Compositional Effects on Indentation Mechanical Properties of Chemically Strengthened TiO2-Doped Soda Lime Silicate Glasses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Glass Compositions
2.2. Ion-Exchange Procedure
2.3. Indentation Characterizations
3. Results
3.1. Hardness and Reduced Elastic Modulus from Nano-Indentation
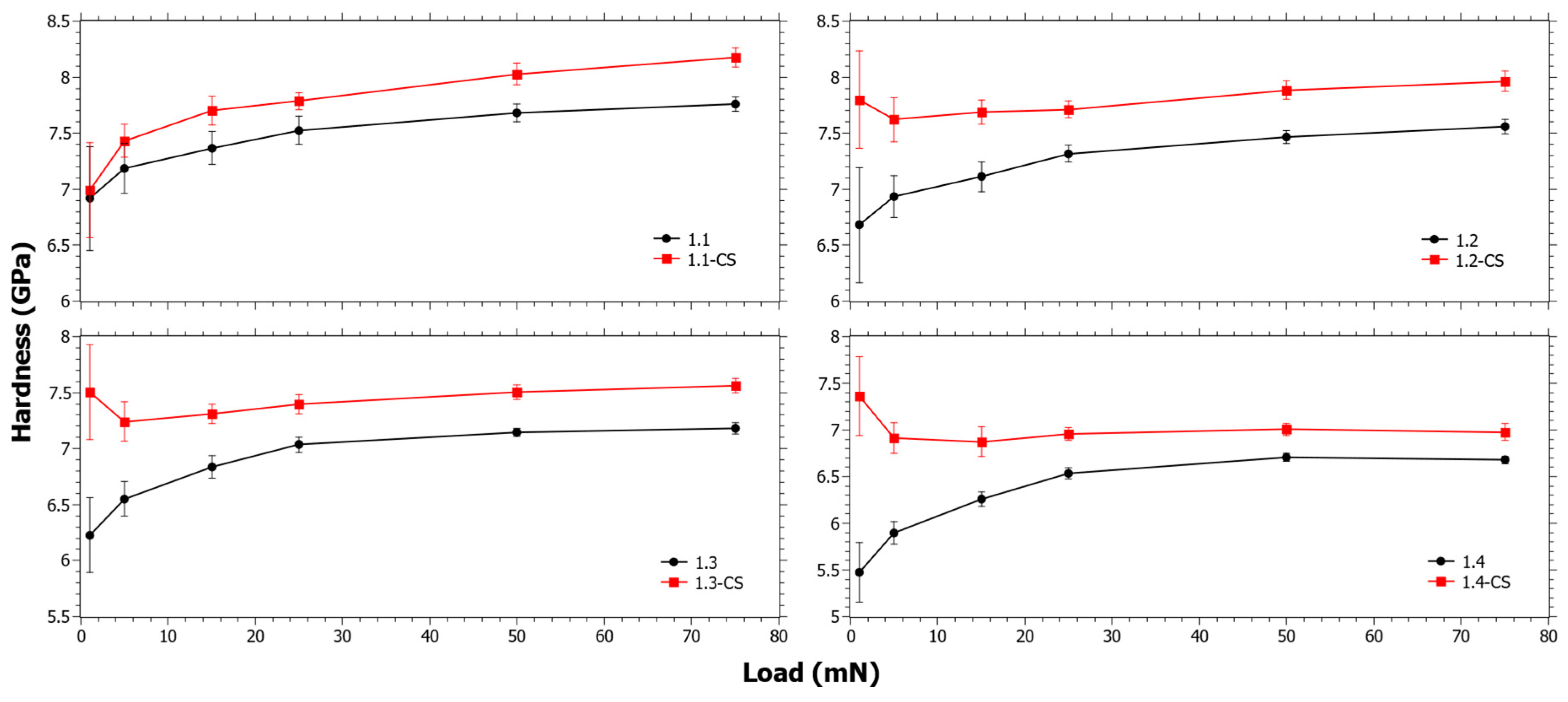
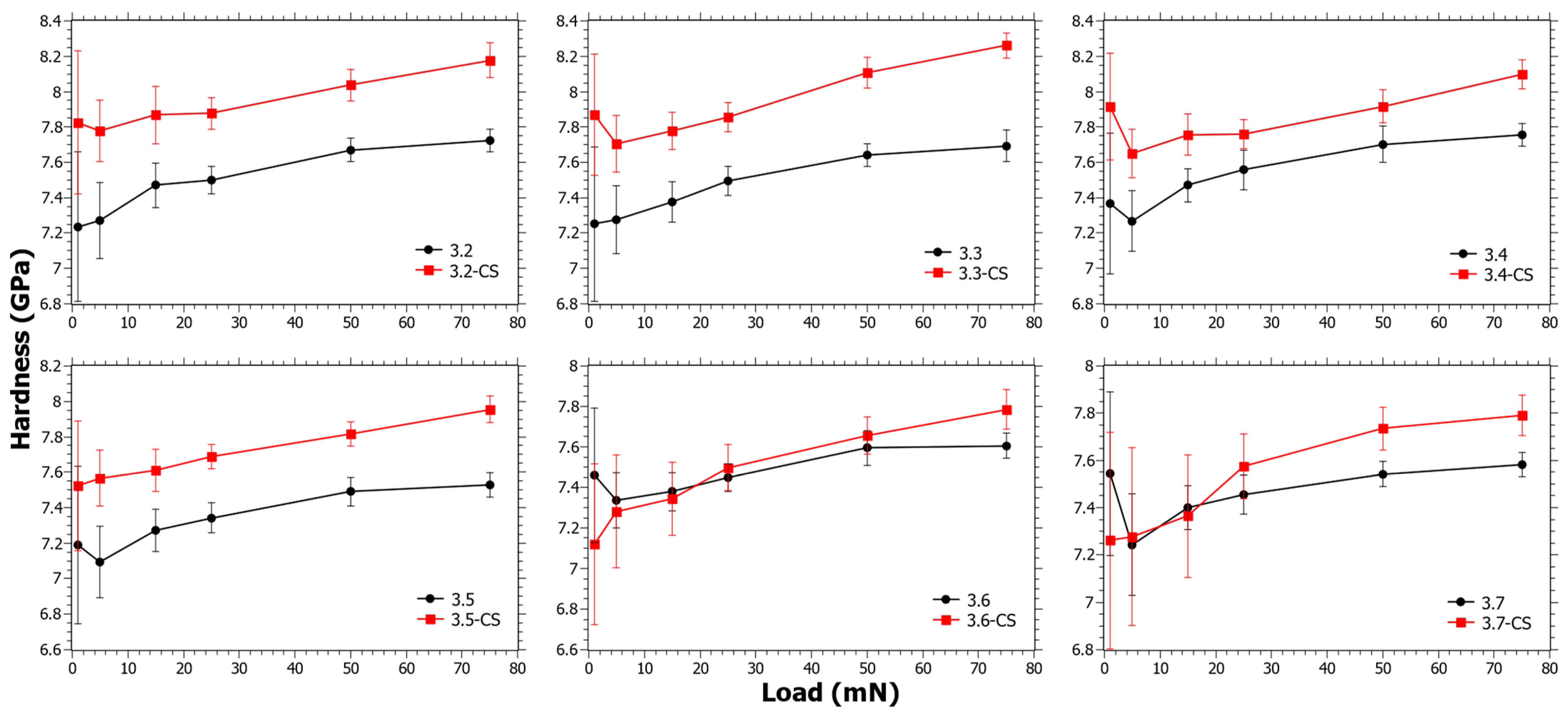
3.2. Hardness as a Function of Indentation Load from Nano-Indentation
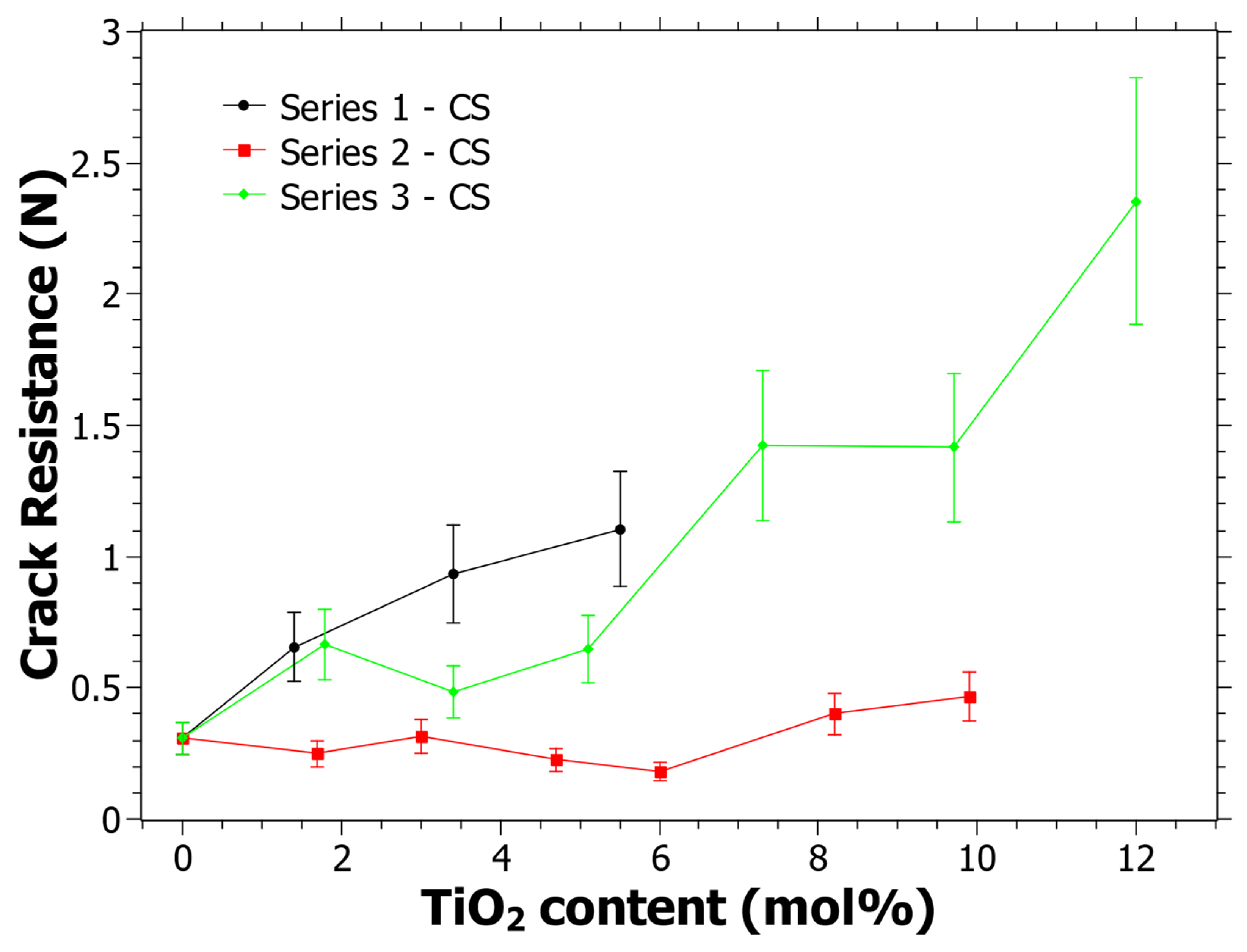
3.3. Crack Resistance
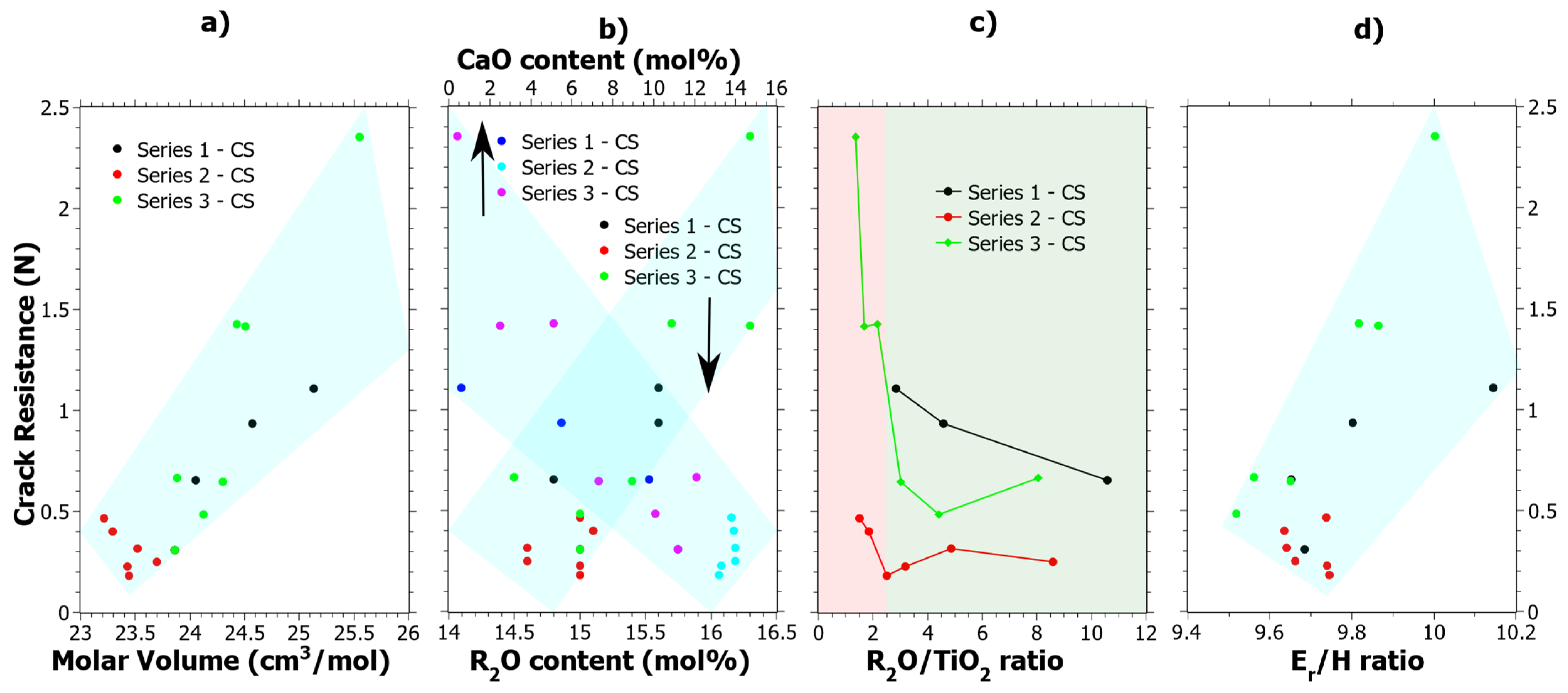
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Axinte, E. Glasses as engineering materials: A review. Mater. Des. 2011, 32, 1717–1732. [Google Scholar] [CrossRef]
- Hand, R.J.; Tadjiev, D.R. Mechanical properties of silicate glasses as a function of composition. J. Non-Cryst. Solids 2010, 356, 2417–2423. [Google Scholar] [CrossRef]
- Wondraczek, L.; Mauro, J.C.; Eckert, J.; Kühn, U.; Horbach, J.; Deubener, J.; Rouxel, T. Towards ultrastrong glasses. Adv. Mater. 2011, 23, 4578–4586. [Google Scholar] [CrossRef]
- Griggs, D.; Stafford-Smith, M.; Gaffney, O.; Rockström, J.; Öhman, M.C.; Shyamsundar, P.; Steffen, W.; Glaser, G.; Kanie, N.; Noble, I. Sustainable development goals for people and planet. Nature 2013, 495, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, S.; Wondraczek, L. Strengthening of oxide glasses. In Encyclopedia for Glass Science, Technology, History and Culture; Richet, P., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Januchta, K.; Youngman, R.E.; Goel, A.; Bauchy, M.; Logunov, S.L.; Rzoska, S.; Bockowski, M.; Jensen, L.R.; Smedskjaer, M.M. Discovery of ultra-crack-resistant oxide glasses with adaptive networks. Chem. Mater. 2017, 29, 5865–5876. [Google Scholar] [CrossRef]
- Molnár, G.; Bojtár, I. Effects of manufacturing inhomogeneities on strength properties of float glass. Mech. Mater. 2013, 59, 1–13. [Google Scholar] [CrossRef]
- Varshneya, A.K.; Spinelli, I.M. High-strength, large-case-depth chemically strengthened lithium aluminosilicate glass. Am. Ceram. Soc. Bull. 2009, 88, 27–33. [Google Scholar]
- Peng, Y.; Ma, W.; Wang, S.; Wang, K.; Gao, G. Investigation of the fracture behaviors of windshield laminated glass used in high-speed trains. Compos. Struct. 2019, 207, 29–40. [Google Scholar] [CrossRef]
