RF Magnetron Sputtering of Substituted Hydroxyapatite for Deposition of Biocoatings
Abstract
:1. Introduction
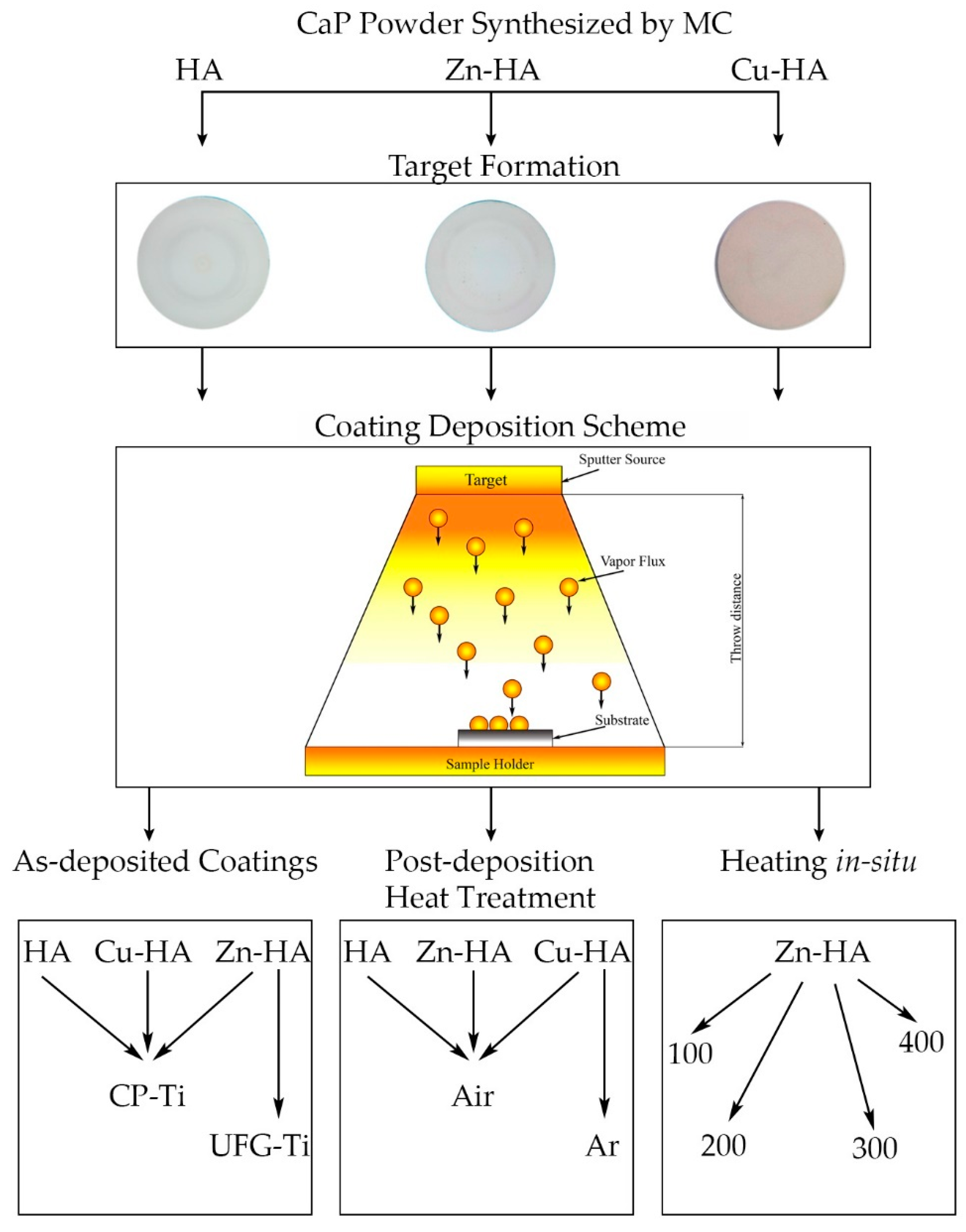
2. Materials and Methods
2.1. RF Magnetron Target Manufacturing
2.2. RF Magnetron Deposition on Titanium Substrates
2.3. Materials Characterization
3. Results
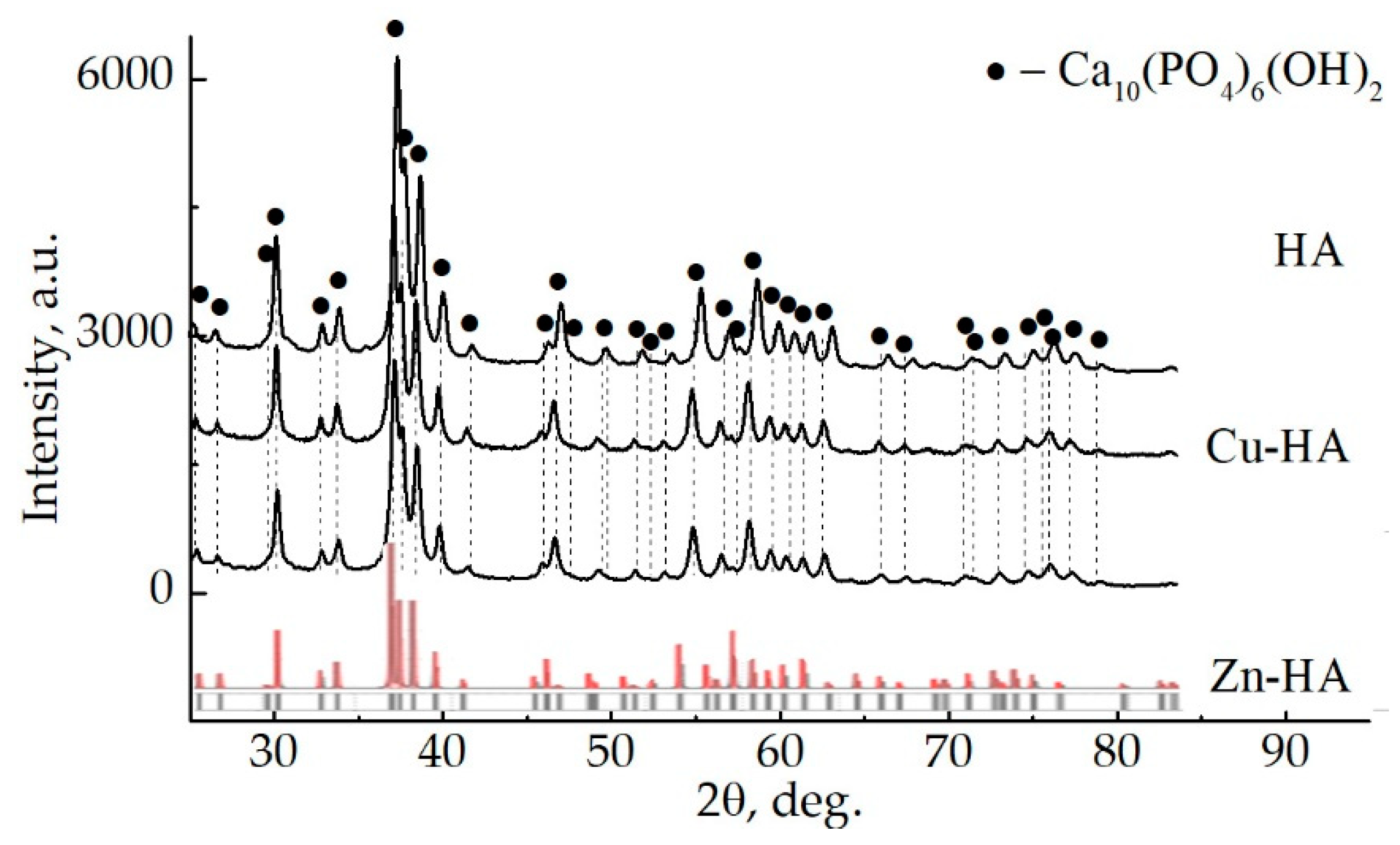
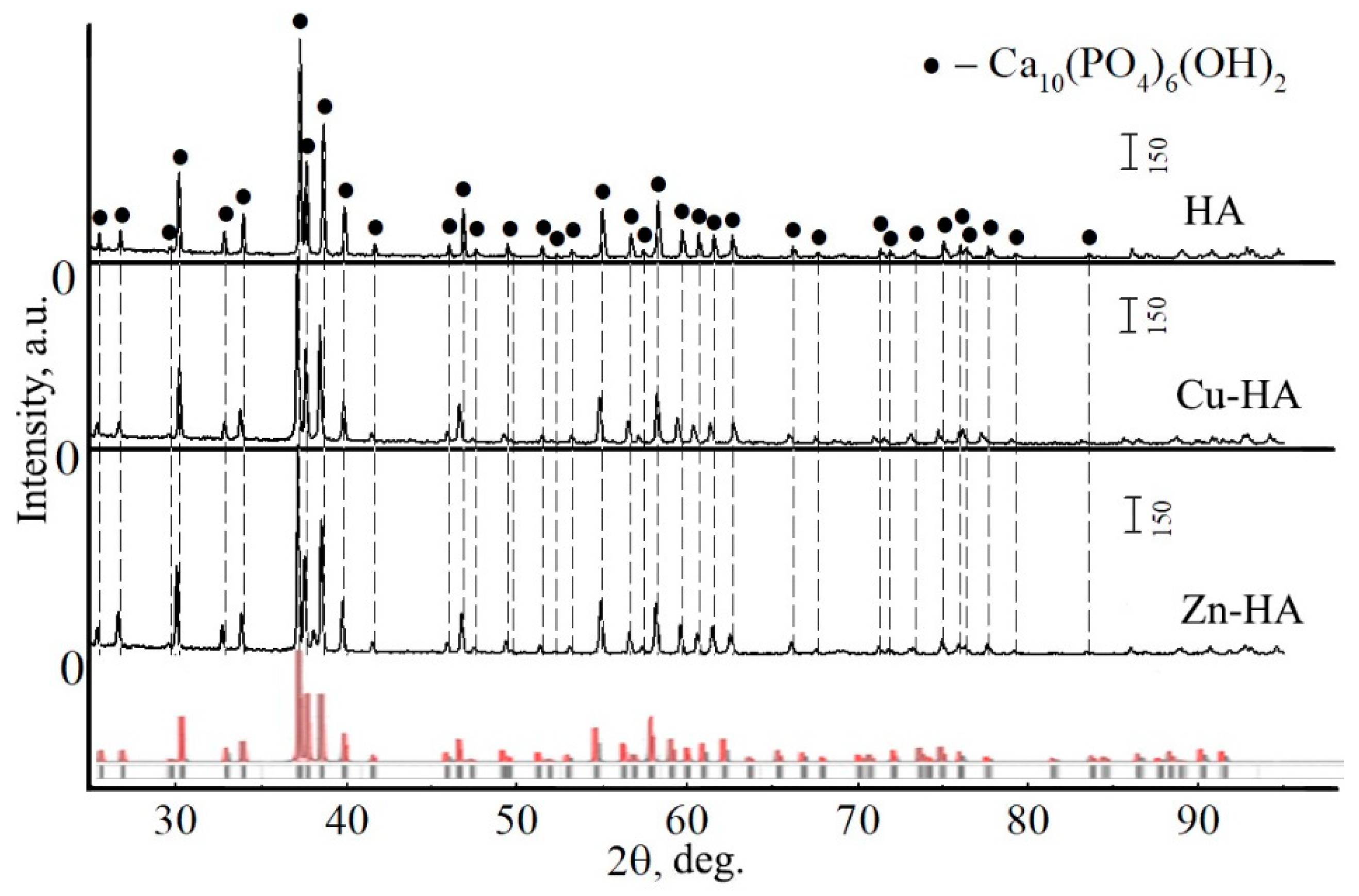
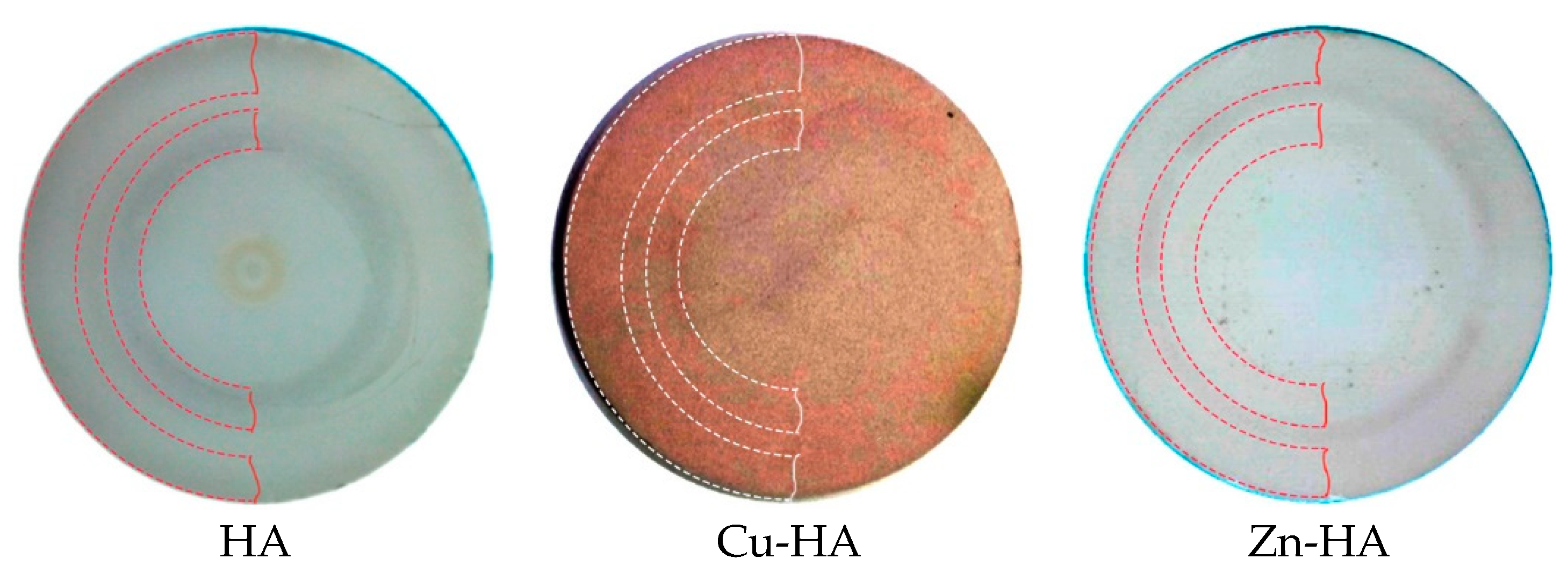
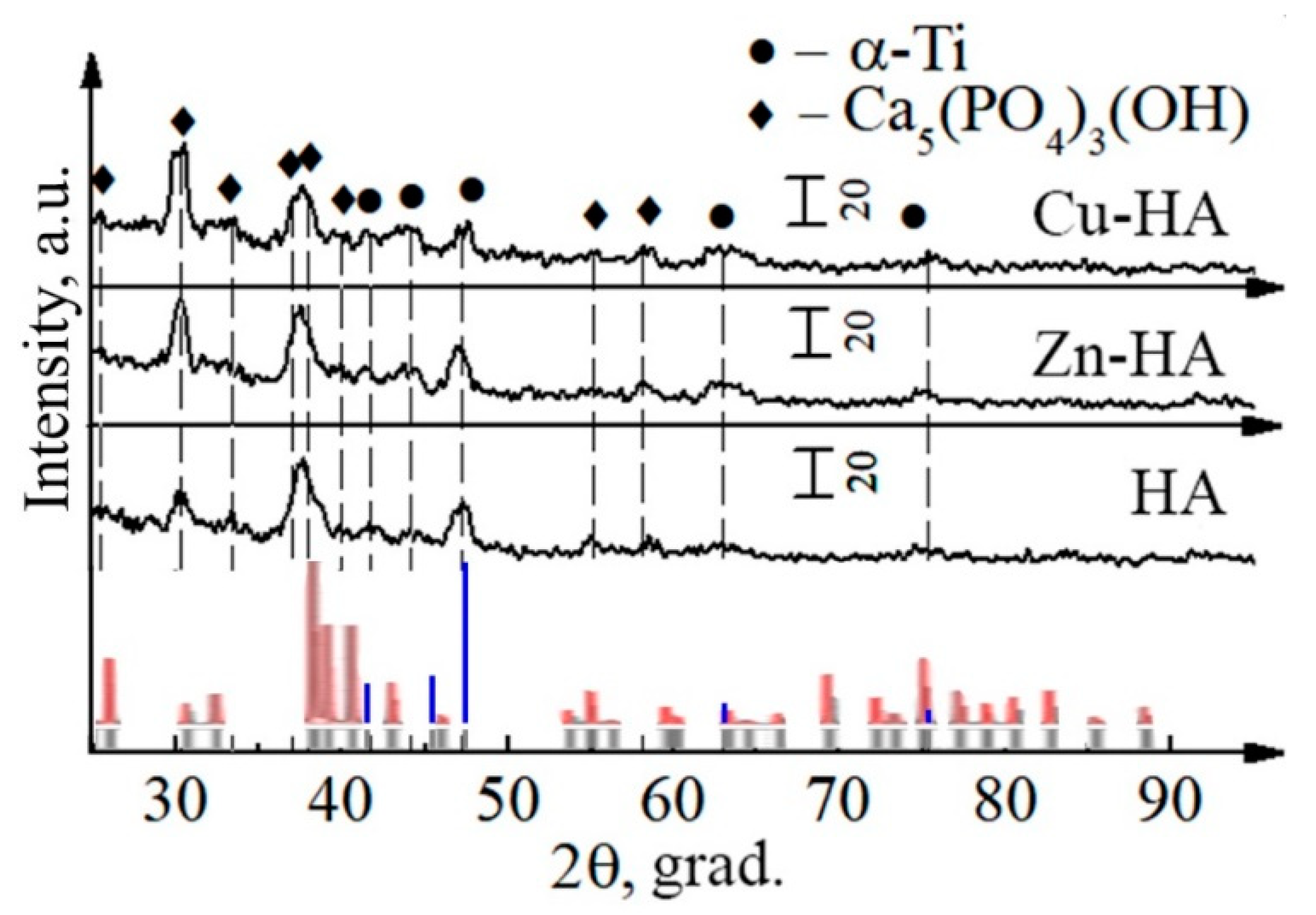
3.1. Targets for RF Magnetron Sputtering
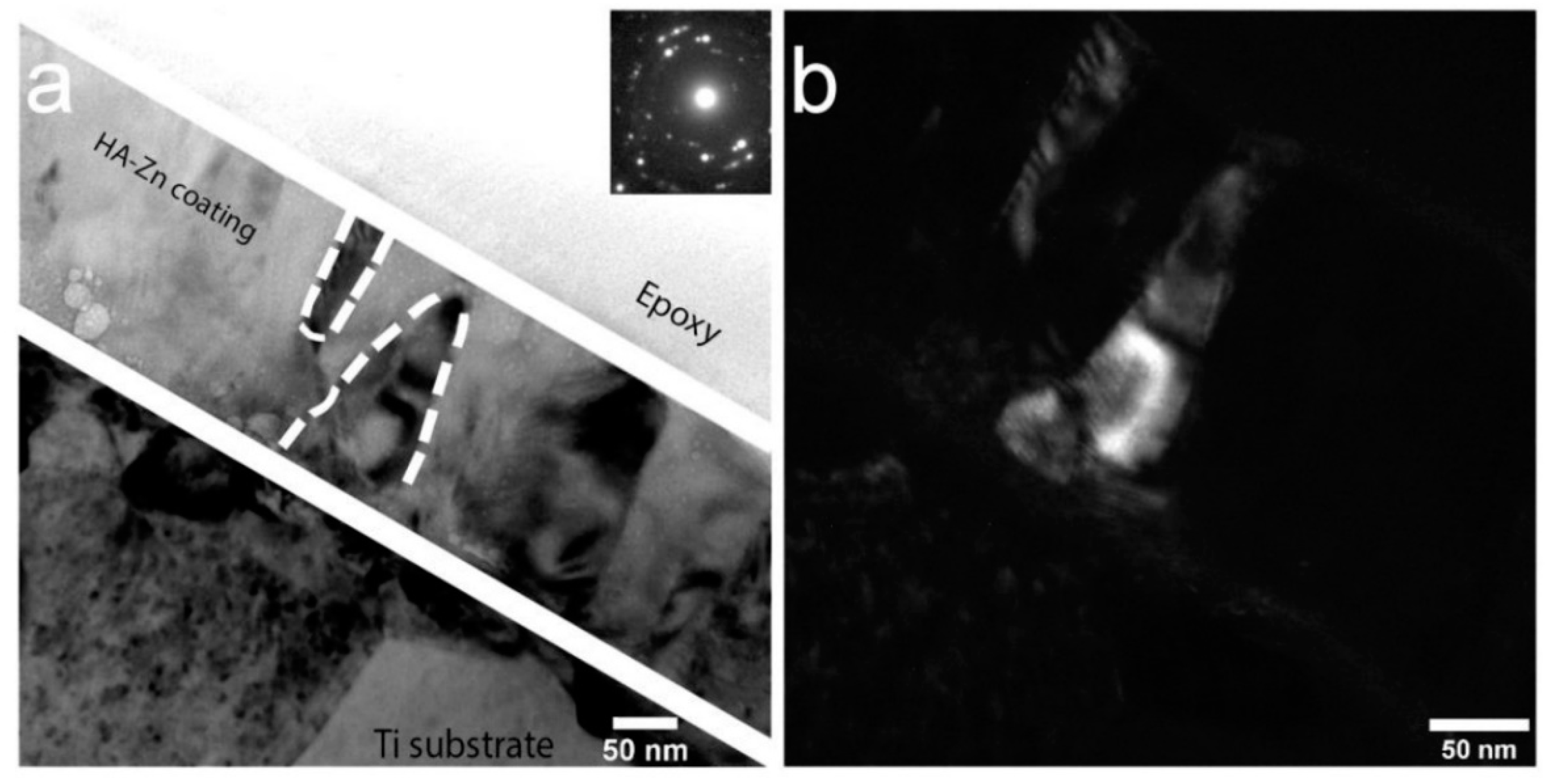
3.2. Substituted HA Sputtering and Resulting ACP Coatings
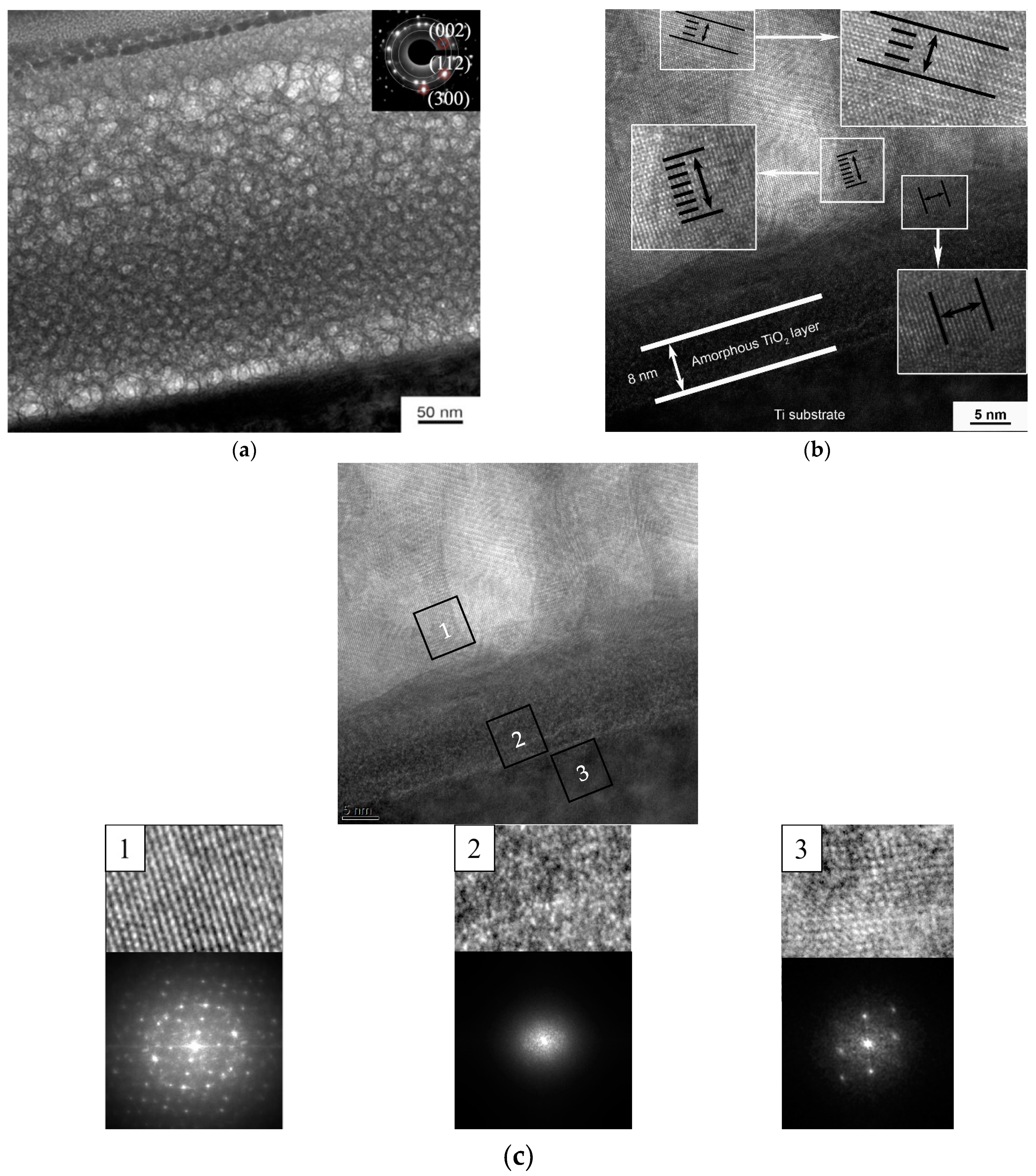
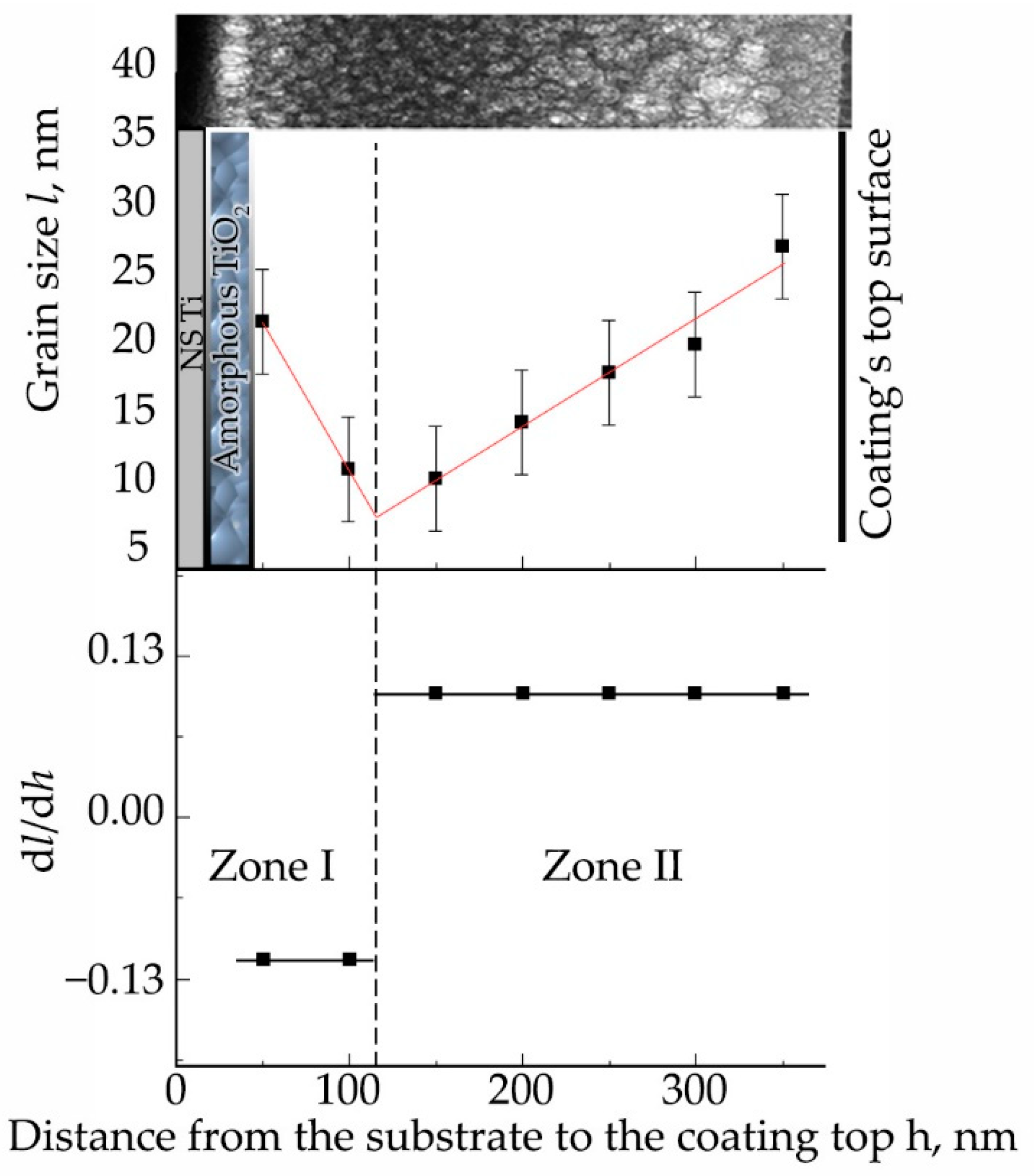
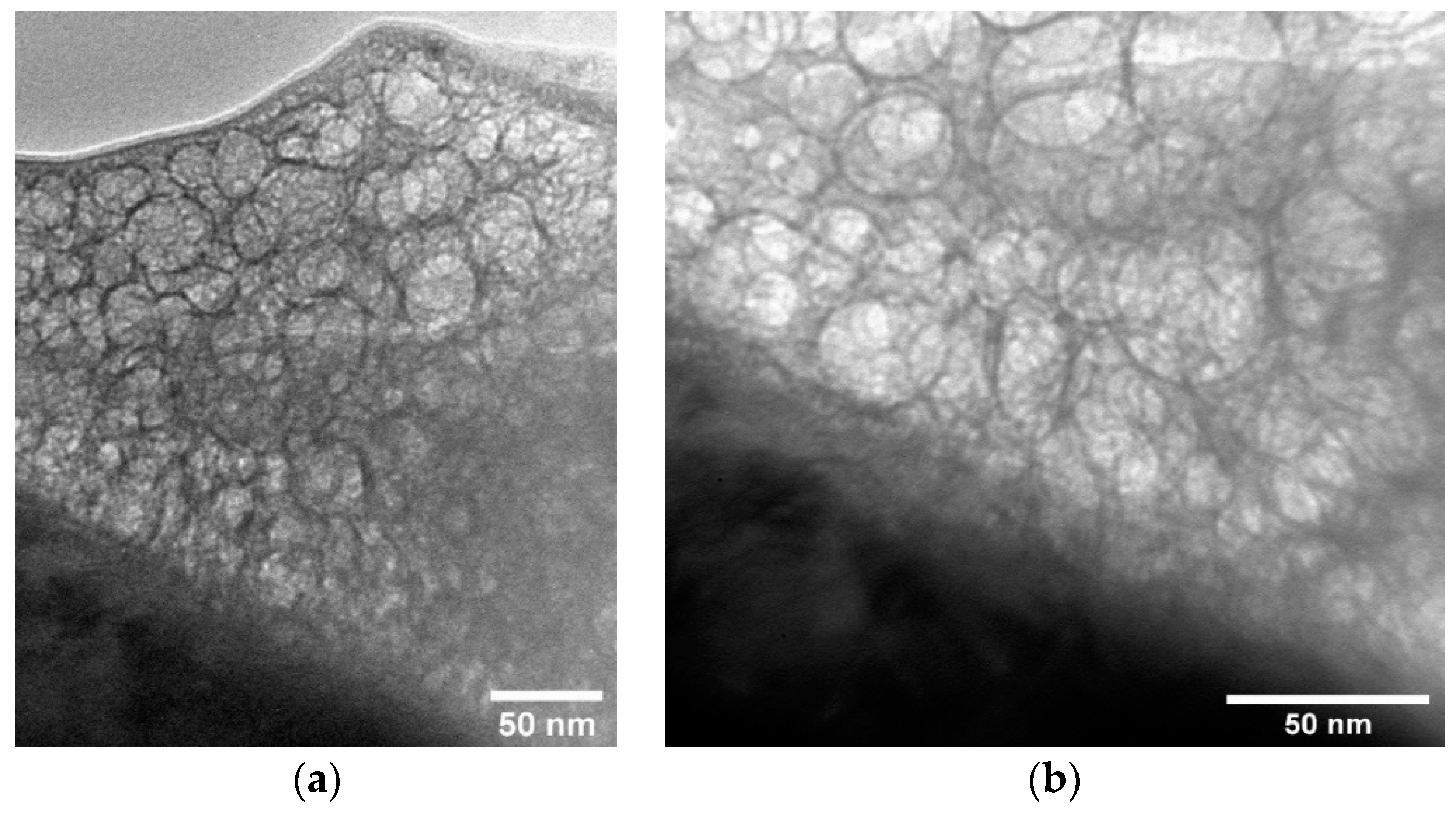
3.3. Formation of Calcium-Phosphate Coatings on a Titanium Substrate in the Nanostructured State
3.4. Influence of Controlled Heating of Substrates during RF Magnetron Sputtering and Post-Deposition Heat Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babuska, V.; Kasi, P.B.; Chocholata, P.; Wiesnerova, L.; Dvorakova, J.; Vrzakova, R.; Nekleionova, A.; Landsmann, L.; Kulda, V. Nanomaterials in Bone Regeneration. Appl. Sci. 2022, 12, 6793. [Google Scholar] [CrossRef]
- Yasenchuk, Y.; Marchenko, E.; Gunther, V.; Radkevich, A.; Kokorev, O.; Gunther, S.; Baigonakova, G.; Hodorenko, V.; Chekalkin, T.; Kang, J.; et al. Biocompatibility and Clinical Application of Porous TiNi Alloys Made by Self-Propagating High-Temperature Synthesis (SHS). Materials 2019, 12, 2405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putlyaev, V.I.; Safronova, T.V. A New Generation of Calcium Phosphate Biomaterials: The Role of Phase and Chemical Compositions. Glass Ceram. 2006, 63, 99–102. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E. In Silico Contact Pressure of Metal-on-Metal Total Hip Implant with Different Materials Subjected to Gait Loading. Metals 2022, 12, 1241. [Google Scholar] [CrossRef]
- Bi, Y.; Seabold, J.M.; Kaar, S.G.; Ragab, A.A.; Goldberg, V.M.; Anderson, J.M.; Greenfield, E.M. Adherent Endotoxin on Orthopedic Wear Particles Stimulates Cytokine Production and Osteoclast Differentiation. J. Bone Miner. Res. 2001, 16, 2082–2091. [Google Scholar] [CrossRef]
