The Effects of MgO and Al2O3 Content in Sinter on the Softening–Melting Properties of Mixed Ferrous Burden
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
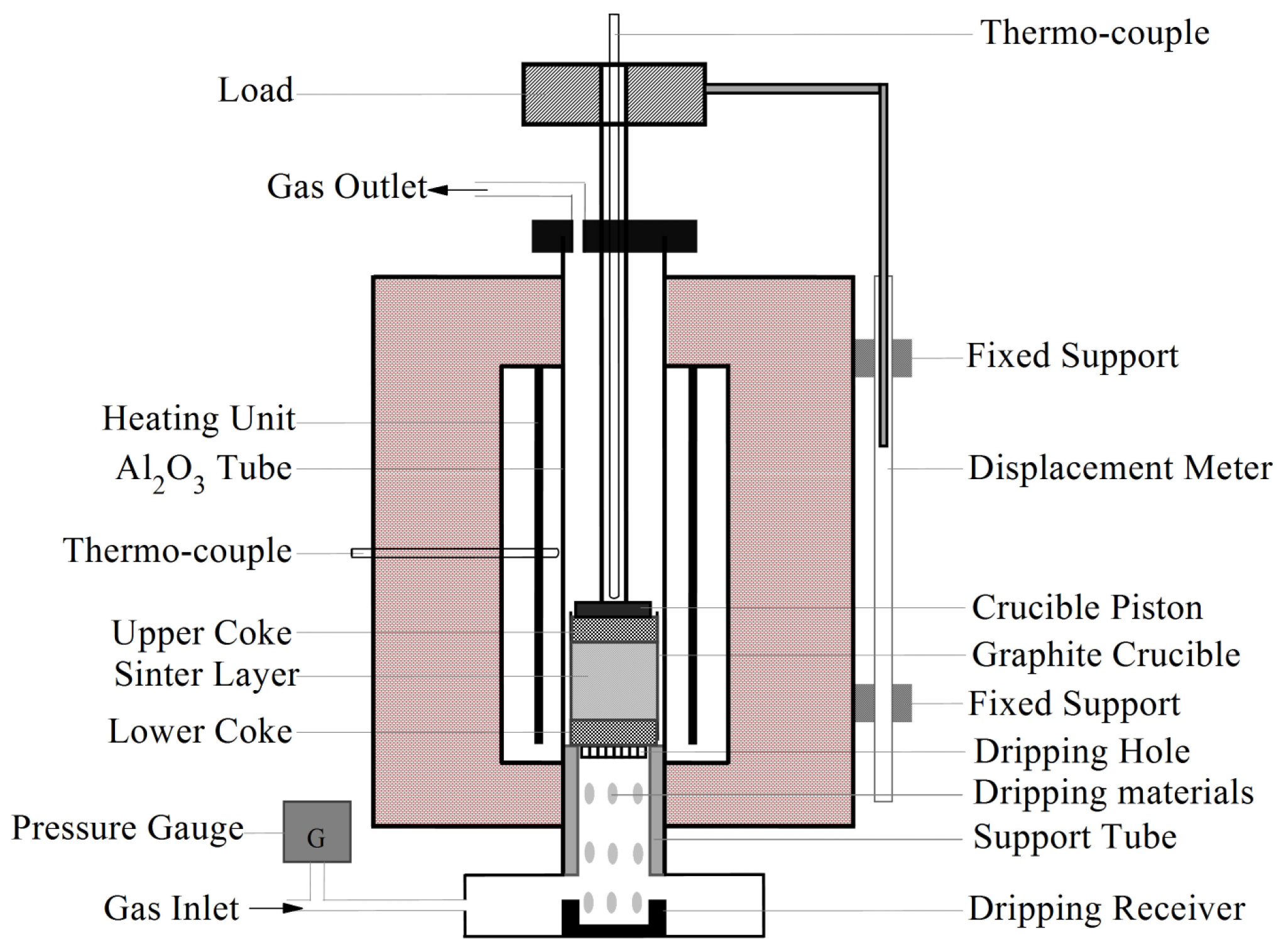
2.2. Experimental Procedure
3. Results and Discussion
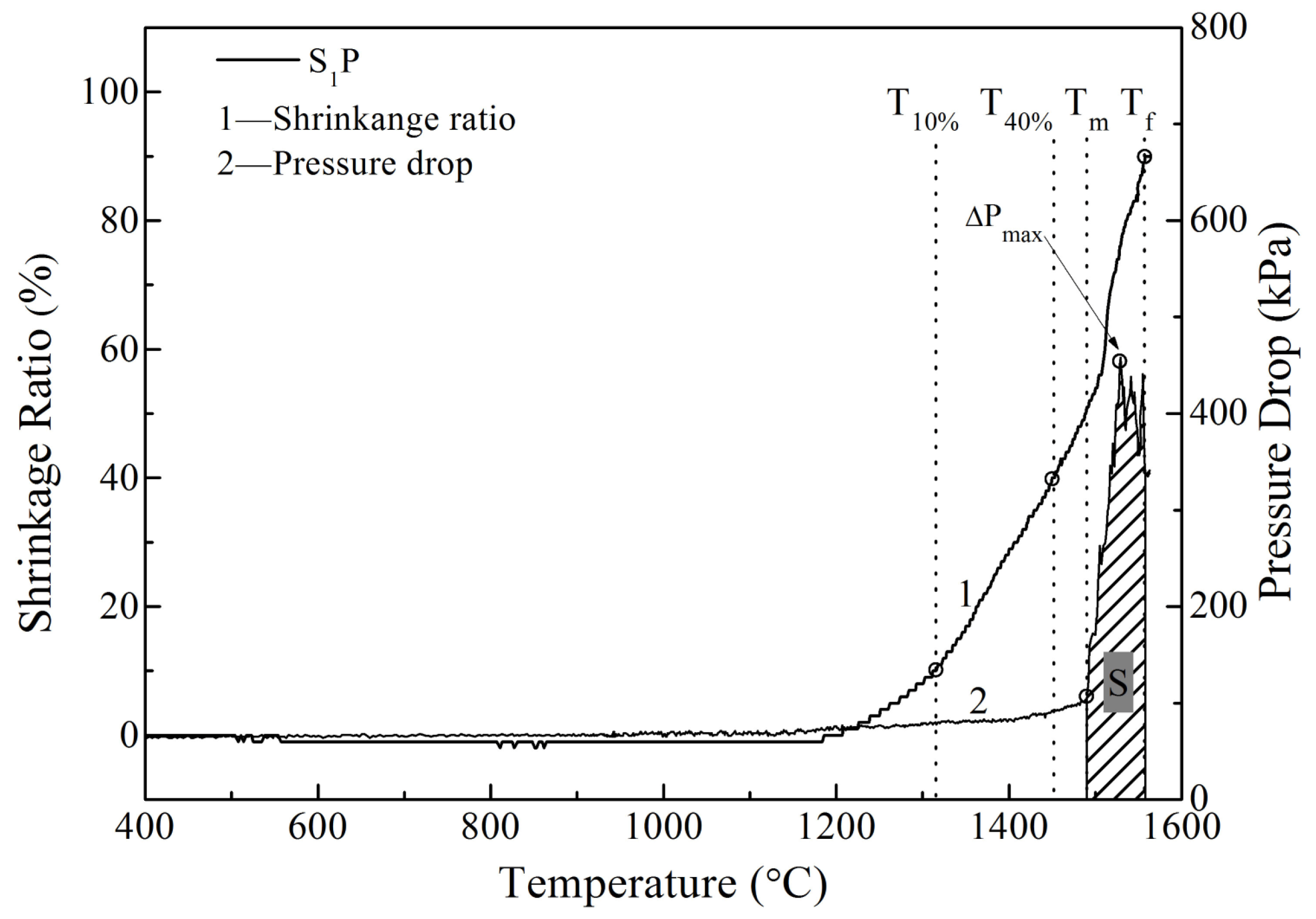
3.1. The Effects of MgO and Al2O3 on Softening Properties
3.2. The Effects of MgO and Al2O3 on Melting Properties
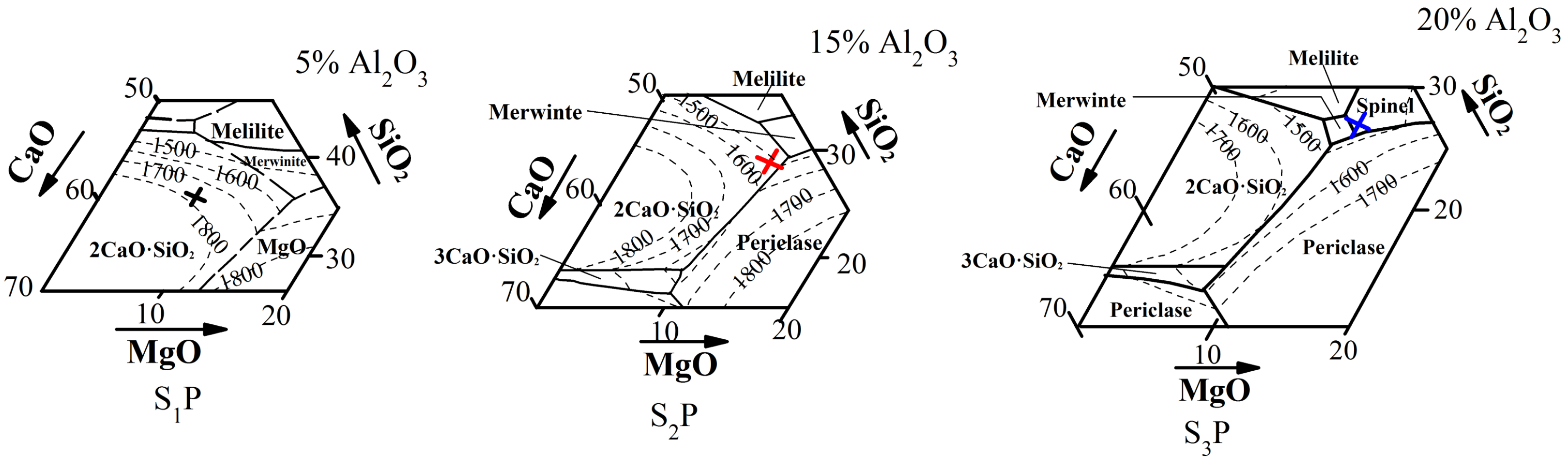
3.3. Roles of MgO and Al2O3 during Melting
4. Conclusions
- (1)
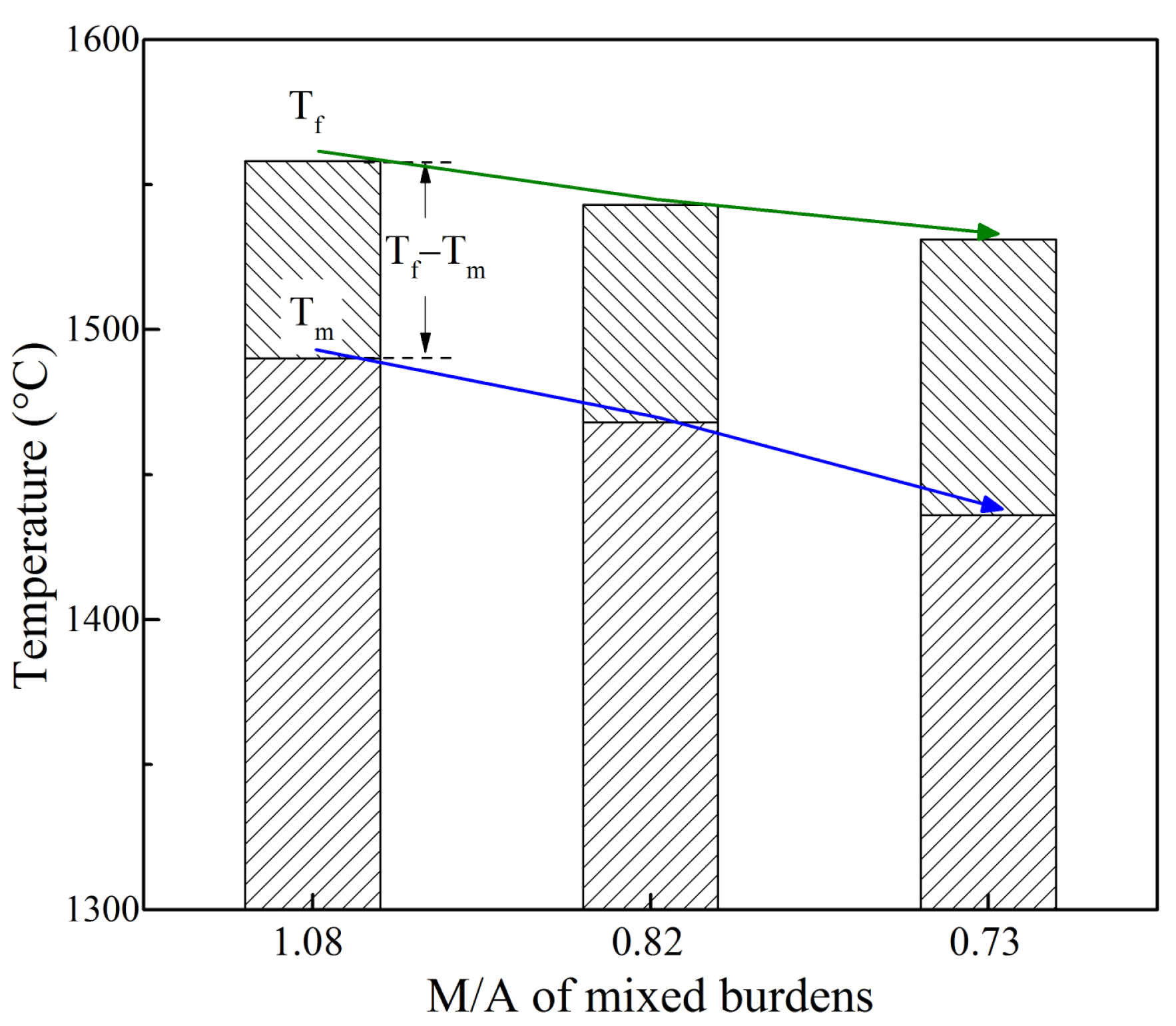
- With decreasing MgO mass%/Al2O3 mass% in mixed burden, the softening–melting characteristic temperatures of mixed burdens tended to decrease. The softening interval widened slightly at first, and then narrowed, while the melting interval first increased slightly, and then increased greatly. The permeability of the melting zone deteriorated once the MgO mass%/Al2O3 mass% was less than a certain content.
- (2)
- The low melting temperature and viscosity of the slag of mixed ferrous burden with a low MgO mass%/Al2O3 mass% was the main reason for the reduction in softening–melting characteristics.
- (3)