- Dziedzic, J.; Inglot, M. Ultrathin glass for the photovoltaic applications. Acta Phys. Pol. A 2017, 132, 176–178. [Google Scholar] [CrossRef]
- Wang, H.F.; Xing, G.Z.; Wang, X.Y.; Zhang, L.L.; Zhang, L.; Li, S. Chemically strengthened protection glasses for the applications of space solar cells. AIP Adv. 2014, 4, 047133. [Google Scholar] [CrossRef]
- Kambe, M.; Hara, K.; Mitarai, K.; Takeda, S.; Fukawa, M.; Ishimaru, N.; Kondo, M. Chemically strengthened cover glass for preventing potential induced degradation of crystalline silicon solar cells. In Proceedings of the 2013 IEEE 39th Photovoltaic Specialists Conference (PVSC), Tampa, FL, USA, 16–21 June 2013; pp. 3500–3503. [Google Scholar]
- Leonhard, T.; Cleary, T.M.; Moore, M.J.; Seyler, S.; Fisher, W.K. Novel Lightweight laminate concept with ultrathin chemically strengthened glass for automotive windshields. SAE Int. J. Passeng. Cars-Mech. Syst. 2015, 8, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Louter, C.; Akilo, M.; Miri, B.; Neeskens, T.; Ribeiro Silveira, R.; Topcu, Ö.; van der Weijde, I.; Zha, C.; Bilow, M.; Turrin, M. Adaptive and composite thin glass concepts for architectural applications. Heron 2018, 63, 199–218. [Google Scholar]
- Macrelli, G.; Varshneya, A.K.; Mauro, J.C. Ultra-thin glass as a substrate for flexible photonics. Opt. Mater. 2020, 106, 109994. [Google Scholar] [CrossRef]
- Varshneya, A.K. Chemical strengthening of glass: Lessons learned and yet to be learned. Int. J. Appl. Glas. Sci. 2010, 1, 131–142. [Google Scholar] [CrossRef]
- Gross, T.M. Chemical strengthening of glass. In Springer Handbook of Glass; Musgraves, J.D., Hu, J., Calvez, L., Eds.; Springer: Cham, Switzerland, 2019; pp. 273–296. [Google Scholar]
- Varshneya, A.K.; Kreski, P.K. The chemistry of chemical strengthening of glass. In Processing, Properties, and Applications of Glass and Optical Materials; John Wiley & Sons: New York, NY, USA, 2012; pp. 107–114. [Google Scholar]
- Toplis, M.J.; Dingwell, D.B.; Lenci, T. Peraluminous viscosity maxima in Na2O-Al2O3-SiO2 liquids: The role of triclusters in tectosilicate melts. Geochim. Cosmochim. Acta 1997, 61, 2605–2612. [Google Scholar] [CrossRef]
- Webb, S.L.; Banaszak, M.; Köhler, U.; Rausch, S.; Raschke, G. The viscosity of Na2O-CaO-Al2O3-SiO2 melts. Eur. J. Miner. 2007, 19, 681–692. [Google Scholar] [CrossRef]