- Kovac, V.; Poljsak, B.; Bergant, M.; Scancar, J.; Mezeg, U.; Primozic, J. Differences in Metal Ions Released from Orthodontic Appliances in an In Vitro and In Vivo Setting. Coatings 2022, 12, 190. [Google Scholar] [CrossRef]
- Falisi, G.; Foffo, G.; Severino, M.; Di Paolo, C.; Bianchi, S.; Bernardi, S.; Pietropaoli, D.; Rastelli, S.; Gatto, R.; Botticelli, G. SEM-EDX Analysis of Metal Particles Deposition from Surgical Burs after Implant Guided Surgery Procedures. Coatings 2022, 12, 240. [Google Scholar] [CrossRef]
- Graziani, G.; Boi, M.; Bianchi, M. A Review on Ionic Substitutions in Hydroxyapatite Thin Films: Towards Complete Biomimetism. Coatings 2018, 8, 269. [Google Scholar] [CrossRef] [Green Version]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, Z.; Huang, J. Substituted Hydroxyapatite: A Recent Development. Mater. Technol. 2020, 35, 785–796. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Shurtakova, D.V.; Vakhin, A.V.; Grishin, P.O.; Gafurov, M.R. Incorporation of Iron(II) and (III) in Hydroxyapatite—A Theoretical Study. Crystals 2021, 11, 1219. [Google Scholar] [CrossRef]
- Šupová, M. Substituted Hydroxyapatites for Biomedical Applications: A Review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Thian, E.S.; Konishi, T.; Kawanobe, Y.; Lim, P.N.; Choong, C.; Ho, B.; Aizawa, M. Zinc-Substituted Hydroxyapatite: A Biomaterial with Enhanced Bioactivity and Antibacterial Properties. J. Mater. Sci. Mater. Med. 2013, 24, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Kalita, S.J.; Bhatt, H.A. Nanocrystalline Hydroxyapatite Doped with Magnesium and Zinc: Synthesis and Characterization. Mater. Sci. Eng. C 2007, 27, 837–848. [Google Scholar] [CrossRef]
- Prosolov, K.A.A.; Belyavskaya, O.A.A.; Bolat-ool, A.A.A.; Khlusov, I.A.A.; Nikolaeva, O.A.A.; Prosolov, A.B.B.; Mitrichenko, D.V.V.; Komkov, A.R.R.; Sharkeev, Y.P.Y.P. Antibacterial Potential of Zn- and Cu-Substituted Hydroxyapatite-Based Coatings Deposited by RF-Magnetron Sputtering. J. Phys. Conf. Ser. 2019, 1393, 012118. [Google Scholar] [CrossRef]
- Szurkowska, K.; Laskus, A.; Kolmas, J. Hydroxyapatite-Based Materials for Potential Use in Bone Tissue Infections. In Hydroxyapatite—Advances in Composite Nanomaterials, Biomedical Applications and Its Technological Facets; InTech: London, UK, 2018. [Google Scholar]
