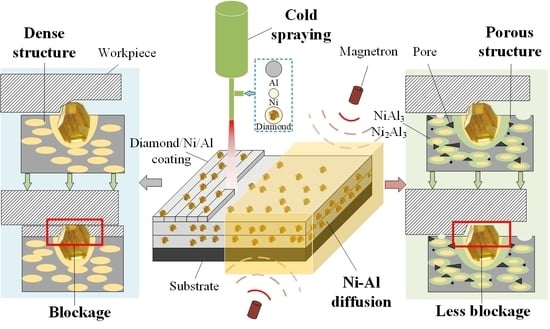
The Fabrication of Porous Metal-Bonded Diamond Coatings Based on Low-Pressure Cold Spraying and Ni-Al Diffusion-Reaction
Abstract
:1. Introduction
2. Experiments
2.1. Material Preparation
2.2. Experimental Procedures
2.3. Coating Deposition
2.4. Post-Spray Heat-Treatment
2.5. Material Characterizations
2.6. Tribological Testing
3. Results and Discussion
3.1. Microstructure Characterizations of As-Sprayed Coatings
3.2. Microstructure Characterizations of Heat-Treated Coatings
3.3. Wear Behavior of As-Sprayed and Porous Heat-Treated Coatings
4. Conclusions
- Diamond/Ni/Al coating was successfully deposited on the YG 20 substrate. The coating was thick (400–600 μm) and dense. Cold spraying could avoid the graphitization of diamond and the Ni-Al diffusion–reaction in the deposition process;
- Pores were successfully produced at the Al site through the Ni-Al in-situ reaction at 400 °C and 500 °C, respectively. The porosities of 400 °C and 500 °C heat-treated coating were 8.8 ± 0.8% and 16.1 ± 0.7%, respectively;
- Both cold-sprayed coating and 500 °C heat-treated coating showed the performance of grinding cemented carbide during the tribology test. The wear mechanism changed from coating worn by cemented carbide to the cemented carbide ground by the coating;
- The porous structure of 500 °C heat-treated coating could benefit the wear performance in the tribology test. The porous coating had large chip space and slight clogging. The surface roughness of cemented carbide ground by 500 °C heat-treated coating was smaller (Sa: 0.30 ± 0.07 μm) than that ground by cold-sprayed coating (Sa: 0.37 ± 0.09 μm). After ultrasonic cleaning, the average exposure height of diamond particles in a wear track of the 500 °C heat-treated coating was 44.5% higher than that of the cold-sprayed coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, P.; He, D.; Wang, L.; Kou, Z.; Li, Y.; Xiong, L.; Hu, Q.; Xu, C.; Lei, L.; Wang, Q.; et al. Diamond-cBN alloy: A universal cutting material. Appl. Phys. Lett. 2015, 107, 101901. [Google Scholar] [CrossRef]
- Loginov, P.A.; Zhassay, U.A.; Bychkova, M.Y.; Petrzhik, M.I.; Mukanov, S.K.; Sidorenko, D.A.; Orekhov, A.S.; Rupasov, S.I.; Levashov, E.A. Chromium-doped Fe-Co-Ni binders for diamond cutting tools: The features of the structure, mechanical properties, and adhesion to diamond. Int. J. Refract. Met. Hard Mater. 2020, 92, 105289. [Google Scholar] [CrossRef]
- Su, H.H.; Xu, H.J.; Xiao, B.; Fu, Y.C.; Xu, J.H. Microstructure and Performance of Porous Ni-Cr Alloy Bonded Diamond Grinding Wheel. Mater. Sci. Forum 2006, 532–533, 373–376. [Google Scholar] [CrossRef]
- Tillmann, W.; Ferreira, M.; Steffen, A.; Rüster, K.; Möller, J.; Bieder, S.; Paulus, M.; Tolan, M. Carbon reactivity of binder metals in diamond-metal composites—Characterization by scanning electron microscopy and X-ray diffraction. Diam. Relat. Mater. 2013, 38, 118–123. [Google Scholar] [CrossRef]
- Sun, Y.X.; Tsai, Y.T.; Lin, K.H. The influence of sintering parameters on the mechanical properties of vitrified bond diamond tools. Mater. Des. 2015, 80, 89–98. [Google Scholar] [CrossRef]
- Du, Z.j.; Zhang, F.l.; Xu, Q.s.; Huang, Y.j.; Li, M.c.; Huang, H.p.; Wang, C.y.; Zhou, Y.m.; Tang, H.q. Selective laser sintering and grinding performance of resin bond diamond grinding wheels with arrayed internal cooling holes. Ceram. Int. 2019, 45, 20873–20881. [Google Scholar] [CrossRef]
- Dai, Q.; Luo, C.; Liao, C. Experimental study on porous metal bonded diamond grinding wheels (II)—Grinding performance of porous wheels. Key Eng. Mater. 2008, 359–360, 48–52. [Google Scholar] [CrossRef]