- Karlsson, S. Viscosity of alumina doped soda lime silicate glasses–observation of anomaly in the linear increase as Al2O3 replaces SiO2. J. Non-Cryst. Solids 2021, 573, 121149. [Google Scholar] [CrossRef]
- Rouxel, T.; Jang, J.-I.; Ramamurty, U. Indentation of glasses. Prog. Mater. Sci. 2021, 121, 100834. [Google Scholar] [CrossRef]
- Dejneka, M.; Kiczenski, T.J. Display glass. In Springer Handbook of Glass; Musgraves, J.D., Hu, J., Calvez, L., Eds.; Springer: Cham, Switzerland, 2019; pp. 1521–1553. [Google Scholar]
- Karlsson, S.; Bäck, L.G.; Kidkhunthod, P.; Lundstedt, K.; Wondraczek, L. Effect of TiO2 on optical properties of glasses in the soda-lime-silicate system. Opt. Mater. Express 2016, 6, 1198. [Google Scholar] [CrossRef] [Green Version]
- Dimitrov, V.; Komatsu, T. Electronic polarizability and average single bond strength of ternary oxide glasses with high TiO2 contents. Phys. Chem. Glasses Eur. J. Glass Sci. Technol. Part B 2011, 52, 225–230. [Google Scholar]
- Abdel-Baki, M.; Wahab, F.A.A.; El-Diasty, F. Optical characterization of xTiO2-(60−x)SiO2-40Na2O glasses: I. Linear and nonlinear dispersion properties. Mater. Chem. Phys. 2006, 96, 201–210. [Google Scholar] [CrossRef]
- Abdel-Baki, M.; El-Diasty, F.; Wahab, F.A.A. Optical characterization of xTiO2-(60−x)SiO2-40Na2O glasses: II. Absorption edge, Fermi level, electronic polarizability and optical basicity. Opt. Commun. 2006, 261, 65–70. [Google Scholar] [CrossRef]
- Higazy, A.A.; Hussein, A.; Ewaida, M.A.; El-Hofy, M. The effect of temperature on the optical absorption edge of the titanium oxide-doped soda-lime silica glasses. J. Mater. Sci. Lett. 1988, 7, 453–456. [Google Scholar] [CrossRef]
- Higazy, A.A.; Hussein, A.; Awaida, M.A. A study of the optical absorption edge in silicate glasses containing TiO2 oxide. J. Mater. Sci. 1989, 24, 2203–2208. [Google Scholar] [CrossRef]
- Hogarth, C.; Khan, M. A study of optical absorption in some sodium titanium silicate glasses. J. Non-Cryst. Solids 1977, 24, 277–281. [Google Scholar] [CrossRef]
- Turnbull, R.C.; Lawrence, W.G. The role of titania in silica glasses. J. Am. Ceram. Soc. 1952, 35, 48–53. [Google Scholar] [CrossRef]
- Weyl, W.A. Coloured Glasses, 2nd ed.; Society of Glass Technology: Sheffield, UK, 1967. [Google Scholar]