- Huang, Y.; Hao, M.; Nian, X.; Qiao, H.; Zhang, X.; Zhang, X.; Song, G.; Guo, J.; Pang, X.; Zhang, H. Strontium and Copper Co-Substituted Hydroxyapatite-Based Coatings with Improved Antibacterial Activity and Cytocompatibility Fabricated by Electrodeposition. Ceram. Int. 2016, 42, 11876–11888. [Google Scholar] [CrossRef]
- Bulina, N.V.; Vinokurova, O.B.; Eremina, N.V.; Prosanov, I.Y.; Khusnutdinov, V.R.; Chaikina, M.V. Features of Solid-Phase Mechanochemical Synthesis of Hydroxyapatite Doped by Copper and Zinc Ions. J. Solid State Chem. 2021, 296, 121973. [Google Scholar] [CrossRef]
- Gross, K.A.; Petzold, C.; Pluduma-LaFarge, L.; Kumermanis, M.; Haugen, H.J. Structural and Chemical Hierarchy in Hydroxyapatite Coatings. Materials 2020, 13, 4447. [Google Scholar] [CrossRef]
- Komarova, E.G.; Sharkeev, Y.P.; Sedelnikova, M.B.; Prosolov, K.A.; Khlusov, I.A.; Prymak, O.; Epple, M. Zn- or Cu-Containing CaP-Based Coatings Formed by Micro-Arc Oxidation on Titanium and Ti-40Nb Alloy: Part I—Microstructure, Composition and Properties. Materials 2020, 13, 4116. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Sharkeev, Y.P.; Tolkacheva, T.V.; Uvarkin, P.V.; Chebodaeva, V.V.; Prosolov, K.A.; Bakina, O.V.; Kashin, A.D.; Shcheglova, N.A.; Panchenko, A.A.; et al. Additively Manufactured Porous Titanium 3D–Scaffolds with Antibacterial Zn-, Ag- Calcium Phosphate Biocoatings. Mater. Charact. 2022, 186, 111782. [Google Scholar] [CrossRef]
- Teghil, R.; Curcio, M.; De Bonis, A. Substituted Hydroxyapatite, Glass, and Glass-Ceramic Thin Films Deposited by Nanosecond Pulsed Laser Deposition (PLD) for Biomedical Applications: A Systematic Review. Coatings 2021, 11, 811. [Google Scholar] [CrossRef]
- Safavi, M.S.; Walsh, F.C.; Surmeneva, M.A.; Surmenev, R.A.; Khalil-Allafi, J. Electrodeposited Hydroxyapatite-Based Biocoatings: Recent Progress and Future Challenges. Coatings 2021, 11, 110. [Google Scholar] [CrossRef]
- Safavi, M.S.; Surmeneva, M.A.; Surmenev, R.A.; Khalil-Allafi, J. RF-Magnetron Sputter Deposited Hydroxyapatite-Based Composite & Multilayer Coatings: A Systematic Review from Mechanical, Corrosion, and Biological Points of View. Ceram. Int. 2021, 47, 3031–3053. [Google Scholar] [CrossRef]
- Kozelskaya, A.; Dubinenko, G.; Vorobyev, A.; Fedotkin, A.; Korotchenko, N.; Gigilev, A.; Shesterikov, E.; Zhukov, Y.; Tverdokhlebov, S. Porous CaP Coatings Formed by Combination of Plasma Electrolytic Oxidation and RF-Magnetron Sputtering. Coatings 2020, 10, 1113. [Google Scholar] [CrossRef]
