Formation and Evolution of sp2 Hybrid Conjugate Structure of Polyacrylonitrile Precursor during Stabilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PAN Thermal-Stabilized Fibers
2.2. Characterizations
3. Results and Discussion
3.1. The Effect of the Temperature on the Formation and Evolution of sp2 Hybrid Conjugate Structures for PAN Fibers
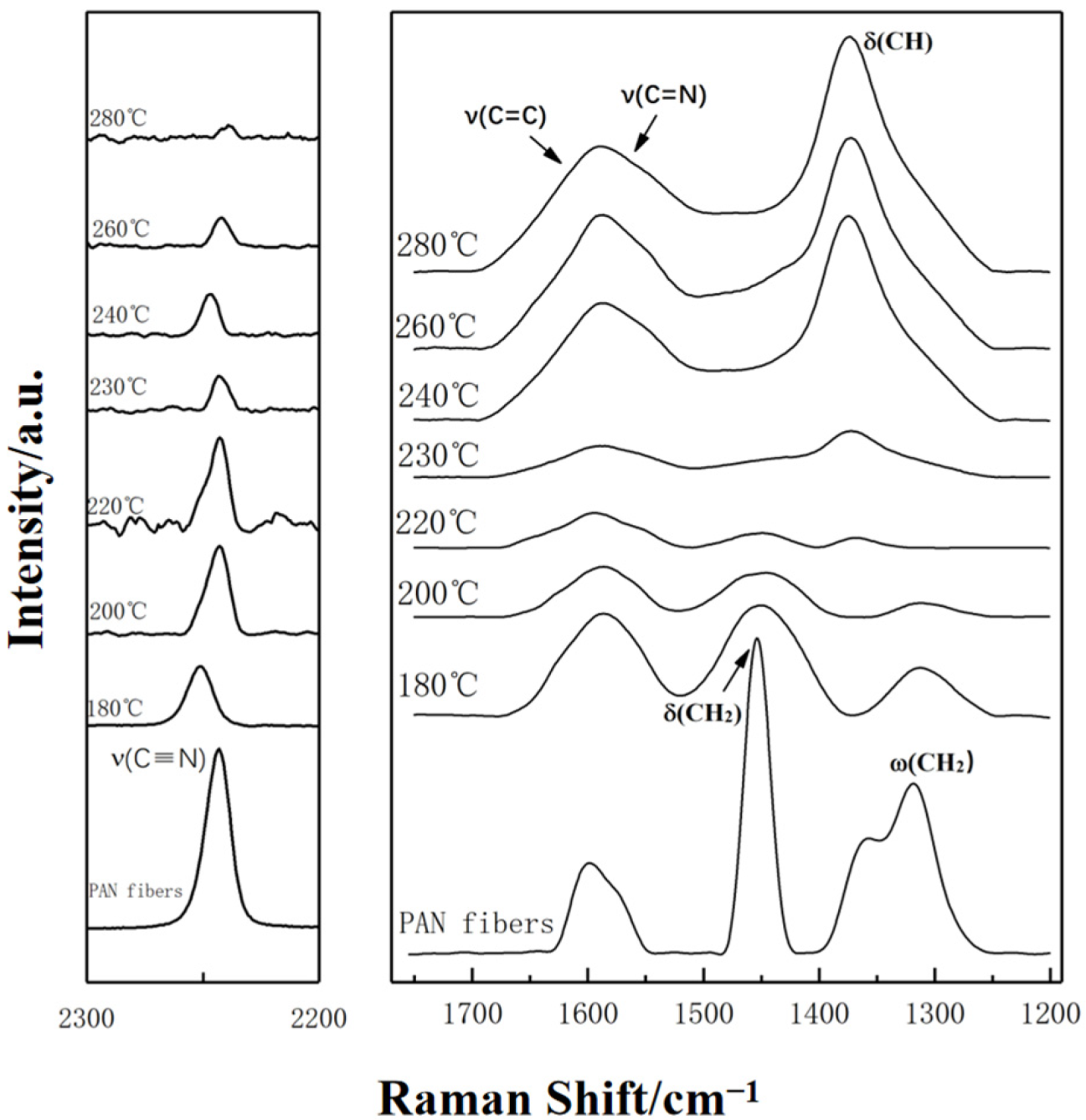
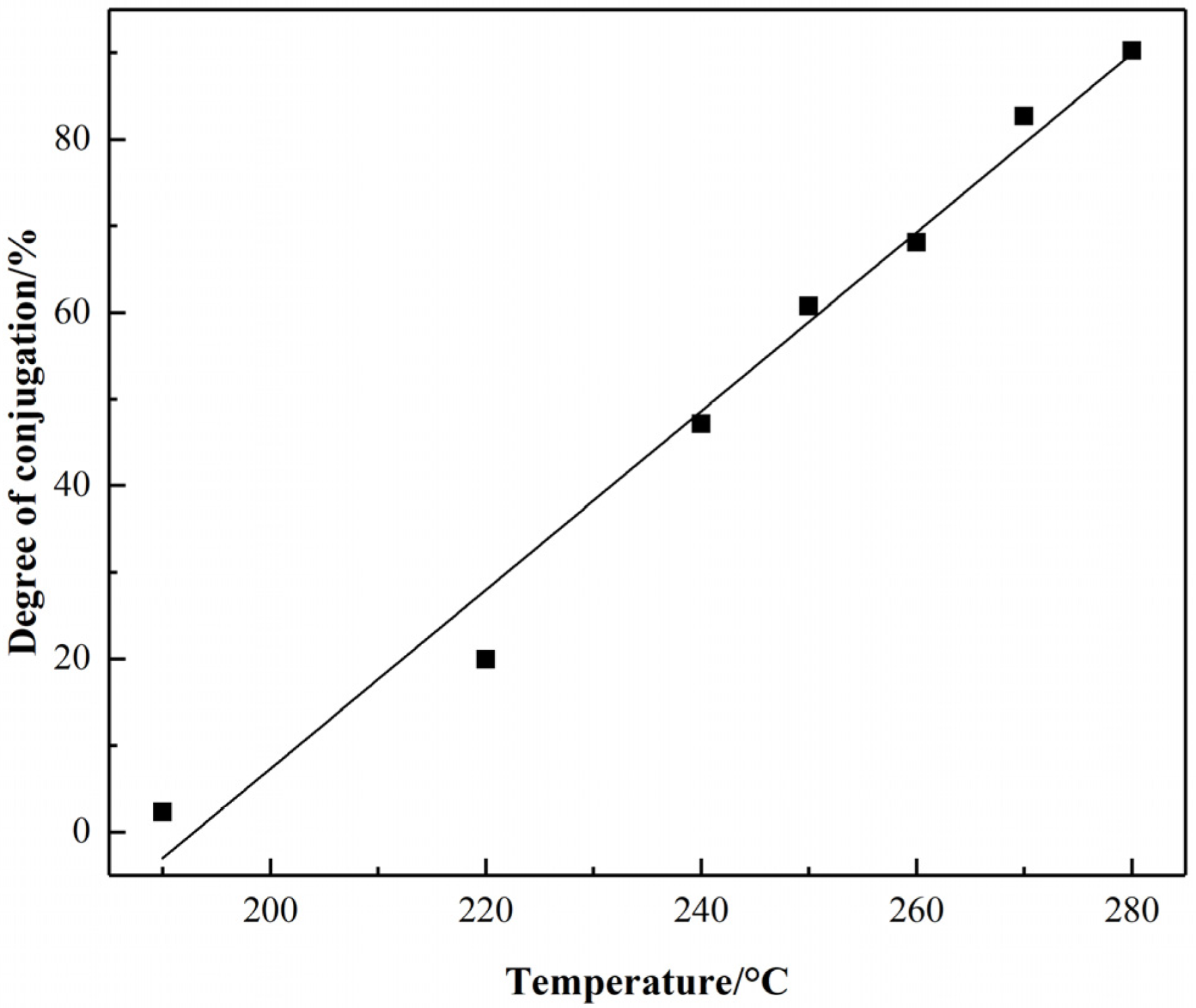
3.1.1. Raman Spectra Analysis
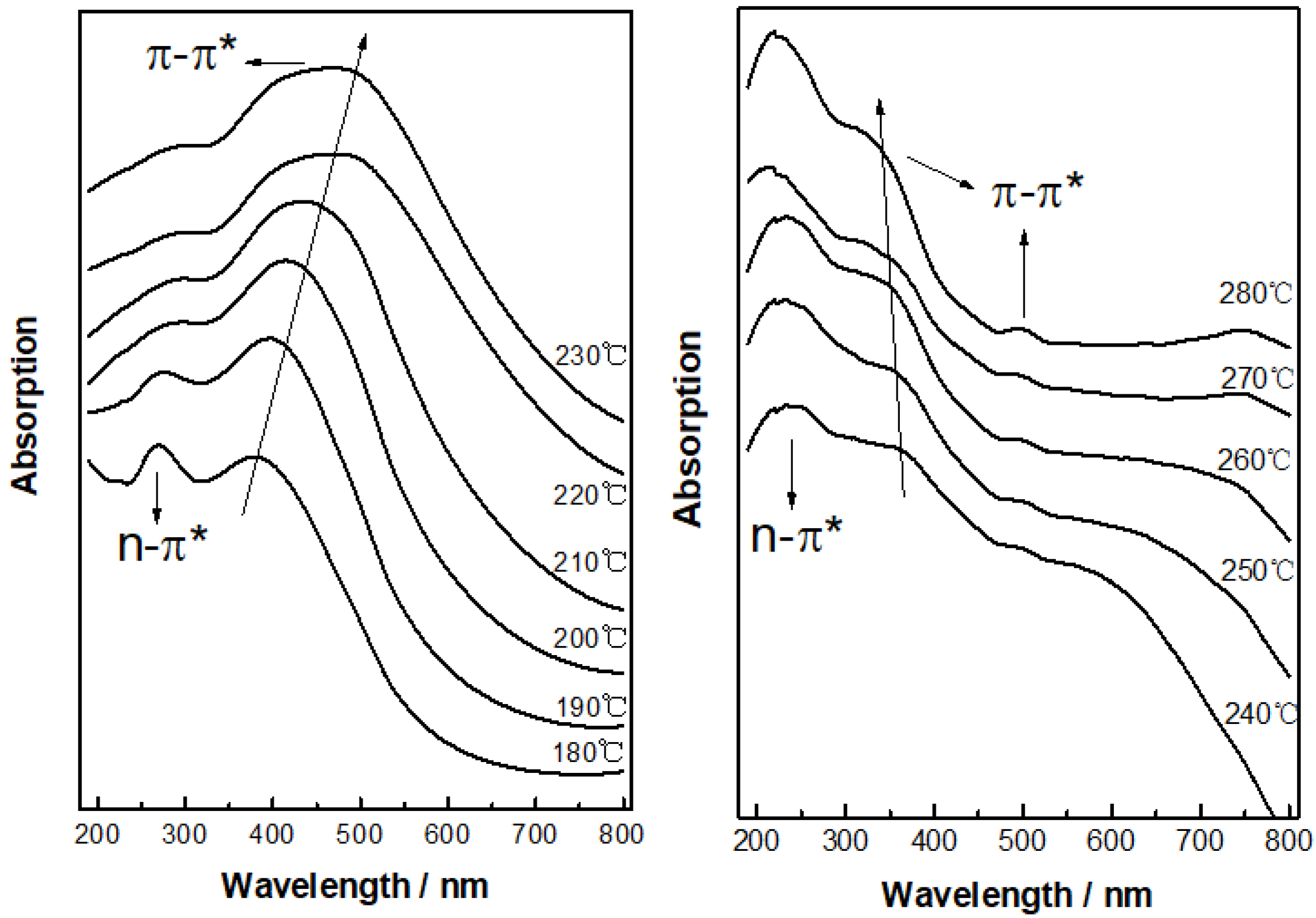
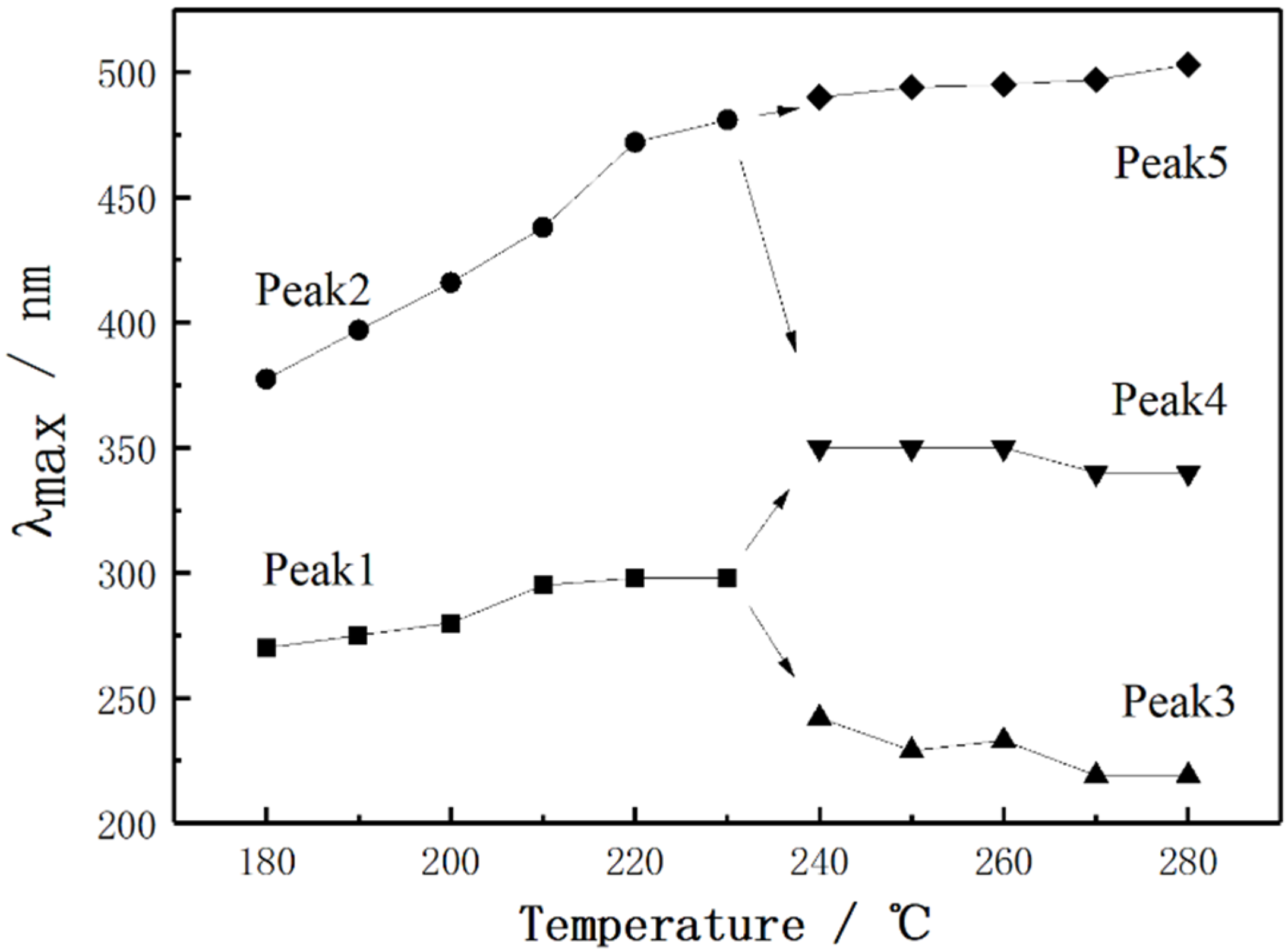
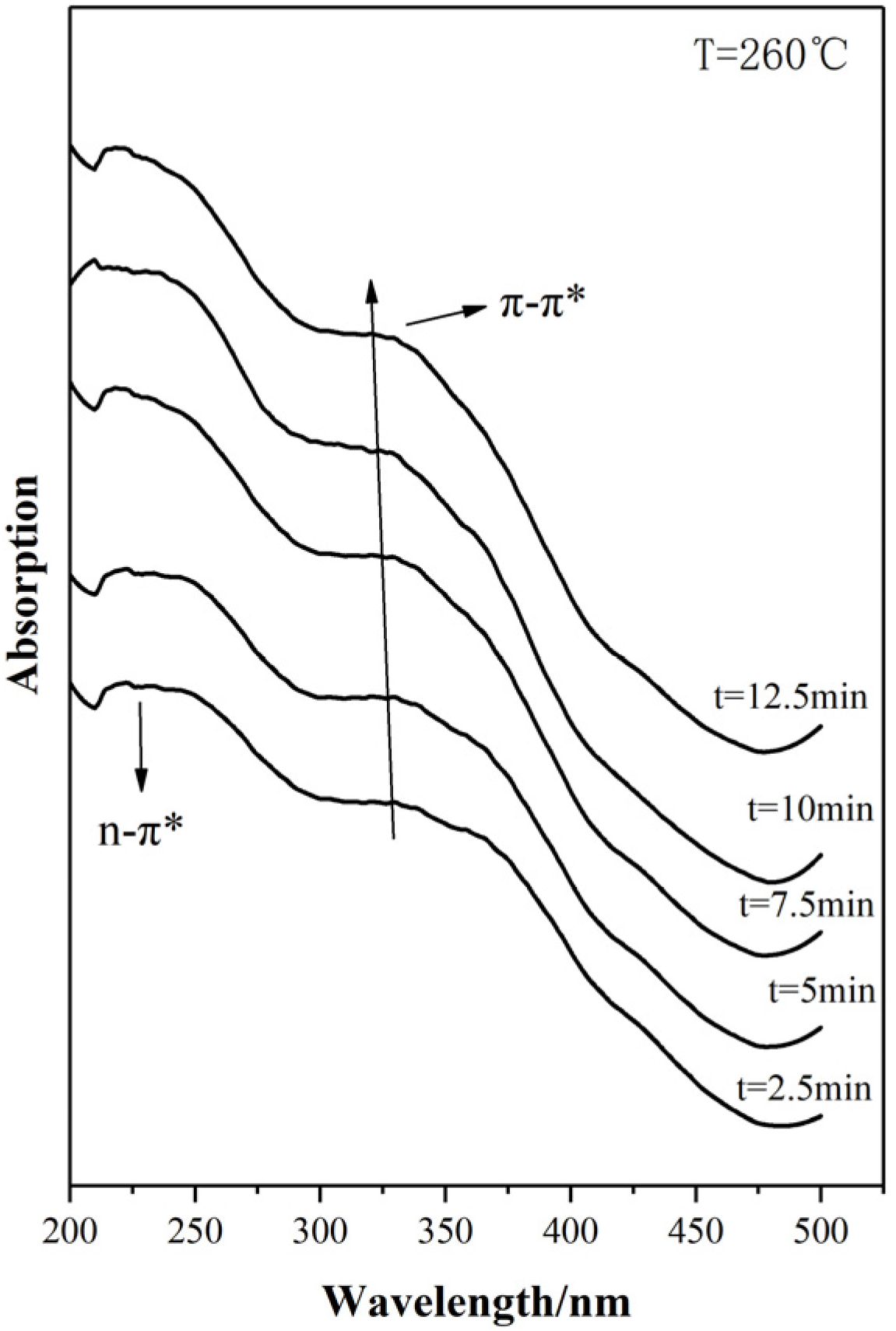
3.1.2. UV-Vis Absorption Spectra Analysis
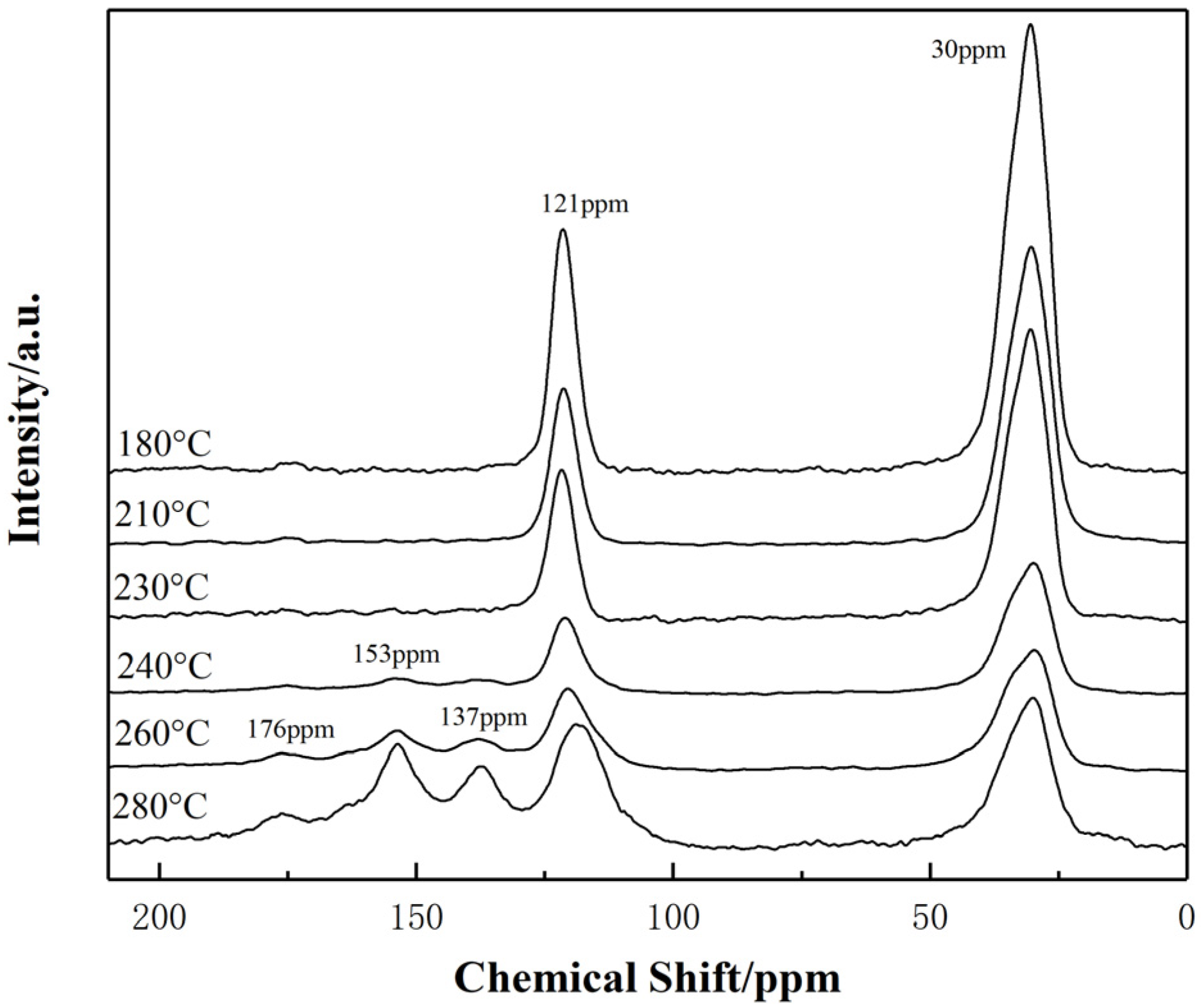
3.1.3. 13C-NMR Analysis
3.2. The Effect of Time on the Formation and Evolution of sp2 Hybrid Conjugate Structure for PAN Fibers
3.2.1. The Characterization of sp2 Hybrid Conjugated Structure for PAN-Stabilized Fibers by Raman Spectrum
3.2.2. UV-vis Spectra Analysis
4. Conclusions
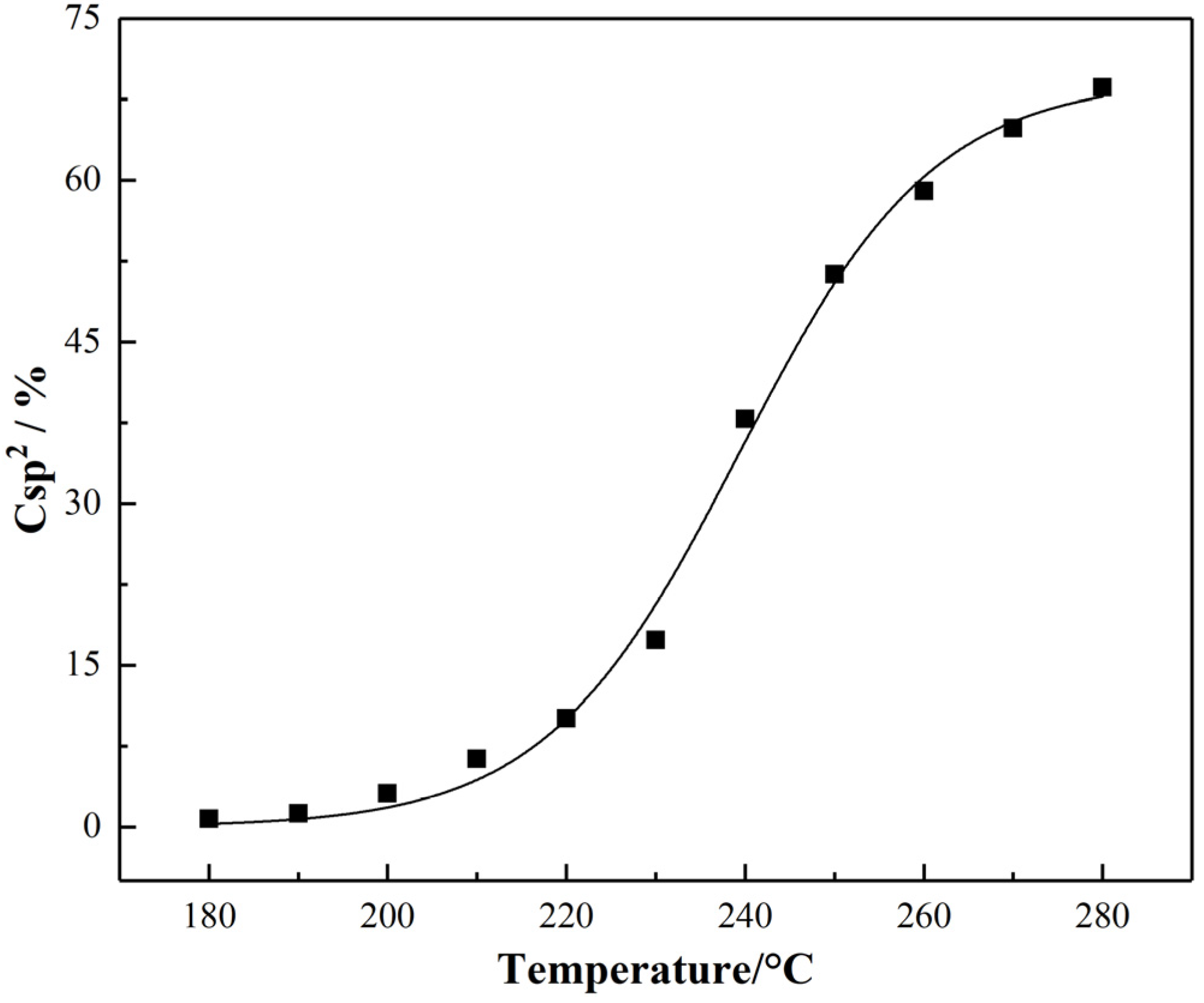