- Falk, T.; Fredriksson, H.; Holmér, G.; Johansson, L.G.; Lang, M.; Sundberg, P. An Introduction to Glass-Craft, Technology and Art; Flygt, E., Ed.; Glafo—The Glass Research Institute: Växjö, Sweden, 2005. [Google Scholar]
- Allsopp, B.L.; Orman, R.; Johnson, S.R.; Baistow, I.; Sanderson, G.; Sundberg, P.; Stålhandske, C.; Grund, L.; Andersson, A.; Booth, J.; et al. Towards improved cover glasses for photovoltaic devices. Prog. Photovoltaics Res. Appl. 2020, 28, 1187–1206. [Google Scholar] [CrossRef]
- Allsopp, B.L.; Christopoulou, G.; Brookfield, A.; Forder, S.D.; Bingham, P.A. Optical and structural properties of d0 ion-doped silicate glasses for photovoltaic applications. Phys. Chem. Glasses Eur. J. Glass Sci. Technol. Part B 2018, 59, 193–202. [Google Scholar] [CrossRef]
- Johansson, W.; Peralta, A.; Jonson, B.; Anand, S.; Österlund, L.; Karlsson, S. Transparent TiO2 and ZnO thin films on glass for UV protection of PV modules. Front. Mater. 2019, 6, 259. [Google Scholar] [CrossRef]
- Hartmann, P.; Jedamzik, R.; Reichel, S.; Schreder, B. Optical glass and glass ceramic historical aspects and recent developments: A Schott view. Appl. Opt. 2010, 49, D157–D176. [Google Scholar] [CrossRef]
- Mukherjee, D.P.; Das, S.K. The influence of TiO2 content on the properties of glass ceramics: Crystallization, microstructure and hardness. Ceram. Int. 2014, 40, 4127–4134. [Google Scholar] [CrossRef]
- Limbach, R.; Karlsson, S.; Scannell, G.; Mathew, R.; Edén, M.; Wondraczek, L. The effect of TiO2 on the structure of Na2O-CaO-SiO2 glasses and its implications for thermal and mechanical properties. J. Non-Cryst. Solids 2017, 471, 6–18. [Google Scholar] [CrossRef]
- Scannell, G.; Koike, A.; Huang, L. Structure and thermo-mechanical response of TiO2-SiO2 glasses to temperature. J. Non-Cryst. Solids 2016, 447, 238–247. [Google Scholar] [CrossRef] [Green Version]
- Scannell, G.; Huang, L.; Rouxel, T. Elastic properties and indentation cracking behavior of Na2O-TiO2-SiO2 glasses. J. Non-Cryst. Solids 2015, 429, 129–142. [Google Scholar] [CrossRef]
- Scannell, G.; Huang, L. Structure and thermo-mechanical response of Na2O-TiO2-SiO2 glasses to temperature. J. Non-Cryst. Solids 2016, 453, 46–58. [Google Scholar] [CrossRef] [Green Version]