- Robinson, L.; Salma-Ancane, K.; Stipniece, L.; Meenan, B.J.; Boyd, A.R. The Deposition of Strontium and Zinc Co-Substituted Hydroxyapatite Coatings. J. Mater. Sci. Mater. Med. 2017, 28, 51. [Google Scholar] [CrossRef]
- López, E.O.; Rossi, A.L.; Bernardo, P.L.; Freitas, R.O.; Mello, A.; Rossi, A.M. Multiscale Connections between Morphology and Chemistry in Crystalline, Zinc-Substituted Hydroxyapatite Nanofilms Designed for Biomedical Applications. Ceram. Int. 2019, 45, 793–804. [Google Scholar] [CrossRef]
- Raţiu, C.A.; Cavalu, S.D.; Miclăuş, V.; Rus, V.; Lăzărescu, G.I. Histological Evidence of Novel Ceramic Implant: Evaluation of Tolerability in Rabbit Femur. Rom. J. Morphol. Embryol. 2015, 56, 1455–1460. [Google Scholar]
- Allegrini, S.; da Silva, A.C.; Tsujita, M.; Salles, M.B.; Gehrke, S.A.; Braga, F.J.C. Amorphous Calcium Phosphate (ACP) in Tissue Repair Process. Microsc. Res. Tech. 2018, 81, 579–589. [Google Scholar] [CrossRef]
- Qadir, M.; Li, Y.; Wen, C. Ion-Substituted Calcium Phosphate Coatings by Physical Vapor Deposition Magnetron Sputtering for Biomedical Applications: A Review. Acta Biomater. 2019, 89, 14–32. [Google Scholar] [CrossRef]
- Ozeki, K.; Aoki, H.; Masuzawa, T. Influence of the Hydrothermal Temperature and PH on the Crystallinity of a Sputtered Hydroxyapatite Film. Appl. Surf. Sci. 2010, 256, 7027–7031. [Google Scholar] [CrossRef]
- Radin, S.; Ducheyne, P.; Berthold, P.; Decker, S. Effect of Serum Proteins and Osteoblasts on the Surface Transformation of a Calcium Phosphate Coating: A Physicochemical and Ultrastructural Study. J. Biomed. Mater. Res. 1998, 39, 234–243. [Google Scholar] [CrossRef]
- Porter, A.E.; Hobbs, L.W.; Rosen, V.B.; Spector, M. The Ultrastructure of the Plasma-Sprayed Hydroxyapatite-Bone Interface Predisposing to Bone Bonding. Biomaterials 2002, 23, 725–733. [Google Scholar] [CrossRef]
- Mak, I.W.Y.; Evaniew, N.; Ghert, M. Lost in Translation: Animal Models and Clinical Trials in Cancer Treatment. Am. J. Transl. Res. 2014, 6, 114–118. [Google Scholar] [PubMed]
- Xue, W.; Tao, S.; Liu, X.; Zheng, X.B.; Ding, C. In Vivo Evaluation of Plasma Sprayed Hydroxyapatite Coatings Having Different Crystallinity. Biomaterials 2004, 25, 415–421. [Google Scholar] [CrossRef]