- Tian, C.; Li, X.; Zhang, S.; Guo, G.; Wang, L.; Rong, Y. Study on design and performance of metal-bonded diamond grinding wheels fabricated by selective laser melting (SLM). Mater. Des. 2018, 156, 52–61. [Google Scholar] [CrossRef]
- Loginov, P.A.; Sidorenko, D.A.; Shvyndina, N.V.; Sviridova, T.A.; Churyumov, A.Y.; Levashov, E.A. Effect of Ti and TiH2 doping on mechanical and adhesive properties of Fe-Co-Ni binder to diamond in cutting tools. Int. J. Refract. Met. Hard Mater. 2019, 79, 69–78. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Rajinikanth, V.; Naveen, T.; Sinha, D.P.; Atiquzzaman; Ray, A.K. Abrasive wear behavior of thermally sprayed diamond reinforced composite coating deposited with both oxy-acetylene and HVOF techniques. Wear 2009, 266, 995–1002. [Google Scholar] [CrossRef]
- Rommel, D.; Scherm, F.; Kuttner, C.; Glatzel, U. Laser cladding of diamond tools: Interfacial reactions of diamond and molten metal. Surf. Coat. Technol. 2016, 291, 62–69. [Google Scholar] [CrossRef]
- Traxel, K.D.; Bandyopadhyay, A. Diamond-reinforced cutting tools using laser-based additive manufacturing. Addit. Manuf. 2021, 37, 101602. [Google Scholar] [CrossRef]
- Yao, J.; Yang, L.; Li, B.; Li, Z. Beneficial effects of laser irradiation on the deposition process of diamond/Ni60 composite coating with cold spray. Appl. Surf. Sci. 2015, 330, 300–308. [Google Scholar] [CrossRef]
- Tillmann, W.; Zajaczkowski, J.; Baumann, I.; Kipp, M.; Biermann, D. Qualification of the Low-pressure Cold Gas Spraying for the Additive Manufacturing of Copper–Nickel–Diamond Grinding Wheels. J. Therm. Spray Technol. 2021. [Google Scholar] [CrossRef]
- Kamaraj, M.; Radhakrishnan, V.M. Cold Spray Coating Diagram: Bonding Properties and Construction Methodology. J. Therm. Spray Technol. 2019, 28, 756–768. [Google Scholar] [CrossRef]
- Assadi, H.; Kreye, H.; Gärtner, F.; Klassen, T. Cold spraying—A materials perspective. Acta Mater. 2016, 116, 382–407. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Li, W.; Qu, L.; Yang, X.; Song, B.; Lupoi, R.; Yin, S. Solid-state cold spraying of FeCoCrNiMn high-entropy alloy: An insight into microstructure evolution and oxidation behavior at 700–900 °C. J. Mater. Sci. Technol. 2021, 68, 172–183. [Google Scholar] [CrossRef]
- Srikanth, A.; Mohammed Thalib Basha, G.; Venkateshwarlu, B. A Brief Review on Cold Spray Coating Process. Mater. Today Proc. 2019, 22, 1390–1397. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, M.; Lee, J.Y. Application of a cold spray technique to the fabrication of a copper canister for the geological disposal of CANDU spent fuels. Nucl. Eng. Des. 2010, 240, 2714–2720. [Google Scholar] [CrossRef]
- Lupoi, R.; O’Neill, W. Deposition of metallic coatings on polymer surfaces using cold spray. Surf. Coat. Technol. 2010, 205, 2167–2173. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Huang, Q.; Wang, X.; Suo, X.; Wang, J.; Li, H. Interfacial metal/ceramic bonding mechanism for metallization of ceramics via cold spraying. J. Mater. Process. Technol. 2021, 288, 116845. [Google Scholar] [CrossRef]
- Gong, Y.D.; Wen, X.L.; Cheng, J.; Yin, G.Q.; Wang, C. Experimental study on fabrication and evaluation of a micro-scale shaft grinding tool. J. Mech. Sci. Technol. 2014, 28, 1027–1037. [Google Scholar] [CrossRef]
- Chen, C.; Xie, Y.; Yan, X.; Ahmed, M.; Lupoi, R.; Wang, J.; Ren, Z.; Liao, H.; Yin, S. Tribology properties of Al/diamond composites produced by cold spray additive manufacturing. Addit. Manuf. 2020, 36, 101434. [Google Scholar] [CrossRef]