- On the whole, with decreasing MgO mass%/Al2O3 mass% in mixed burden, the softening properties were improved whereas the melting properties were worsened. Considering the iron ore reduction and thermal state of blast furnace hearth, mixed burden had the optimal softening–melting properties at an MgO mass%/Al2O3 mass% of 0.82.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geerdes, M.; Chaigneau, R.; Kurunov, I.; Lingiardi, O.; Ricketts, J. Modern Blast Furnace Ironmaking: An Introduction; Ios Press: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Bao, J.W.; Chu, M.S.; Liu, Z.G.; Han, D.; Guo, J.; Zhang, L.F. Influence mechanism of different reactive iron carbon agglomerates on softening–melting–dropping properties of BF mixed burdens. Ironmak. Steelmak. 2022, 50, 571–584. [Google Scholar] [CrossRef]
- Flores, I.V.; Matos, O.; Lima da Silva, A.; Covcevich Bagatini, M. Microstructure and porosity evolution during the reduction, softening and melting of iron-bearing materials. Metall. Mater. Trans. B 2021, 52, 1716–1738. [Google Scholar] [CrossRef]
- Lu, Y.N.; Wu, S.L.; Du, B.B.; Zhou, H. Increasing the softening as well as melting behaviors for iron ore materials within the blast furnace cohesive zone through the high-temperature interactivity. ISIJ Int. 2020, 60, 1461–1468. [Google Scholar] [CrossRef]