- Corno, M.; Chiatti, F.; Pedone, A.; Ugliengo, P. In Silico Study of Hydroxyapatite and Bioglass®: How Computational Science Sheds Light on Biomaterials. In Biomaterials—Physics and Chemistry; Pignatello, R., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-418-4. [Google Scholar]
- Bystrov, V.; Paramonova, E.; Avakyan, L.; Coutinho, J.; Bulina, N. Simulation and Computer Study of Structures and Physical Properties of Hydroxyapatite with Various Defects. Nanomaterials 2021, 11, 2752. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Surmeneva, M.A.; Tyurin, A.I.; Surmenev, R.A. Correlation between Structural and Mechanical Properties of RF Magnetron Sputter Deposited Hydroxyapatite Coating. Mater. Charact. 2018, 142, 261–269. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Surmeneva, M.A.; Surmenev, R.A.; Depla, D. Structural Evolution and Growth Mechanisms of RF-Magnetron Sputter-Deposited Hydroxyapatite Thin Films on the Basis of Unified Principles. Appl. Surf. Sci. 2017, 425, 497–506. [Google Scholar] [CrossRef]
- Thian, E.S.; Huang, J.; Best, S.M.; Barber, Z.H.; Bonfield, W. Magnetron Co-Sputtered Silicon-Containing Hydroxyapatite Thin Films—An in Vitro Study. Biomaterials 2005, 26, 2947–2956. [Google Scholar] [CrossRef]
- Feng, F.; Wu, Y.; Xin, H.; Chen, X.; Guo, Y.; Qin, D.; An, B.; Diao, X.; Luo, H. Surface Characteristics and Biocompatibility of Ultrafine-Grain Ti after Sandblasting and Acid Etching for Dental Implants. ACS Biomater. Sci. Eng. 2019, 5, 5107–5115. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Nagumothu, R.; Parfenov, E.; Valiev, R. Development of Nanostructured Titanium Implants for Biomedical Implants—A Short Review. Mater. Today Proc. 2021, 46, 1195–1200. [Google Scholar] [CrossRef]
- Chaikina, M.V.; Bulina, N.V.; Prosanov, I.Y.; Vinokurova, O.B.; Ishchenko, A.V. Structure Formation of Zinc-Substituted Hydroxyapatite during Mechanochemical Synthesis. Inorg. Mater. 2020, 56, 402–408. [Google Scholar] [CrossRef]
- Orlovskii, V.P.; Komlev, V.S.; Barinov, S.M. Hydroxyapatite and Hydroxyapatite-Based Ceramics. Inorg. Mater. 2002, 38, 973–984. [Google Scholar] [CrossRef]