- Yin, S.; Xie, Y.; Cizek, J.; Ekoi, E.J.; Hussain, T.; Dowling, D.P.; Lupoi, R. Advanced diamond-reinforced metal matrix composites via cold spray: Properties and deposition mechanism. Compos. Part B Eng. 2017, 113, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Xiang, G.; Li, W.; Zhang, F.; Zhou, Y.; Long, W. Research on resin-bonded diamond grinding wheel based on brazing-coated diamond and its perfomances. Diam. Abras. Eng. 2020, 40, 36–41. [Google Scholar] [CrossRef]
- Kapłonek, W.; Nadolny, K.; Rokosz, K.; Marciano, J.; Mia, M.; Pimenov, D.Y.; Kulik, O.; Gupta, M.K. Internal cylindrical grinding process of INCONEL® alloy 600 using grinding wheels with sol-gel alumina and a synthetic organosilicon polymer-based impregnate. Micromachines 2020, 11, 115. [Google Scholar] [CrossRef] [Green Version]
- Kapłonek, W.; Nadolny, K.; Sutowska, M.; Mia, M.; Pimenov, D.Y.; Gupta, M.K. Experimental Studies on MoS2-Treated Grinding Wheel Active Surface Condition after High-Efficiency Internal Cylindrical Grinding Process of INCONEL® Alloy 718. Micromachines 2019, 10, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Li, X.; Tian, C.; Guo, G.; Wang, L.; Liu, X. The design and fabrication of porous sintered grinding wheel based on Selective Laser Melting technology. J. Phys. Conf. Ser. 2018, 1074, 012157. [Google Scholar] [CrossRef] [Green Version]
- Tian, C.; Li, X.; Zhang, S.; Guo, G.; Ziegler, S.; Schleifenbaum, J.H.; Wang, L.; Rong, Y. Porous structure design and fabrication of metal-bonded diamond grinding wheel based on selective laser melting (SLM). Int. J. Adv. Manuf. Technol. 2019, 100, 1451–1462. [Google Scholar] [CrossRef]
- Xu, H.; Liao, C.J.; Weng, Q. Experimental study on porous metal bonded diamond grinding wheels—The selection of porosity inducers and agglomeration’s parameter. Adv. Mater. Res. 2012, 415–417, 594–597. [Google Scholar] [CrossRef]
- Wang, C.; Wang, D.; Tian, C.; Wang, L.; Rong, Y.; Li, X. Grinding performance evaluation of 3D-printed porous metal-bonded grinding wheel in BK7 glass grinding. Int. J. Adv. Manuf. Technol. 2021, 117, 1445–1457. [Google Scholar] [CrossRef]
- White, B.C.; Story, W.A.; Brewer, L.N.; Jordon, J.B. Fatigue behavior of freestanding AA2024 and AAA7075 cold spray deposits. Int. J. Fatigue 2018, 112, 355–360. [Google Scholar] [CrossRef]
- Sample, C.M.; Champagne, V.K.; Nardi, A.T.; Lados, D.A. Factors governing static properties and fatigue, fatigue crack growth, and fracture mechanisms in cold spray alloys and coatings/repairs: A review. Addit. Manuf. 2020, 36, 101371. [Google Scholar] [CrossRef]
- Moridi, A.; Stewart, E.J.; Wakai, A.; Assadi, H.; Gartner, F.; Guagliano, M.; Klassen, T.; Dao, M. Solid-state additive manufacturing of porous Ti-6Al-4V by supersonic impact. Appl. Mater. Today 2020, 21, 100865. [Google Scholar] [CrossRef]
- Bagherifard, S.; Monti, S.; Zuccoli, M.V.; Riccio, M.; Kondás, J.; Guagliano, M. Cold spray deposition for additive manufacturing of freeform structural components compared to selective laser melting. Mater. Sci. Eng. A 2018, 721, 339–350. [Google Scholar] [CrossRef]
- Spencer, K.; Zhang, M.X. The use of kinetic metallization to form intermetallic reinforced composite coatings by post-spray heat treatment. Surf. Coat. Technol. 2009, 203, 3019–3025. [Google Scholar] [CrossRef]
- Dong, H.X.; Jiang, Y.; He, Y.H.; Song, M.; Zou, J.; Xu, N.P.; Huang, B.Y.; Liu, C.T.; Liaw, P.K. Formation of porous Ni-Al intermetallics through pressureless reaction synthesis. J. Alloys Compd. 2009, 484, 907–913. [Google Scholar] [CrossRef]
- Diab, M.; Pang, X.; Jahed, H. The effect of pure aluminum cold spray coating on corrosion and corrosion fatigue of magnesium (3% Al–1% Zn) extrusion. Surf. Coat. Technol. 2017, 309, 423–435. [Google Scholar] [CrossRef]