- Iljana, M.; Kemppainen, A.; Paananen, T.; Mattila, O.; Heikkinen, E.P.; Fabritius, T. Evaluating the reduction-softening behaviour of blast furnace burden with an advanced test. ISIJ Int. 2016, 56, 1705–1714. [Google Scholar] [CrossRef] [Green Version]
- Ju, J.T.; Ma, K.; Xing, X.D.; Zhao, G.Q.; Li, Q.D. Investigate the oxidation induration mechanism of manganese-bearing magnetite concentrate pellets. Ironmak. Steelmak. 2022, 49, 588–595. [Google Scholar] [CrossRef]
- Kamireddy, V.; Wang, D.Q.; Pan, W.; Chen, S.G.; Evans, T.; Tang, F.Q.; Zhao, B.J.; Ma, X.D. Liquid formation in sinters and its correlation with softening behaviour. Metals 2022, 12, 885. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, X.; Shen, F.M.; Zheng, H.Y.; Gao, Q.J.; Zhang, X. Influence of SiO2 on the compressive strength and reduction-melting of pellets. Metals 2019, 9, 852. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.G.; Kim, W.H.; Min, D.J. A Study on the effect of alumina on the morphology and reduction behavior of sinter by in situ observation. Metals 2021, 11, 740. [Google Scholar] [CrossRef]