- The degree of the sp2 hybrid conjugate for PAN-stabilized fibers increased “linearly” approximately with the increase of stabilization temperature from the Raman spectra analysis. It could also be increased gradually with the extension of heat-treatment time. As the results from 13C-NMR show, the amount of sp2 hybrid carbon atoms increased in a “S-type” tendency with the increase of stabilized temperature.
- When the thermal stabilization temperature was in the range of 180–230 °C, the conjugated structure of PAN-stabilized fibers changed from a single ring structure plus two double-bond structures to a conjugated double-ring structure, two conjugated rings, and two double-bond changed to triple-ring conjugated structures. In the temperature range of 230–280 °C, the dissociation and transformation of the double-ring structure and triple-ring structure occurred, resulting in the formation of three types of conjugated structures at same time. These were the single-ring and double-bond, double-ring and double-bond, triple-ring and double-bond structures, respectively.
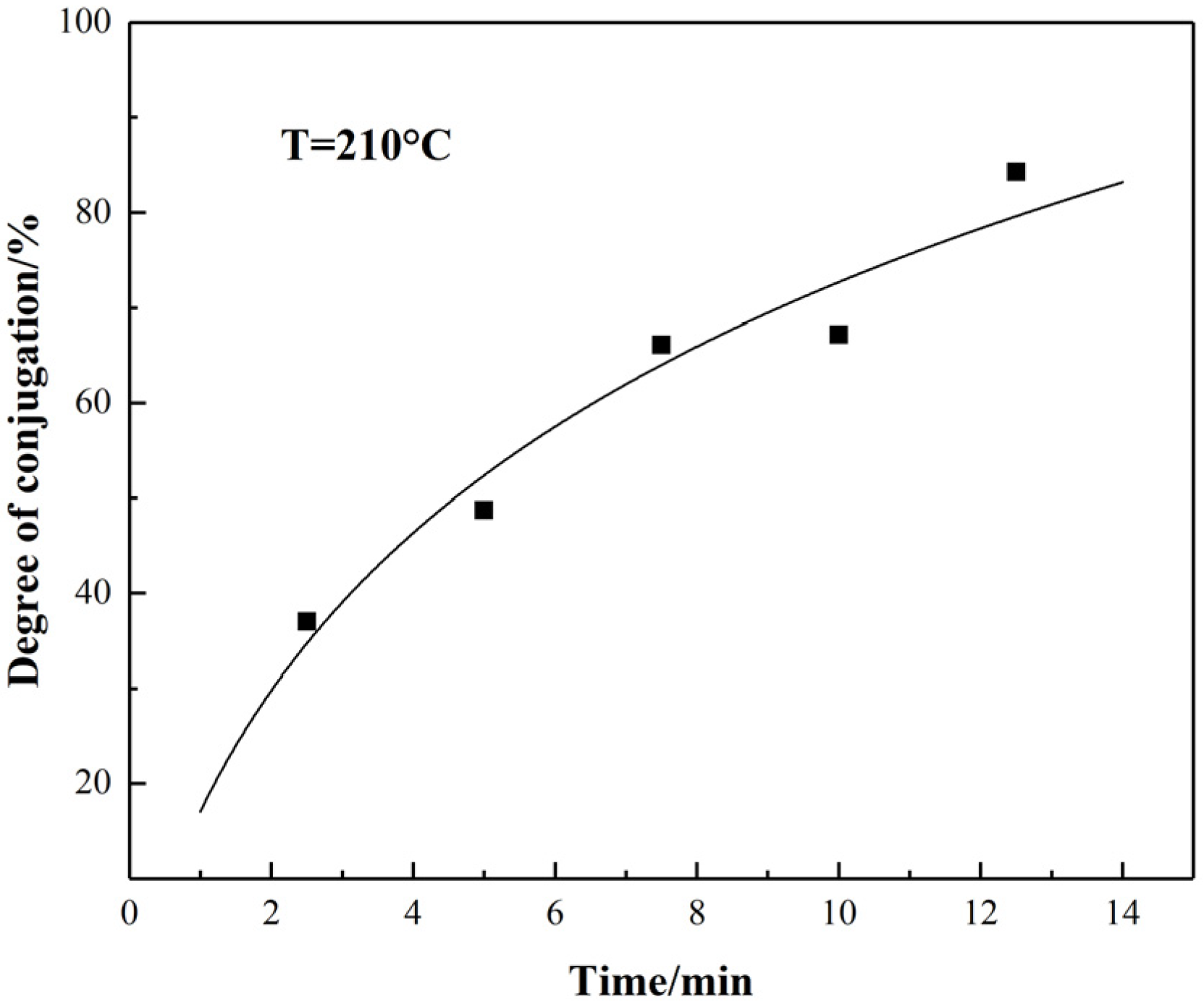