- Sharkeev, Y.P.; Eroshenko, A.Y.; Danilov, V.I.; Tolmachev, A.I.; Uvarkin, P.V.; Abzaev, Y.A. Microstructure and Mechanical Properties of Nanostructured and Ultrafine-Grained Titanium and the Zirconium Formed by the Method of Severe Plastic Deformation. Russ. Phys. J. 2014, 56, 1156–1162. [Google Scholar] [CrossRef]
- Kweh, S.W.K.; Khor, K.A.; Cheang, P. Plasma-Sprayed Hydroxyapatite (HA) Coatings with Flame-Spheroidized Feedstock: Microstructure and Mechanical Properties. Biomaterials 2000, 21, 1223–1234. [Google Scholar] [CrossRef]
- Yang, Y.C.; Chang, E. The Bonding of Plasma-Sprayed Hydroxyapatite Coatings to Titanium: Effect of Processing, Porosity and Residual Stress. Thin Solid Films 2003, 444, 260–275. [Google Scholar] [CrossRef]
- Miranda, P.; Saiz, E.; Gryn, K.; Tomsia, A.P. Sintering and Robocasting of β-Tricalcium Phosphate Scaffolds for Orthopaedic Applications. Acta Biomater. 2006, 2, 457–466. [Google Scholar] [CrossRef]
- Rau, J.V.; Wu, V.M.; Graziani, V.; Fadeeva, I.V.; Fomin, A.S.; Fosca, M.; Uskoković, V. The Bone Building Blues: Self-Hardening Copper-Doped Calcium Phosphate Cement and Its in Vitro Assessment against Mammalian Cells and Bacteria. Mater. Sci. Eng. C 2017, 79, 270–279. [Google Scholar] [CrossRef]
- Miyaji, F.; Kono, Y.; Suyama, Y. Formation and Structure of Zinc-Substituted Calcium Hydroxyapatite. Mater. Res. Bull. 2005, 40, 209–220. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Gupta, A.; Verma, M.; Murugan, P.A.; Sengupta, P.; Matheshwaran, S.; Manna, I.; Balani, K. Site-Specific Antibacterial Efficacy and Cyto/Hemo-Compatibility of Zinc Substituted Hydroxyapatite. Ceram. Int. 2019, 45, 12225–12233. [Google Scholar] [CrossRef]
- Kazin, P.E.; Zykin, M.A.; Tret’yakov, Y.D.; Jansen, M. Synthesis and Properties of Colored Copper-Containing Apatites of Composition Ca5(PO4)3CuyO y + δ(OH)0.5 − y − ΔX0.5 (X = OH, F, Cl). Russ. J. Inorg. Chem. 2008, 53, 362–366. [Google Scholar] [CrossRef]
- Gomes, S.; Vichery, C.; Descamps, S.; Martinez, H.; Kaur, A.; Jacobs, A.; Nedelec, J.-M.; Renaudin, G. Cu-Doping of Calcium Phosphate Bioceramics: From Mechanism to the Control of Cytotoxicity. Acta Biomater. 2018, 65, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Baikie, T.; Ng, G.M.H.; Madhavi, S.; Pramana, S.S.; Blake, K.; Elcombe, M.; White, T.J. The Crystal Chemistry of the Alkaline-Earth Apatites A10(PO4)6CuxOy(H)z (A = Ca, Sr and Ba). Dalton Trans. 2009, 34, 6722. [Google Scholar] [CrossRef]
