Biocompatibility and Microstructure-Based Stress Analyses of TiNbZrTa Composite Films
Abstract
:1. Introduction
2. Materials and Methods
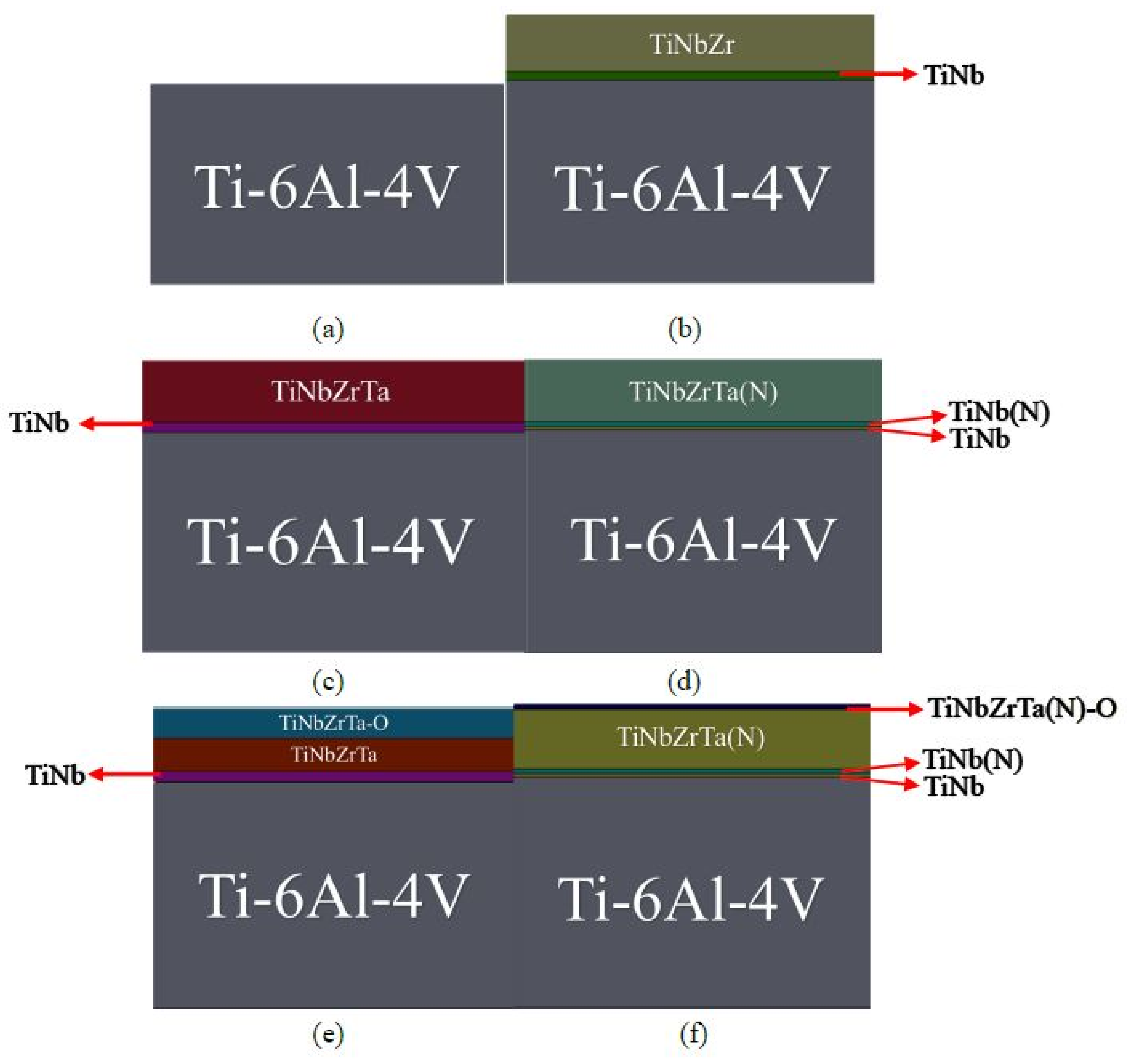
2.1. Experimental Sample
2.2. Cytotoxicity Analysis (Extract Test)
2.3. Cell Viability Analysis (Direct Contact Method)
2.4. Alkaline Phosphatase Analysis