- With the extension of thermal stabilization time, the amount of conjugated ring structures increased, but the dimension of conjugated ring structures did not change. Therefore, the dimension of conjugated ring structures mainly depended on the stabilized temperature. Stabilization time did not play an important role in this.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Z.; Zhu, J.; Rajabpour, S.; Kaushik, J. Graphene reinforced carbon fibers. Sci. Adv. 2020, 6, 2375–2548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.; Chien, A.T.; Liu, H.C.; Wang, P.H.; Newcomb, B.A.; Kumar, S. Gel Spinning of Polyacrylonitrile/Cellulose Nanocrystal Composite Fibers. ACS Biomater. Sci. Eng. 2015, 1, 610–616. [Google Scholar] [CrossRef]
- Golova, L.K.; Bondarenko, G.N.; Makarov, I.S.; Kuznetsova, L.K.; Vinogradov, M.I.; Kulichikhin, V.G. Peculiarities of Dissolving Polyacrylonitrile Copolymer Containing Methylsulfo Groups in N-Methylmorpholine-N-Oxide. Polym. Sci. Ser. A 2020, 62, 597–606. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Vinogradov, M.I.; Levin, I.S.; Sorokin, S.E. Structure of Polyacrylonitrile Fibers Produced from N-Methylmorpholine-N-Oxide Solutions. Fibre Chem. 2019, 50, 508–513. [Google Scholar] [CrossRef]