- Ji, Z.Y.; Zhao, Y.J.; Gan, M.; Fan, X.H.; Chen, X.L.; Hu, L. Microstructure and minerals evolution of iron ore sinter: Influence of SiO2 and Al2O3. Minerals 2019, 9, 449. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Matsuura, H.; Tsukihashi, F.; Wang, W.L.; Min, D.J.; Sohn, I. Effect of Al2O3 and CaO/SiO2 on the viscosity of calcium-silicate-based slags containing 10 Mass Pct MgO. Metall. Mater. Trans. B 2013, 44, 5–12. [Google Scholar] [CrossRef]
- Wang, Z.J.; Sun, Y.Q.; Sridhar, S.; Zhang, M.; Guo, M.; Zhang, Z.T. Effect of Al2O3 on the viscosity and structure of CaO-SiO2-MgO-Al2O3-FetO slags. Metall. Mater. Trans. B 2015, 46, 537–541. [Google Scholar] [CrossRef]
- Yao, L.; Ren, S.; Wang, X.Q.; Liu, Q.C.; Dong, L.Y.; Yang, J.F.; Liu, J.B. Effect of Al2O3, MgO, and CaO/SiO2 on viscosity of high alumina blast furnace slag. Steel Res. Int. 2016, 87, 241–249. [Google Scholar] [CrossRef]
- Sun, C.Y.; Liu, X.H.; Li, J.; Yin, X.T.; Song, S.; Wang, Q. Influence of Al2O3 and MgO on the viscosity and stability of CaO-MgO-SiO2-Al2O3 slags with CaO/SiO2 = 1.0. ISIJ Int. 2017, 57, 978–982. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.Y.; Chen, Y.C.; Li, J.; Wang, Q.; Liu, X.H.; Sheng, X.X. Influence and dynamics analysis of MgO content on the desulfurization of blast furnace slag with high Al2O3 content. J. Chongqing Univ. 2016, 39, 82–87. (In Chinese) [Google Scholar]