- Shanker, G.; Prathap, P.; Srivatsa, K.M.K.; Singh, P. Effect of Balanced and Unbalanced Magnetron Sputtering Processes on the Properties of SnO2 Thin Films. Curr. Appl. Phys. 2019, 19, 697–703. [Google Scholar] [CrossRef]
- Monaghan, D.P.; Teer, D.G.; Logan, P.A.; Efeoglu, I.; Arnell, R.D. Deposition of Wear Resistant Coatings Based on Diamond like Carbon by Unbalanced Magnetron Sputtering. Surf. Coat. Technol. 1993, 60, 525–530. [Google Scholar] [CrossRef]
- Zubizarreta, C.; G-Berasategui, E.; Ciarsolo, I.; Barriga, J.; Gaspar, D.; Martins, R.; Fortunato, E. The Influence of Target Erosion Grade in the Optoelectronic Properties of AZO Coatings Growth by Magnetron Sputtering. Appl. Surf. Sci. 2016, 380, 218–222. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium Phosphate Ceramics in Bone Tissue Engineering: A Review of Properties and Their Influence on Cell Behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef] [PubMed]
- Syromotina, D.S.; Surmeneva, M.A.; Gorodzha, S.N.; Pichugin, V.F.; Ivanova, A.A.; Grubova, I.Y.; Kravchuk, K.S.; Gogolinskii, K.V.; Prymak, O.; Epple, M.; et al. Physical-Mechanical Characteristics of RF Magnetron Sputter-Deposited Coatings Based on Silver-Doped Hydroxyapatite. Russ. Phys. J. 2014, 56, 1198–1205. [Google Scholar] [CrossRef]
- Haque, S.M.; Rao, K.D.; Misal, J.S.; Tokas, R.B.; Shinde, D.D.; Ramana, J.V.; Rai, S.; Sahoo, N.K. Study of Hafnium Oxide Thin Films Deposited by RF Magnetron Sputtering under Glancing Angle Deposition at Varying Target to Substrate Distance. Appl. Surf. Sci. 2015, 353, 459–468. [Google Scholar] [CrossRef]
- Prosolov, K.A.; Mitrichenko, D.V.; Prosolov, A.B.; Nikolaeva, O.O.; Lastovka, V.V.; Belyavskaya, O.A.; Chebodaeva, V.A.; Glukhov, I.A.; Litvinova, L.S.; Shupletsova, V.V.; et al. Zn-Doped CaP-Based Coatings on Ti–6Al–4V and Ti–6Al–7Nb Alloys Prepared by Magnetron Sputtering: Controllable Biodegradation, Bacteriostatic, and Osteogenic Activities. Coatings 2021, 11, 809. [Google Scholar] [CrossRef]
- Prosolov, K.A.; Lastovka, V.V.; Belyavskaya, O.A.; Lychagin, D.V.; Schmidt, J.; Sharkeev, Y.P. Tailoring the Surface Morphology and the Crystallinity State of Cu- and Zn-Substituted Hydroxyapatites on Ti and Mg-Based Alloys. Materials 2020, 13, 4449. [Google Scholar] [CrossRef]