- Deurbergue, A.; Oberlin, A. Stabilization and carbonization of PAN-based carbon fibers as related to mechanical properties. Carbon 1991, 29, 621–628. [Google Scholar] [CrossRef]
- Fu, Z.; Gui, Y.; Cao, C. Structure evolution and mechanism of polyacrylonitrile and related copolymers during the stabilization. J. Mater. Sci. 2014, 49, 2864–2874. [Google Scholar] [CrossRef]
- Liu, C.; Ni, X.; Chen, H.; Yu, H. High molecular weight poly(acrylonitrile-co-3-aminocarbonyl-3-butenoic acid methyl ester) prepared by mixed solvent polymerization I. effect of monomer feed ratios on polymerization and stabilization. J. Polym. Res. 2019, 26, 1–11. [Google Scholar] [CrossRef]
- Ise, M.; Nakayama, I.; Endo, M. Carbon Fiber, Process for Production of Polyacrylonitrile-Base Precursor Fiber for Carbon Fiber Production, and Process for Production of Carbon Fiber. U.S. Patent 8137810, 20 March 2012. [Google Scholar]
- Zeng, Z.P.; Shao, Z.C.; Xiao, R. Structure evolution mechanism of poly(acrylonitrile/itaconic acid/acrylamide) during thermal oxidative stabilization process. Chin. J. Polym. Sci. 2017, 35, 1020–1034. [Google Scholar] [CrossRef]
- Wang, B.; Li, C.G.; Cao, W.Y. Effect of Polyacrylonitrile Precursor Orientation on the Structures and Properties of Thermally Stabilized Carbon Fiber. Materials 2021, 14, 3237. [Google Scholar] [CrossRef]
- Yusof, N.; Ismail, A.F.; Jaafar, J.; Salleh, W.; Hasbullah, H. Effects of stabilization temperature on the chemical and the physical properties of polyacrylonitrile stabilized fibers. Adv. Mater. Res. 2015, 1112, 402–405. [Google Scholar] [CrossRef]
- Xiao, H.; Lu, Y.; Zou, W. The effect of heat treatment temperature and time on the microstructure and mechanical properties of PAN-based carbon fibers. J. Mater. Sci. 2014, 49, 794–804. [Google Scholar] [CrossRef]
- Berlin, A.A.; Dubinskaya, A.M.; Moshkovskii, Y.S. Heat treatment of polyacrylonitrile in solution in dimethylformamide. Polym. Sci. USSR 1964, 6, 2145–2151. [Google Scholar] [CrossRef]