- Scannell, G.; Barra, S.; Huang, L. Structure and properties of Na2O-TiO2-SiO2 glasses: Role of Na and Ti on modifying the silica network. J. Non-Cryst. Solids 2016, 448, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Bengtsson, F.; Bayrak Pehlivan, İ.; Österlund, L.; Karlsson, S. Alkali Ion diffusion and structure of chemically strengthened TiO2 doped soda-lime silicate glass. J. Non-Cryst. Solids, 2022; submitted. [Google Scholar]
- Rouxel, T. Elastic Properties and short-to medium-range order in glasses. J. Am. Ceram. Soc. 2007, 90, 3019–3039. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Cormier, L.; Neuville, D. Ca and Na environments in Na2O-CaO-Al2O3-SiO2 glasses: Influence of cation mixing and cation-network interactions. Chem. Geol. 2004, 213, 103–113. [Google Scholar] [CrossRef]
- Ray, N.H. Composition-property relationships in inorganic oxide glasses. J. Non-Cryst. Solids 1974, 15, 423–434. [Google Scholar] [CrossRef]
- Grammes, T.; Limbach, R.; Bruns, S.; Van Wüllen, L.; De Ligny, D.; Kamitsos, E.I.; Durst, K.; Wondraczek, L.; Brauer, D.S. Tailoring the mechanical properties of metaluminous aluminosilicate glasses by phosphate incorporation. Front. Mater. 2020, 7, 115. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Sundberg, P.; Bäck, L.G.; Orman, R.; Booth, J.; Karlsson, S. Simultaneous chemical vapor deposition and thermal strengthening of glass. Thin Solid Films 2018, 669, 487–493. [Google Scholar] [CrossRef]
- Kato, Y.; Yamazaki, H.; Yoshida, S.; Matsuoka, J. Effect of densification on crack initiation under Vickers indentation test. J. Non-Cryst. Solids 2010, 356, 1768–1773. [Google Scholar] [CrossRef]
- Wada, M.; Furukawa, H.; Fujita, K. Crack resistance of glass on Vickers indentation. In Proceedings of the X International Congress on Glass (ICG), Kyoto, Japan, 8–12 July 1974; pp. 39–46. [Google Scholar]
- Day, D.E. Mixed alkali glasses—Their properties and uses. J. Non-Cryst. Solids 1976, 21, 343–372. [Google Scholar] [CrossRef]
- Karlsson, S.; Mathew, R.; Ali, S.; Edén, M. Investigations on ion exchange strengthened alumina doped soda lime silicate glasses: 27Al and 23Na NMR, DTA and indentation mechanical properties. 2021; submitted. [Google Scholar]
- Karlsson, S. The viscosity effect of TiO2 on soda-lime-silicate bearing glass. In Proceedings of the 4th International Workshop on Glass and Entropy and 9th International Otto Schott Colloquium, Jena, Germany, 9–12 September 2019. [Google Scholar]