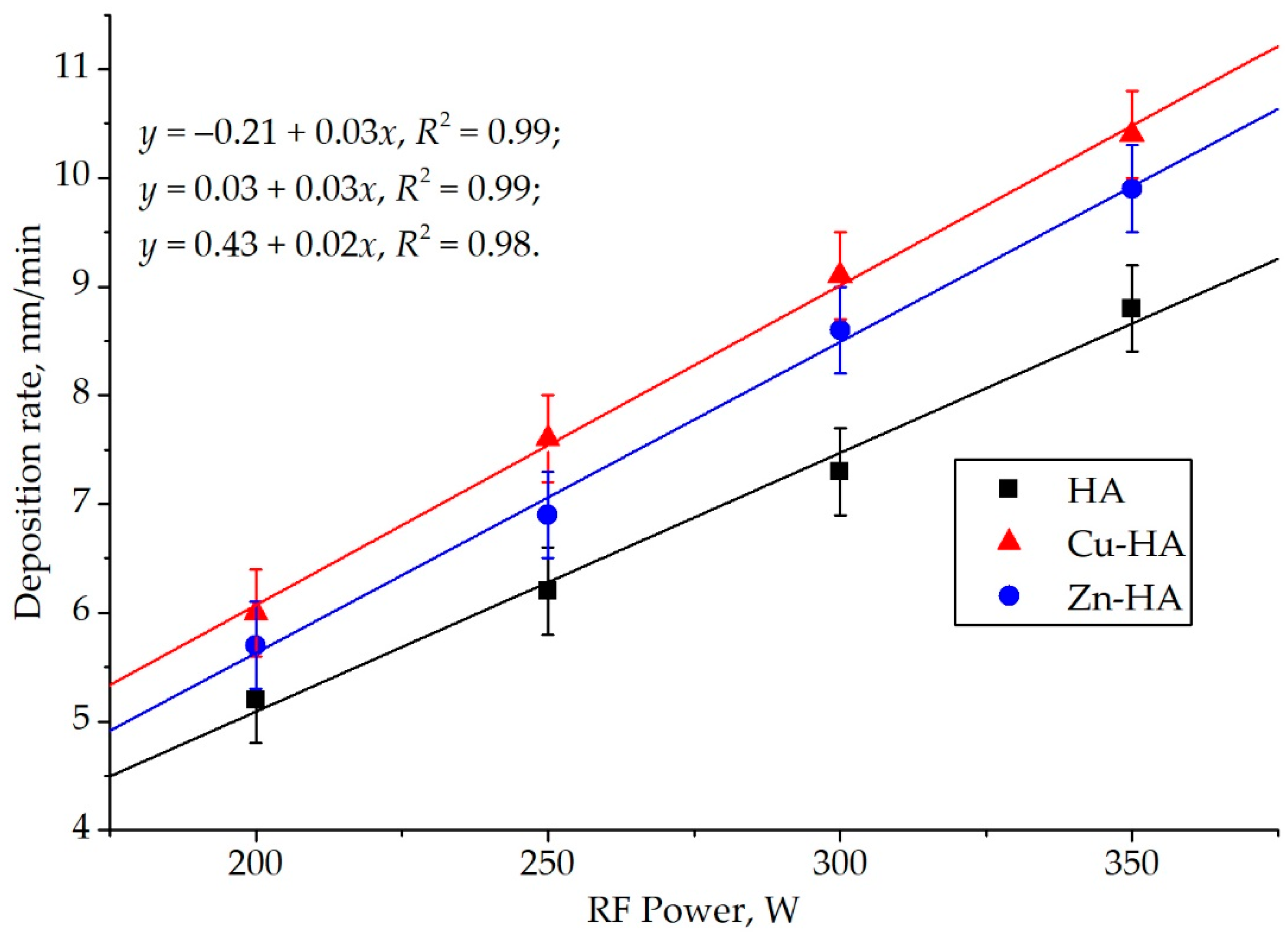
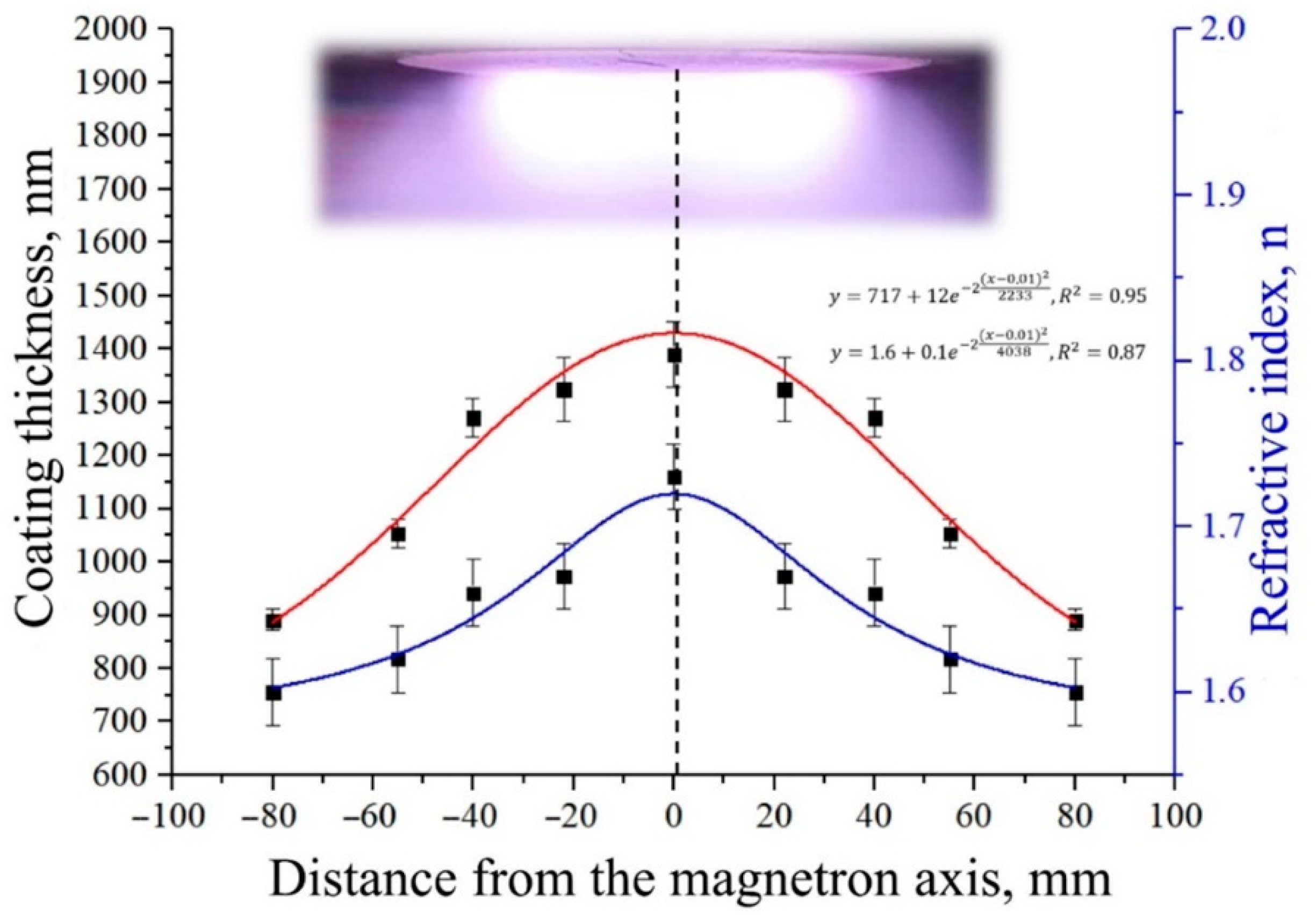


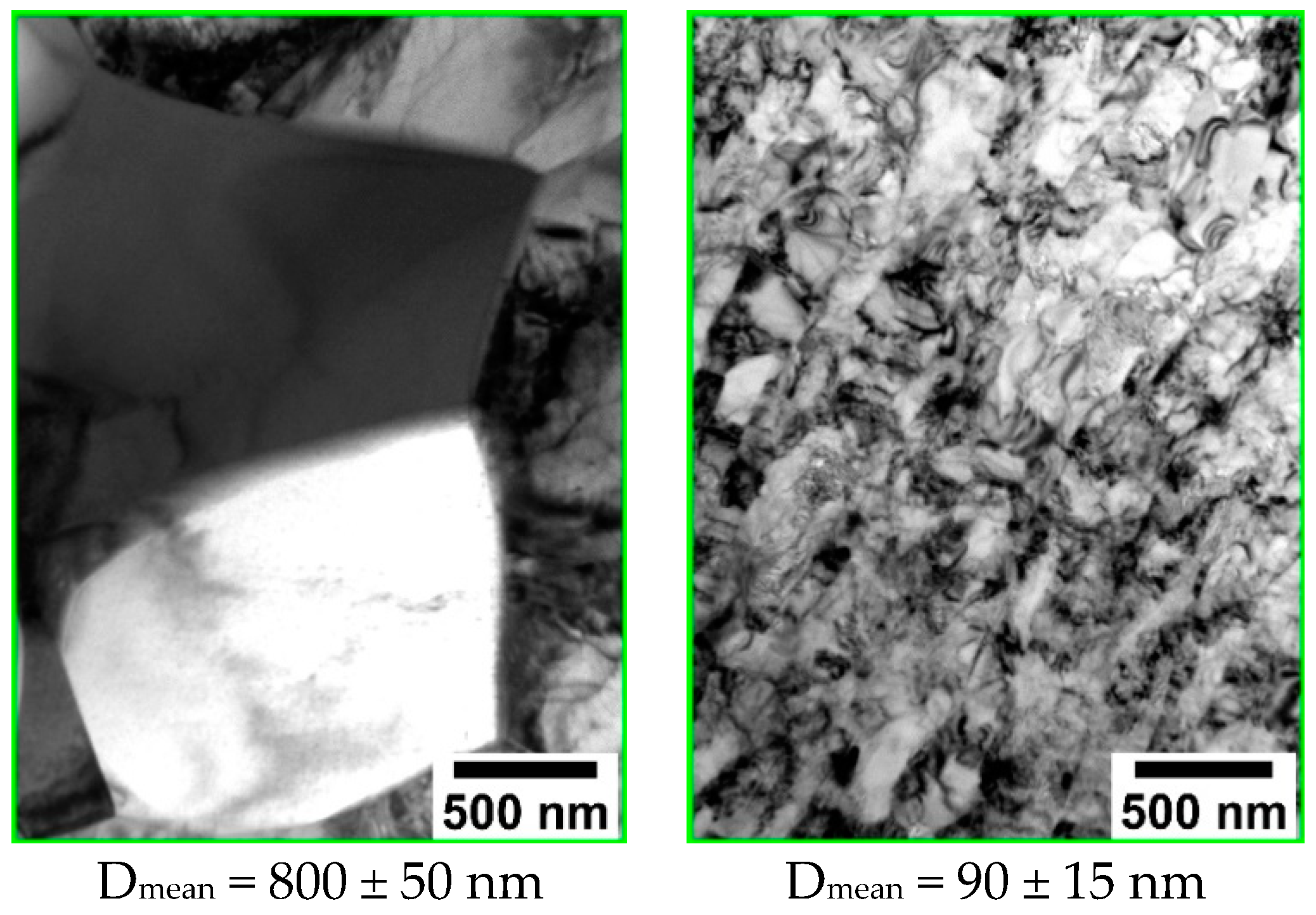
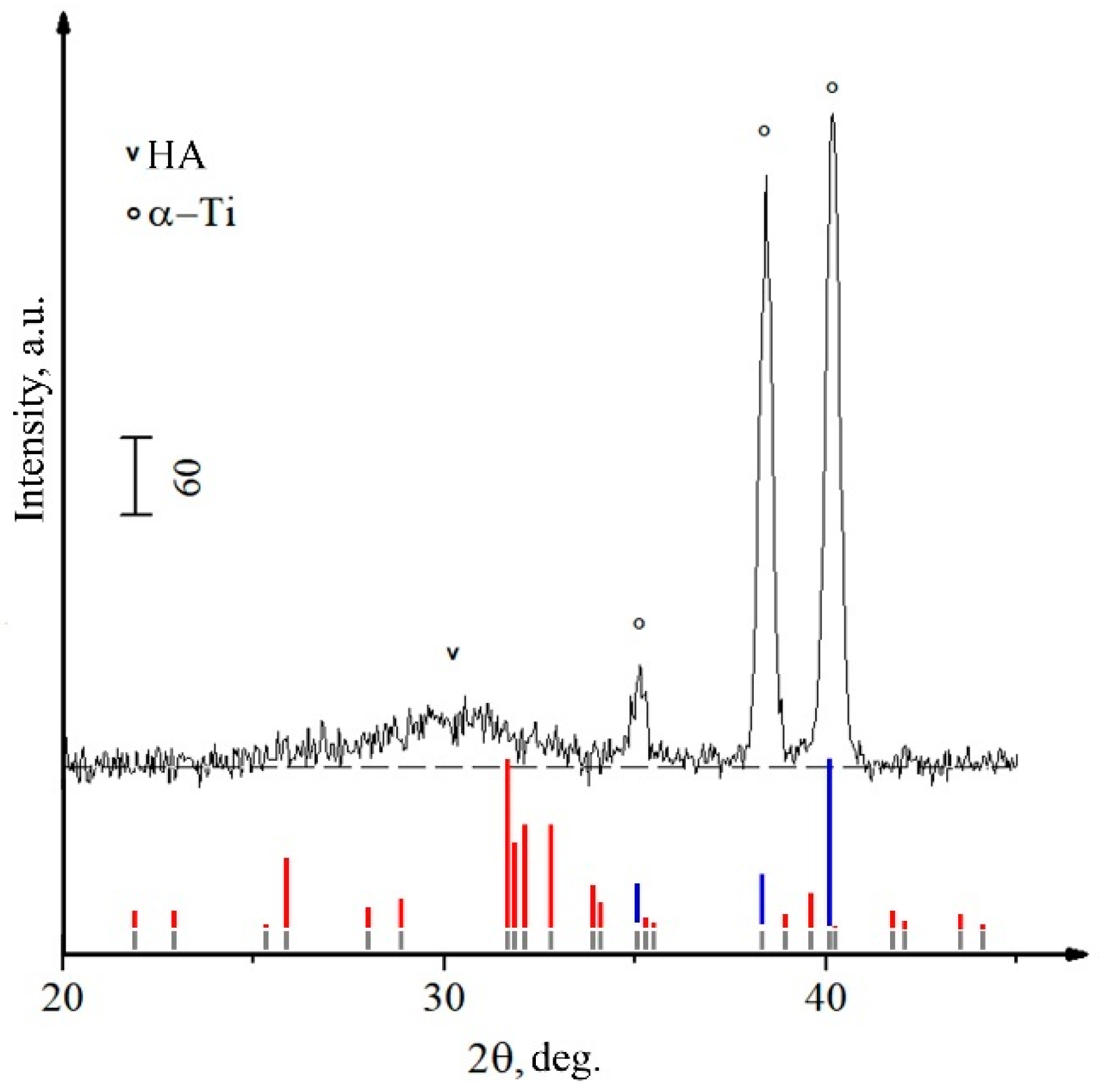



| Powder | a = b, Å | c, Å | V, Å3 | CSR, nm |
|---|---|---|---|---|
| HA | 9.4166 ± 0.0021 | 6.8775 ± 0.005 | 528.1 | 94 ± 21 |
| Cu-HA | 9.4201 ± 0.0611 | 6.7659 ± 0.2546 | 519.9 | 74 ± 13 |
| Zn-HA | 9.4311 ± 0.0074 | 6.6649 ± 0.425 | 513.4 | 76 ± 29 |
| ICDD | 9.422 | 6.881 | 533.7 |
| Target | a = b, Å | c, Å | V, Å3 | , g/cm3 | , g/cm3 |
|---|---|---|---|---|---|
| HA | 9.4175 ± 0.0035 | 6.8767 ± 0.0082 | 528.1 | 2.64 0.12 | 3.158 |
| Cu-HA | 9.4169 ± 0.0912 | 6.8796 ± 0.0153 | 519.9 | 2.8 0.08 | 3.172 |
| Zn-HA | 9.4158 ± 0.0782 | 6.8911 ± 0.0096 | 513.4 | 2.83 0.14 | 3.185 |
| ICDD | 9.422 | 6.881 | 533.7 | - | 3.154 |
| Target Material | Ca/P | Zn, wt.% | Cu, wt.% |
|---|---|---|---|
| HA | 1.9 | - | - |
| Cu-HA | 2.5 | - | 0.3 |
| Zn-HA | 2.25 | 0.8 | - |
| Target Material | Lattice Parameters | Crystallinity Index (%) |
|---|---|---|
| HA | a = b = 9.529 Å, c = 6.841 Å | 18 ± 12 |
| Cu-HA | a = b = 9.659 Å, c = 6.869 Å | 25 ± 17 |
| Zn-HA | a = b = 9.587 Å, c = 6.913 Å | 29 ± 14 |
| Annealing Temperature, °C | Lattice Parameters | CSR, nm | Crystallinity Index (%) |
|---|---|---|---|
| - | Amorphous state | - | - |
| 400 | a = b = 9.547Å, c = 6.866 Å | 47 | 27 ± 12 |
| 700 | a = b = 9.534 Å, c = 6.783 Å | 13 | 43 ± 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prosolov, K.A.; Lastovka, V.V.; Khimich, M.A.; Chebodaeva, V.V.; Khlusov, I.A.; Sharkeev, Y.P. RF Magnetron Sputtering of Substituted Hydroxyapatite for Deposition of Biocoatings. Materials 2022, 15, 6828. https://doi.org/10.3390/ma15196828
Prosolov KA, Lastovka VV, Khimich MA, Chebodaeva VV, Khlusov IA, Sharkeev YP. RF Magnetron Sputtering of Substituted Hydroxyapatite for Deposition of Biocoatings. Materials. 2022; 15(19):6828. https://doi.org/10.3390/ma15196828
Chicago/Turabian StyleProsolov, Konstantin A., Vladimir V. Lastovka, Margarita A. Khimich, Valentina V. Chebodaeva, Igor A. Khlusov, and Yurii P. Sharkeev. 2022. "RF Magnetron Sputtering of Substituted Hydroxyapatite for Deposition of Biocoatings" Materials 15, no. 19: 6828. https://doi.org/10.3390/ma15196828