- Wu, S.L.; Lu, Y.N.; Hong, Z.B.; Zhou, H. Improving the softening and melting properties of ferrous burden with high Al2O3 content for blast furnace by ore blending. ISIJ Int. 2020, 60, 1504–1511. [Google Scholar] [CrossRef]
- Li, T.L.; Sun, C.Y.; Liu, X.; Song, S.; Wang, Q. The Effects of MgO and Al2O3 behaviours on softening-melting properties of high basicity sinter. Ironmak. Steelmak. 2018, 45, 755–763. [Google Scholar] [CrossRef]
- Ma, L.M.; Zhang, J.L.; Wang, Y.Z.; Lu, M.; Cai, Q.Y.; Xu, C.Y.; Li, Z.; Liu, Z.J. Mixed burden softening-melting property optimization based on high-silica fluxed pellets. Powder Technol. 2022, 412, 117979. [Google Scholar] [CrossRef]
- Guo, H.; Shen, F.M.; Liu, M.X.; Zhang, L.; Zheng, H.Y.; Jiang, X. Effects of MgO and burden charging pattern of the sinter ore on melting and dropping. J. Mater. Metall. 2017, 16, 13–18. (In Chinese) [Google Scholar]
- Guo, H.; Shen, F.M.; Zhang, H.Y.; Gao, Q.J.; Jiang, X. High-temperature reduction and melting mechanism of sinter with different MgO content. Metals 2019, 9, 510. [Google Scholar] [CrossRef] [Green Version]