- Varshneya, A.K.; Olson, G.A.; Kreski, P.K.; Gupta, P.K. Buildup and relaxation of stress in chemically strengthened glass. J. Non-Cryst. Solids 2015, 427, 91–97. [Google Scholar] [CrossRef]
- Ragoen, C.; Sen, S.; Lambricht, T.; Godet, S. Effect of Al2O3 content on the mechanical and interdiffusional properties of ion-exchanged Na-aluminosilicate glasses. J. Non-Cryst. Solids 2017, 458, 129–136. [Google Scholar] [CrossRef]
- Shelby, J.E. Introduction to Glass Science and Technology, 2nd ed.; The Royal Society of Chemistry: Cambridge, UK, 2005; pp. 249–264. [Google Scholar]
- Yoshida, S. Indentation deformation and cracking in oxide glass—Toward understanding of crack nucleation. J. Non-Cryst. Solids X 2019, 1, 100009. [Google Scholar] [CrossRef]
- Sheth, N.; Greenley, C.; Bermejo, R.; Mauro, J.C.; Pantano, C.G.; Kim, S.H. Effects of acid leaching treatment of soda-lime silicate glass on crack initiation and fracture. J. Am. Ceram. Soc. 2021, 104, 4550–4558. [Google Scholar] [CrossRef]
- To, T.; Jensen, L.R.; Smedskjaer, M.M. On the relation between fracture toughness and crack resistance in oxide glasses. J. Non-Cryst. Solids 2020, 534, 119946. [Google Scholar] [CrossRef]
- Sellappan, P.; Rouxel, T.; Célarié, F.; Becker, E.; Houizot, P.; Conradt, R. Composition dependence of indentation deformation and indentation cracking in glass. Acta Mater. 2013, 61, 5949–5965. [Google Scholar] [CrossRef]
- Rouxel, T.; Sellappan, P.; Célarié, F.; Houizot, P.; Sanglebœuf, J.-C. Toward glasses with better indentation cracking resistance. Comptes Rendus Mécanique 2014, 342, 46–51. [Google Scholar] [CrossRef]
- Logrado, M.; Eckert, H.; Murata, T.; Nakane, S.; Yamazaki, H. Structure-property relations in crack-resistant alkaline-earth aluminoborosilicate glasses studied by solid state NMR. J. Am. Ceram. Soc. 2021, 104, 2250–2267. [Google Scholar] [CrossRef]
- Liu, P.; Youngman, R.E.; Jensen, L.R.; Bockowski, M.; Smedskjaer, M.M. Achieving ultrahigh crack resistance in glass through humid aging. Phys. Rev. Mater. 2020, 4, 063606. [Google Scholar] [CrossRef]