- Wu, S.; Qin, X. Effects of the stabilization temperature on the structure and properties of polyacrylonitrile-based stabilized electrospun nanofiber microyarns. J. Therm. Anal. Calorim. 2014, 116, 303–308. [Google Scholar] [CrossRef]
- Zhou, L.X. The Cross-Linked Structure of PAN Fiber during Stabilized Process; Donghua University: Shanghai, China, 2016. [Google Scholar]
- Liu, J.; Wang, X.; Ma, Z.; Liang, J. Function of temperature effect on the radial pervasion of oxygen elements during the stabilization of polyacrylonitrile fibers. Acta Mater. Compos. Sin. 2012, 29, 26–34. [Google Scholar]
- Coleman, M.M.; Sivy, G.T. Fourier transform IR studies of the degradation of polyacrylonitrile copolymers—III: Acrylonitrile/vinyl acetate copolymers. Carbon 1981, 19, 133–135. [Google Scholar] [CrossRef]
- Lei, S.; Zhang, X.; Zhong, S.; Liu, Z.; Cao, W.; Xu, L. Degradation Behavior of Thermal Stabilized Polyacrylonitrile Fibers. J. Mater. Eng. 2017, 45, 59–63. [Google Scholar]
- Shimada, I.; Takahagi, T.; Fukuhara, M.; Morita, K.; Ishitani, A. FT-IR study of the stabilization reaction of polyacrylonitrile in the production of carbon fibers. J. Polym. Sci. Part A Polym. Chem. 2010, 24, 1989–1995. [Google Scholar] [CrossRef]