- Li, S.D.; Zhang, J.L.; Wang, Y.Z.; Lu, F.L.; Niu, L.L.; Li, Z.; Liu, Z.J. Dual effect of MgO on softing–melting performance of high gangue sinter: Changes of slag viscosity and phase composition. Ironmak. Steelmak. 2023. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.L.; Zuo, H.B.; Gao, B.; Li, F.G.; Wang, R.B. Influence of Al2O3 content on softening-melting property of high basicity sinter. Iron Steel 2015, 50, 20–25+76. (In Chinese) [Google Scholar]
- Iljana, M.; Paananen, T.; Mattila, O.; Kondrakov, M.; Fabritius, T. Effect of iron ore pellet size on metallurgical properties. Metals 2022, 12, 302. [Google Scholar] [CrossRef]
- Iljana, M.; Heikkinen, E.P.; Fabritius, T. Estimation of iron ore pellet softening in a blast furnace with computational thermodynamics. Metals 2021, 11, 1515. [Google Scholar] [CrossRef]
- Chen, B.J.; Jiang, T.; Wen, J.; Li, L.; Hu, P. Review of pellets and blast furnace slag research progress: The effects of MgO on metallurgical properties. Ironmak. Steelmak. 2023. [Google Scholar] [CrossRef]
- Liu, Z.G.; Chu, M.S.; Wang, H.T.; Zhao, W.; Xue, X.X. Effect of MgO content in sinter on the softening-melting behavior of mixed burden made from chromium-bearing vanadium-titanium magnetite. Int. J. Miner. Metall. Mater. 2016, 23, 25–32. [Google Scholar] [CrossRef]
- Bai, K.K.; Pan, Y.Z.; Wang, J.S.; Zuo, H.B.; Xue, Q.G. Optimizing MgO distribution between sinter and pellet based on melting behavior and interaction. Metall. Res. Technol. 2021, 118, 318. [Google Scholar] [CrossRef]