- Limbach, R.; Winterstein-Beckmann, A.; Dellith, J.; Möncke, D.; Wondraczek, L. Plasticity, crack initiation and defect resistance in alkali-borosilicate glasses: From normal to anomalous behavior. J. Non-Cryst. Solids 2015, 417-418, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Koike, A.; Akiba, S.; Sakagami, T.; Hayashi, K.; Ito, S. Difference of cracking behavior due to Vickers indentation between physically and chemically tempered glasses. J. Non-Cryst. Solids 2012, 358, 3438–3444. [Google Scholar] [CrossRef]
- Januchta, K.; Stepniewska, M.; Jensen, L.R.; Zhang, Y.; Somers, M.A.J.; Bauchy, M.; Yue, Y.; Smedskjaer, M.M. Breaking the limit of micro-ductility in oxide glasses. Adv. Sci. 2019, 6, 1901281. [Google Scholar] [CrossRef] [Green Version]
- Januchta, K.; Smedskjaer, M.M. Indentation deformation in oxide glasses: Quantification, structural changes, and relation to cracking. J. Non-Cryst. Solids X 2019, 1, 100007. [Google Scholar] [CrossRef]
- Hasdemir, I.; Striepe, S.; Deubener, J.; Simon, K. A 2000-year perspective on indentation crack resistance and brittleness of glass. J. Non-Cryst. Solids 2015, 408, 51–56. [Google Scholar] [CrossRef]
- Gross, T.; Wu, J.; Baker, D.; Price, J.; Yongsunthon, R. Crack-resistant glass with high shear band density. J. Non-Cryst. Solids 2018, 494, 13–20. [Google Scholar] [CrossRef]
- Wondraczek, L.; Mauro, J.C. Advancing glasses through fundamental research. J. Eur. Ceram. Soc. 2009, 29, 1227–1234. [Google Scholar] [CrossRef]
- Mauro, J.C. Grand challenges in glass science. Front. Mater. 2014, 1, 20. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Jiang, L.; Mohagheghian, I.; Dear, J.P.; Li, L.; Yan, Y. New insights into nanoindentation crack initiation in ion-exchanged sodium aluminosilicate glass. J. Am. Ceram. Soc. 2018, 101, 2930–2940. [Google Scholar] [CrossRef]
- Ponader, C.W.; Boek, H.; Dickinson, J.E. X-ray absorption study of the coordination of titanium in sodium-titanium-silicate glasses. J. Non-Cryst. Solids 1996, 201, 81–94. [Google Scholar] [CrossRef]
- Duffy, J.A. Refractivity and coordination number changes of the Ti4+ ion in glass. Phys. Chem. Glasses Eur. J. Glass Sci. Technol. Part B 2006, 47, 582–587. [Google Scholar]
- Rouxel, T. Driving force for indentation cracking in glass: Composition, pressure and temperature dependence. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373, 20140140. [Google Scholar] [CrossRef] [Green Version]
