156.1 |
| VN/CFC | SIBs | 0.1 | 100 | 368.4 |
| 2 | 1000 | 227.0 | ||
| PIBs | 0.1 | 150 | 204.9 | |
| 1 | 1000 | 125.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Zhang, H.; Yanghe, J.; Yang, J.; Li, W.; Zhao, X.; Liu, S. Vanadium Nitride Nanoparticles Grown on Carbon Fiber Cloth as an Advanced Binder-Free Anode for the Storage of Sodium and Potassium Ions. Materials 2023, 16, 5820. https://doi.org/10.3390/ma16175820
Qin Y, Zhang H, Yanghe J, Yang J, Li W, Zhao X, Liu S. Vanadium Nitride Nanoparticles Grown on Carbon Fiber Cloth as an Advanced Binder-Free Anode for the Storage of Sodium and Potassium Ions. Materials. 2023; 16(17):5820. https://doi.org/10.3390/ma16175820
Chicago/Turabian StyleQin, Yiwei, Haimin Zhang, Jiachen Yanghe, Jing Yang, Wei Li, Xiaojun Zhao, and Sainan Liu. 2023. "Vanadium Nitride Nanoparticles Grown on Carbon Fiber Cloth as an Advanced Binder-Free Anode for the Storage of Sodium and Potassium Ions" Materials 16, no. 17: 5820. https://doi.org/10.3390/ma16175820