- Li, T.L.; Sun, C.Y.; Lan, D.; Song, J.; Song, S.; Wang, Q. Effect of mineral elements migration on softening–melting properties of ti–bearing high basicity sinter. ISIJ Int. 2019, 59, 245–252. [Google Scholar] [CrossRef]
- Tsutsumi, T.; Wang, Z.; Sasaki, Y.; Kashiwaya, Y.; Ishii, K.; Konno, N. Heating-Up reduction and high temperature properties of high alumina sintered ores. Tetsu-to-Hagane 1998, 84, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Nandy, B.; Chandra, S.; Bhattacharjee, D.; Ghosh, D. Assessment of blast furnace behaviour through softening-melting test. Ironmak. Steelmak. 2006, 33, 111–119. [Google Scholar] [CrossRef]
- Eisenhüttenleute, V.D. Slag Atlas; Verlag Stahleisen Press: Dusseldorf, Germany, 1995. [Google Scholar]
- Lee, Y.S.; Min, D.J.; Jung, S.M.; Yi, S.H. Influence of basicity and FeO content on viscosity of blast furnace type slags containing FeO. ISIJ Int. 2004, 44, 1283–1290. [Google Scholar] [CrossRef] [Green Version]


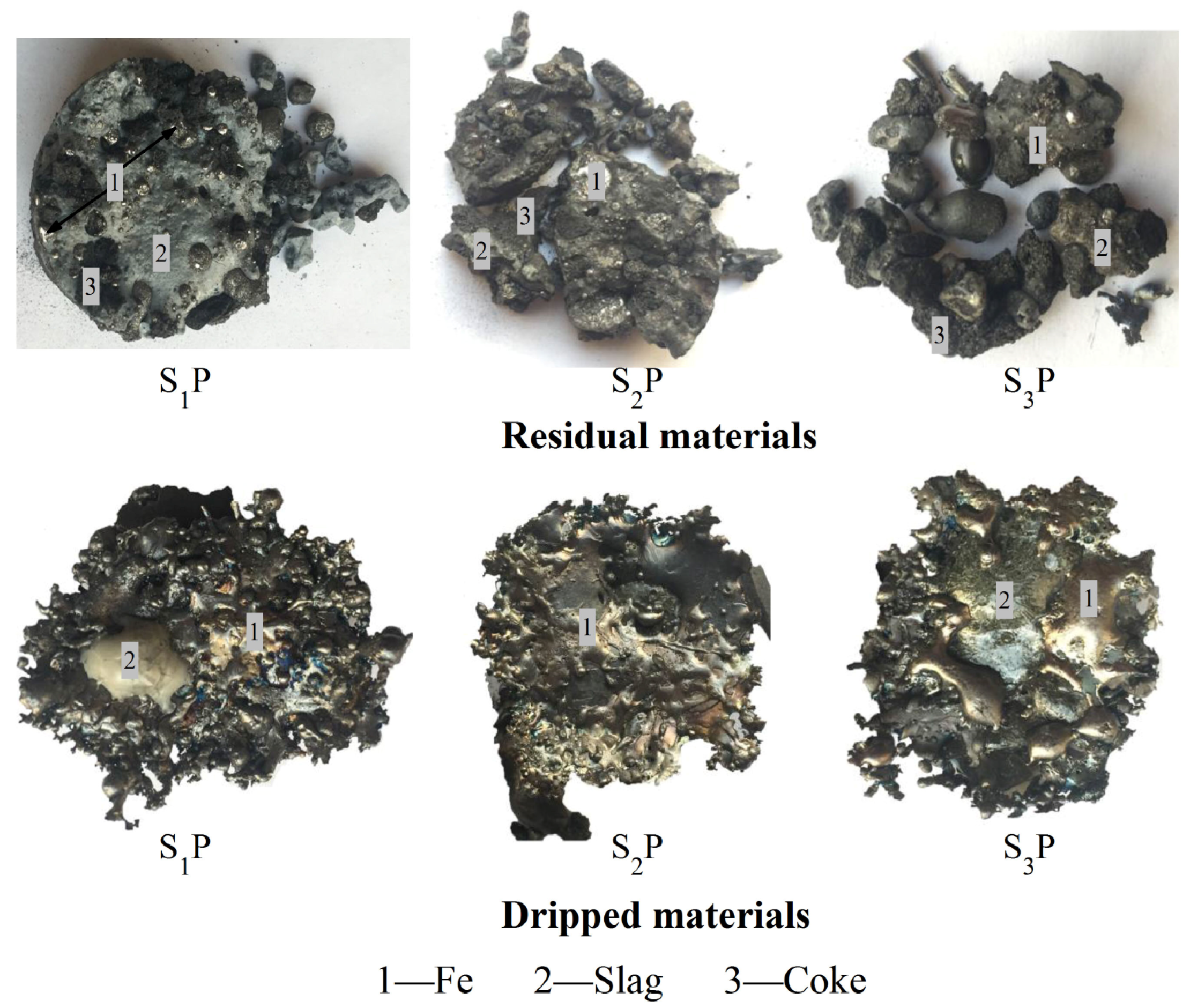
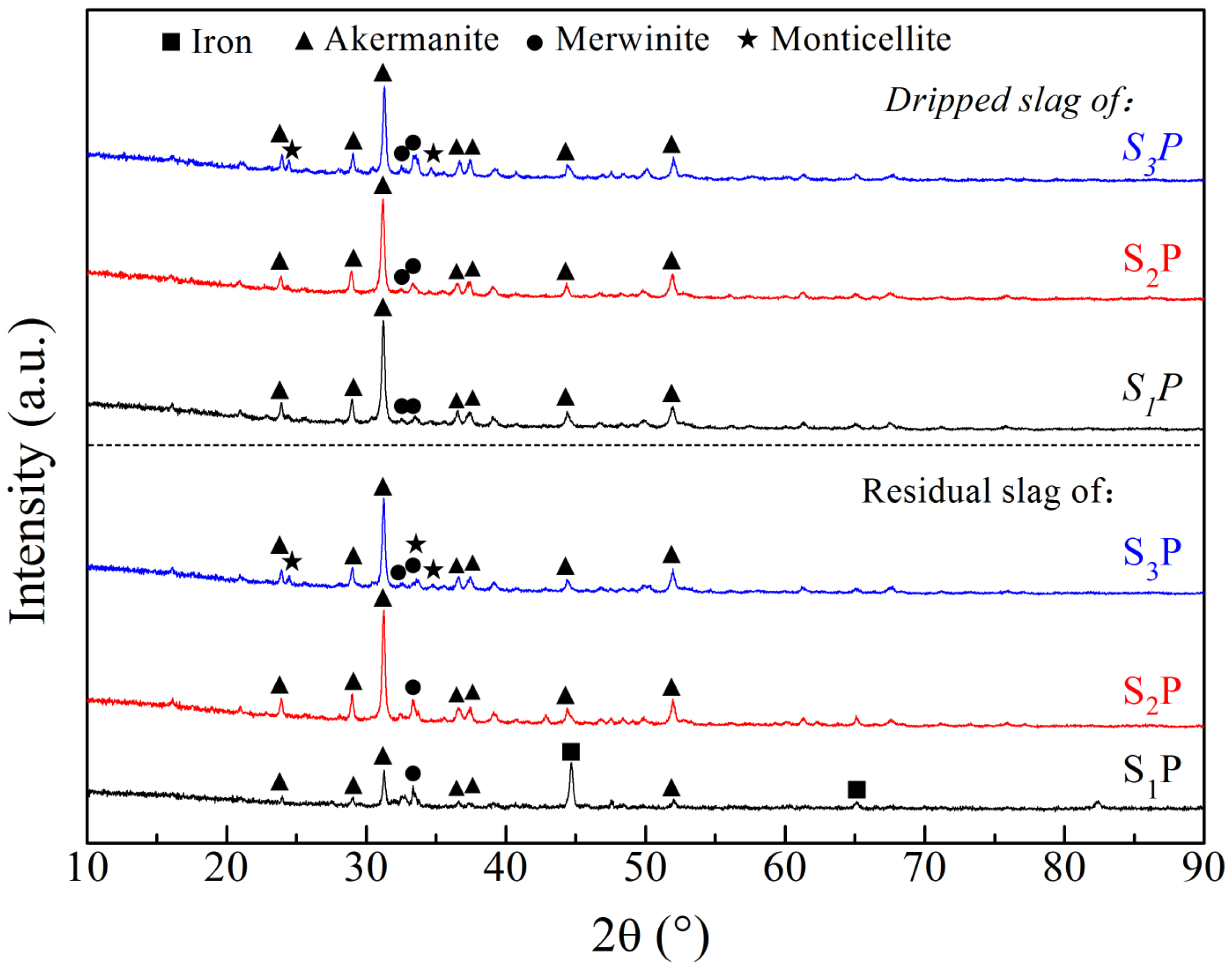
| Materials | TFe 1 | FeO | SiO2 | CaO | MgO | Al2O3 | M/A 2 | C/S 3 |
|---|---|---|---|---|---|---|---|---|