- Lee, H.Y.; Jung, S.H.; Lee, S.Y.; You, Y.H.; Ko, K.H. Correlation between Al 2 O 3 particles and interface of Al-Al 2 O 3 coatings by cold spray. Appl. Surf. Sci. 2005, 252, 1891–1898. [Google Scholar] [CrossRef]
- Yin, S.; Cizek, J.; Chen, C.; Jenkins, R.; O’Donnell, G.; Lupoi, R. Metallurgical bonding between metal matrix and core-shelled reinforcements in cold sprayed composite coating. Scr. Mater. 2020, 177, 49–53. [Google Scholar] [CrossRef]
- Feng, C.; Guipont, V.; Jeandin, M.; Amsellem, O.; Pauchet, F.; Saenger, R.; Bucher, S.; Iacob, C. B4C/Ni Composite coatings prepared by cold spray of blended or CVD-coated powders. J. Therm. Spray Technol. 2012, 21, 561–570. [Google Scholar] [CrossRef]
- Na, H.; Bae, G.; Shin, S.; Kumar, S.; Kim, H.; Lee, C. Advanced deposition characteristics of kinetic sprayed bronze/diamond composite by tailoring feedstock properties. Compos. Sci. Technol. 2009, 69, 463–468. [Google Scholar] [CrossRef]
- Lee, H.; Shin, H.; Ko, K. Effects of gas pressure of cold spray on the formation of Al-based intermetallic compound. J. Therm. Spray Technol. 2010, 19, 102–109. [Google Scholar] [CrossRef]
- Morsi, K. Review: Reaction synthesis processing of Ni-Al intermetallic materials. Mater. Sci. Eng. A 2001, 299, 1–15. [Google Scholar] [CrossRef]
- Jin, Y.M.; Cho, J.H.; Park, D.Y.; Kim, J.H.; Lee, K.A. Manufacturing and macroscopic properties of cold sprayed Cu-In coating material for sputtering target. J. Therm. Spray Technol. 2011, 20, 497–507. [Google Scholar] [CrossRef]
- Zhao, B.; Ding, W.; Xiao, G.; Zhao, J.; Li, Z. Effects of open pores on grinding performance of porous metal-bonded aggregated cBN wheels during grinding Ti–6Al–4V alloys. Ceram. Int. 2021, 47, 31311–31318. [Google Scholar] [CrossRef]












| Type | Size (μm) | Shape | Supplier |
|---|---|---|---|
| Ni (99.99%) | −10 + 5 | Irregular morphology | Nangong Xindun Alloy Welding Material Spraying Co. Ltd., Xingtai, China |
| Al (99.98%) | d10 = 13 | Spherical morphology | Henan Yuanyang Powder Technology Co. Ltd., Xinxiang, China |
| d50 = 20 | |||
| d90 = 30 | |||
| Ni-coateddiamond | diamond core | Irregular morphology | Henan Ruizhong New Material Technology Co., Ltd., Zhengzhou, China |
| d10 = 20 | |||
| d50 = 28 | |||
| d90 = 38 |
| Parameter | Value |
|---|---|
| Process gas | Compressed air |
| Powder feeding gas | Compressed air |
| Process gas pressure | 0.7 MPa |
| Temperature | 600 °C |
| Standoff distance | 10 mm |
| Traverse speed | 10 mm/s |
| Number of gun passes | 4 |
| Composition | Volume Content in the Feedstock | Volume Content in the Coating |
|---|---|---|
| Ni | 37.3% | 39.9 ± 1.9% |
| Al | 31.2% | 45.4 ± 2.3% |
| diamond | 31.5% | 14.7 ± 1.1% |
| As-Sprayed HV200 | Heated at 400 °C HV200 | Heated at 500 °C HV200 | |
|---|---|---|---|
| Hardness | 144 ± 17 | 179 ± 13 | 168 ± 10 |
| Porosity | tiny | 8.8 ± 0.8% | 16.1 ± 0.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Liu, Z.; Ge, H.; Wang, B.; Cai, Y.; Song, Q. The Fabrication of Porous Metal-Bonded Diamond Coatings Based on Low-Pressure Cold Spraying and Ni-Al Diffusion-Reaction. Materials 2022, 15, 2234. https://doi.org/10.3390/ma15062234
Zhang Z, Liu Z, Ge H, Wang B, Cai Y, Song Q. The Fabrication of Porous Metal-Bonded Diamond Coatings Based on Low-Pressure Cold Spraying and Ni-Al Diffusion-Reaction. Materials. 2022; 15(6):2234. https://doi.org/10.3390/ma15062234
Chicago/Turabian StyleZhang, Zhicheng, Zhanqiang Liu, Hui Ge, Bing Wang, Yukui Cai, and Qinghua Song. 2022. "The Fabrication of Porous Metal-Bonded Diamond Coatings Based on Low-Pressure Cold Spraying and Ni-Al Diffusion-Reaction" Materials 15, no. 6: 2234. https://doi.org/10.3390/ma15062234