- Rahaman, M.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Usami, T.; Itoh, T.; Ohtani, H.; Tsuge, S. Structural study of polyacrylonitrile fibers during oxidative thermal degradation by pyrolysis-gas chromatography, solid-state carbon-13 NMR, and Fourier-transform infrared spectroscopy. Macromolecules 2002, 23, 2460–2465. [Google Scholar] [CrossRef]
- Dalton, S.; Hatley, F.; Budd, P.M. Thermal stabilization of polyacrylonitrile fibres. Polymer 1999, 40, 5531–5543. [Google Scholar] [CrossRef]
- Kubasova, N.A.; Shishkina, M.V.; Zaliznaya, N.F.; Geiderikh, M.A. Thermal conversion of polyacrylonitrile (PAN) in solution. Polym. Sci. USSR 1968, 10, 1537–1542. [Google Scholar] [CrossRef]
- Kubasova, N.A.; Din, D.S.; Heiderich, M.A.; Shishkina, M.V. Effect of the stereoregularity of polyacrylonitrile macromolecules on the formation of a conjugated bond system. Polym. Sci. USSR 1971, 13, 184–190. [Google Scholar] [CrossRef]
- Lei, S.; Cao, W.; Fu, Z.; Xu, L. The conjugated plane formed in polyacrylonitrile during thermal stabilization. J. Appl. Polym. Sci. 2016, 133, 233–243. [Google Scholar] [CrossRef]
- Jaffe, H.H.; Orchin, M. The Theory and Applications of Ultraviolet Spectroscopy; Wiley: Hoboken, NJ, USA, 1963. [Google Scholar]
- Huang, L.; Yu, D. UV Spectrum: Application in Organic Chemistry; Science Press: Beijing, China, 1988. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, R.; Wu, J.; You, T.; Cao, W. Formation and Evolution of sp2 Hybrid Conjugate Structure of Polyacrylonitrile Precursor during Stabilization. Materials 2022, 15, 30. https://doi.org/10.3390/ma15010030
Dong R, Wu J, You T, Cao W. Formation and Evolution of sp2 Hybrid Conjugate Structure of Polyacrylonitrile Precursor during Stabilization. Materials. 2022; 15(1):30. https://doi.org/10.3390/ma15010030
Chicago/Turabian StyleDong, Ruihao, Jianglu Wu, Ting You, and Weiyu Cao. 2022. "Formation and Evolution of sp2 Hybrid Conjugate Structure of Polyacrylonitrile Precursor during Stabilization" Materials 15, no. 1: 30. https://doi.org/10.3390/ma15010030