| S1 | 54.39 | 9.54 | 6.63 | 13.15 | 1.70 | 1.14 | 1.49 | 1.98 |
| S2 | 54.70 | 9.40 | 5.53 | 10.26 | 2.84 | 3.08 | 0.92 | 1.86 |
| S3 | 52.30 | 9.00 | 6.16 | 11.31 | 3.16 | 4.03 | 0.78 | 1.84 |
| P | 63.80 | 0.18 | 3.38 | 0.34 | 0.48 | 1.43 | 0.69 | 0.10 |
| Mixed Burdens | TFe 1 | FeO | SiO2 | CaO | MgO | Al2O3 | M/A 2 |
|---|---|---|---|---|---|---|---|
| S1P | 57.21 | 6.73 | 5.66 | 9.31 | 1.33 | 1.23 | 1.08 |
| S2P | 57.43 | 6.63 | 4.89 | 7.28 | 2.13 | 2.59 | 0.82 |
| S3P | 55.75 | 6.35 | 5.33 | 8.02 | 2.36 | 3.25 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, T.; Sun, C.; Yang, S.; Wang, Q. The Effects of MgO and Al2O3 Content in Sinter on the Softening–Melting Properties of Mixed Ferrous Burden. Materials 2023, 16, 5490. https://doi.org/10.3390/ma16155490
Li Z, Li T, Sun C, Yang S, Wang Q. The Effects of MgO and Al2O3 Content in Sinter on the Softening–Melting Properties of Mixed Ferrous Burden. Materials. 2023; 16(15):5490. https://doi.org/10.3390/ma16155490
Chicago/Turabian StyleLi, Zhexi, Tingle Li, Changyu Sun, Songtao Yang, and Qi Wang. 2023. "The Effects of MgO and Al2O3 Content in Sinter on the Softening–Melting Properties of Mixed Ferrous Burden" Materials 16, no. 15: 5490. https://doi.org/10.3390/ma16155490