- Machacek, J.; Gedeon, O.; Liska, M. Molecular approach to the 5-coordinated silicon atoms in silicate glasses. Phys. Chem. Glasses Eur. J. Glass Sci. Part B 2007, 48, 345–353. [Google Scholar]
- Zhang, Y.; Huang, L.; Shi, Y. Silica glass toughened by consolidation of glassy nanoparticles. Nano Lett. 2019, 19, 5222–5228. [Google Scholar] [CrossRef]


| Label | SiO2 | CaO | Na2O | TiO2 | ρ (g/cm3) | Vm (cm3/mol) | Cg | COx |
|---|---|---|---|---|---|---|---|---|
| Series 1 | ||||||||
| 1.1 | 73.9 | 11.2 | 15.0 | 0.0 | 2.514 | 23.86 | 0.499 | 0.07292 |
| 1.2 | 73.9 | 9.8 | 14.8 | 1.4 | 2.503 | 24.05 | 0.497 | 0.07286 |
| 1.3 | 75.6 | 5.5 | 15.6 | 3.4 | 2.478 | 24.57 | 0.494 | 0.07289 |
| 1.4 | 78.3 | 0.6 | 15.6 | 5.5 | 2.443 | 25.13 | 0.490 | 0.07315 |
| Series 2 | ||||||||
| 2.2 | 69.8 | 14.0 | 14.6 | 1.7 | 2.560 | 23.70 | 0.499 | 0.07242 |
| 2.3 | 68.3 | 14.0 | 14.6 | 3.0 | 2.566 | 23.52 | 0.502 | 0.07280 |
| 2.4 | 67.0 | 13.3 | 15.0 | 4.7 | 2.594 | 23.43 | 0.505 | 0.07329 |
| 2.5 | 65.8 | 13.2 | 15.0 | 6.0 | 2.605 | 23.44 | 0.505 | 0.07328 |
| 2.6 | 62.8 | 13.9 | 15.1 | 8.2 | 2.638 | 23.29 | 0.507 | 0.07342 |
| 2.7 | 61.3 | 13.8 | 15.0 | 9.9 | 2.661 | 23.21 | 0.509 | 0.07376 |
| Series 3 | ||||||||
| 3.2 | 71.6 | 12.1 | 14.5 | 1.8 | 2.519 | 23.88 | 0.497 | 0.07261 |
| 3.3 | 71.5 | 10.1 | 15.0 | 3.4 | 2.520 | 24.12 | 0.495 | 0.07251 |
| 3.4 | 72.3 | 7.3 | 15.4 | 5.1 | 2.526 | 24.30 | 0.497 | 0.07306 |
| 3.5 | 71.9 | 5.1 | 15.7 | 7.3 | 2.525 | 24.43 | 0.497 | 0.07334 |
| 3.6 | 71.6 | 2.5 | 16.3 | 9.7 | 2.535 | 24.51 | 0.500 | 0.07402 |
| 3.7 | 71.3 | 0.4 | 16.3 | 12.0 | 2.535 | 25.55 | 0.497 | 0.07174 |
| Uncertainties | ±1.3 | ±0.3 | ±0.3 | ±0.2 | ±0.2% | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karlsson, S. Compositional Effects on Indentation Mechanical Properties of Chemically Strengthened TiO2-Doped Soda Lime Silicate Glasses. Materials 2022, 15, 577. https://doi.org/10.3390/ma15020577
Karlsson S. Compositional Effects on Indentation Mechanical Properties of Chemically Strengthened TiO2-Doped Soda Lime Silicate Glasses. Materials. 2022; 15(2):577. https://doi.org/10.3390/ma15020577
Chicago/Turabian StyleKarlsson, Stefan. 2022. "Compositional Effects on Indentation Mechanical Properties of Chemically Strengthened TiO2-Doped Soda Lime Silicate Glasses" Materials 15, no. 2: 577. https://doi.org/10.3390/ma15020577