2.5. Scanning Electron Microscope
2.6. RT-qPCR
2.7. Statistical Analysis
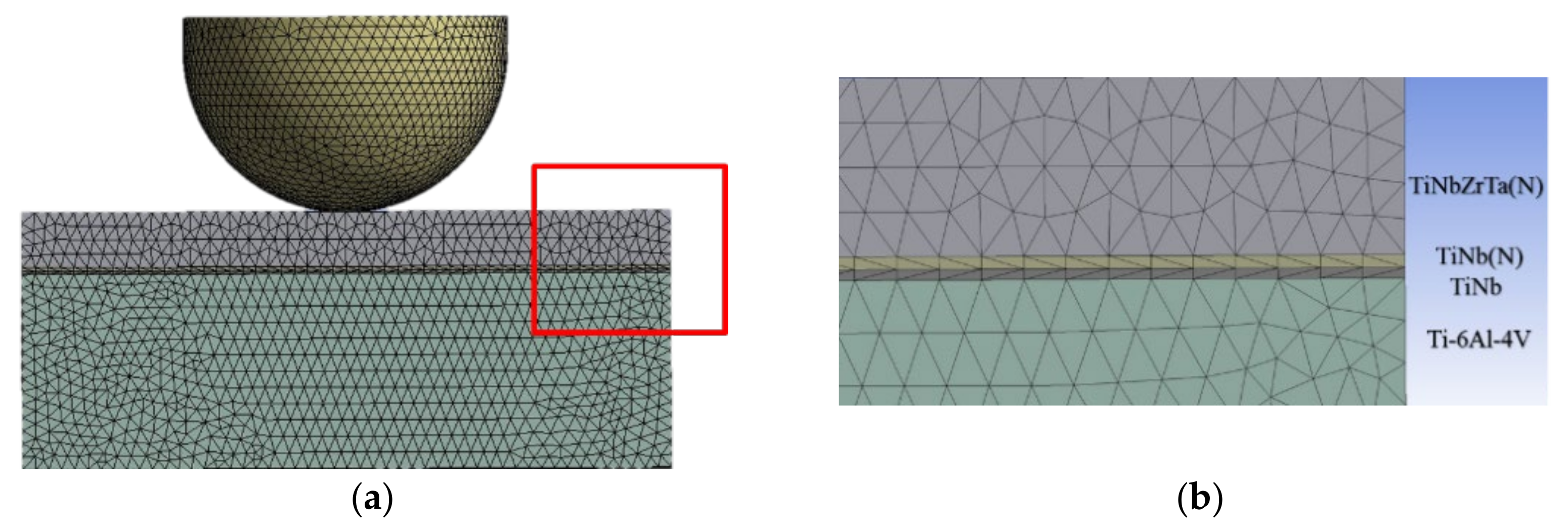
2.8. Computer Simulation Analysis
3. Results and Discussion
3.1. Cytotoxicity Analysis (Extract Test)
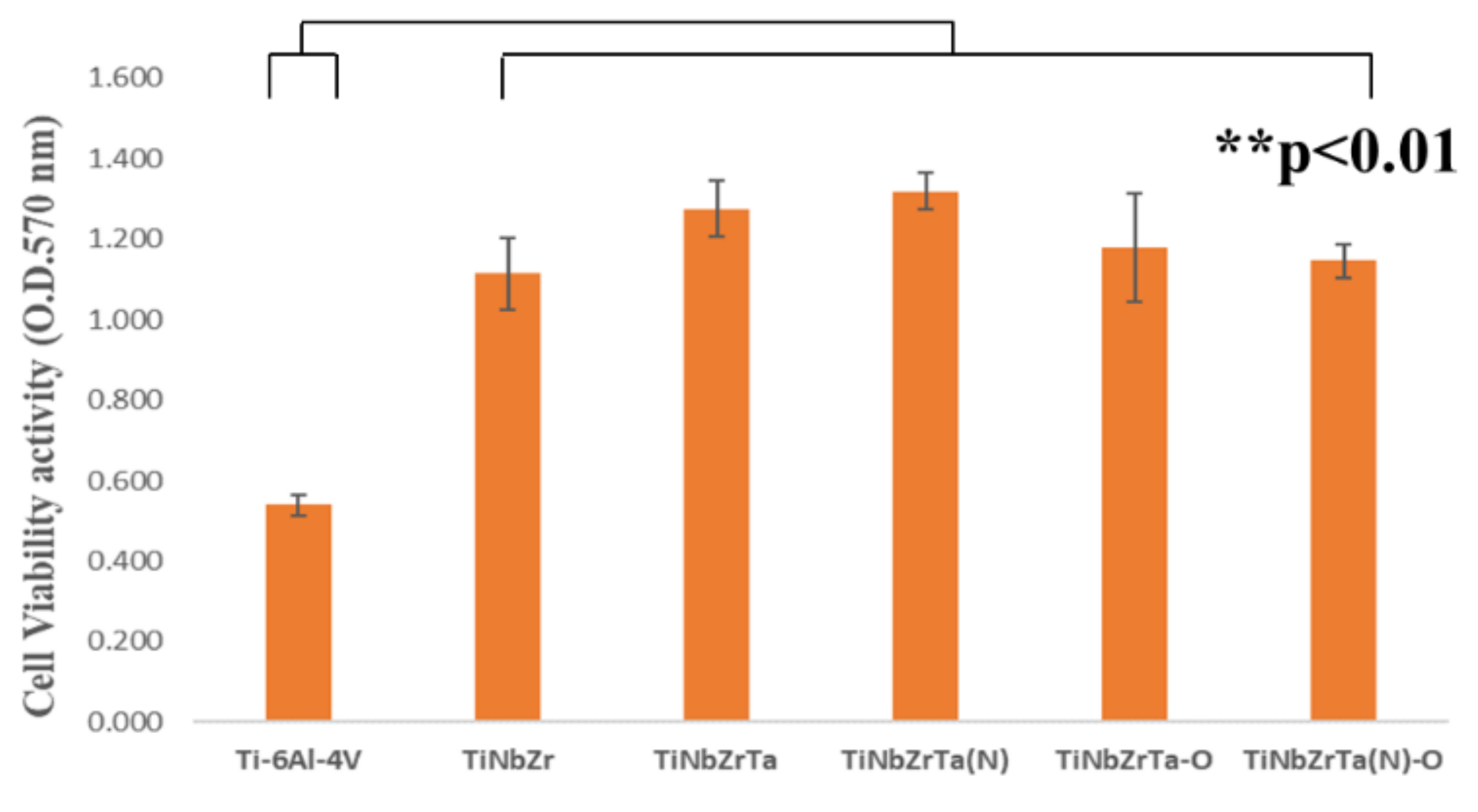
3.2. Cell Viability Analyses (Direct Contact Method)
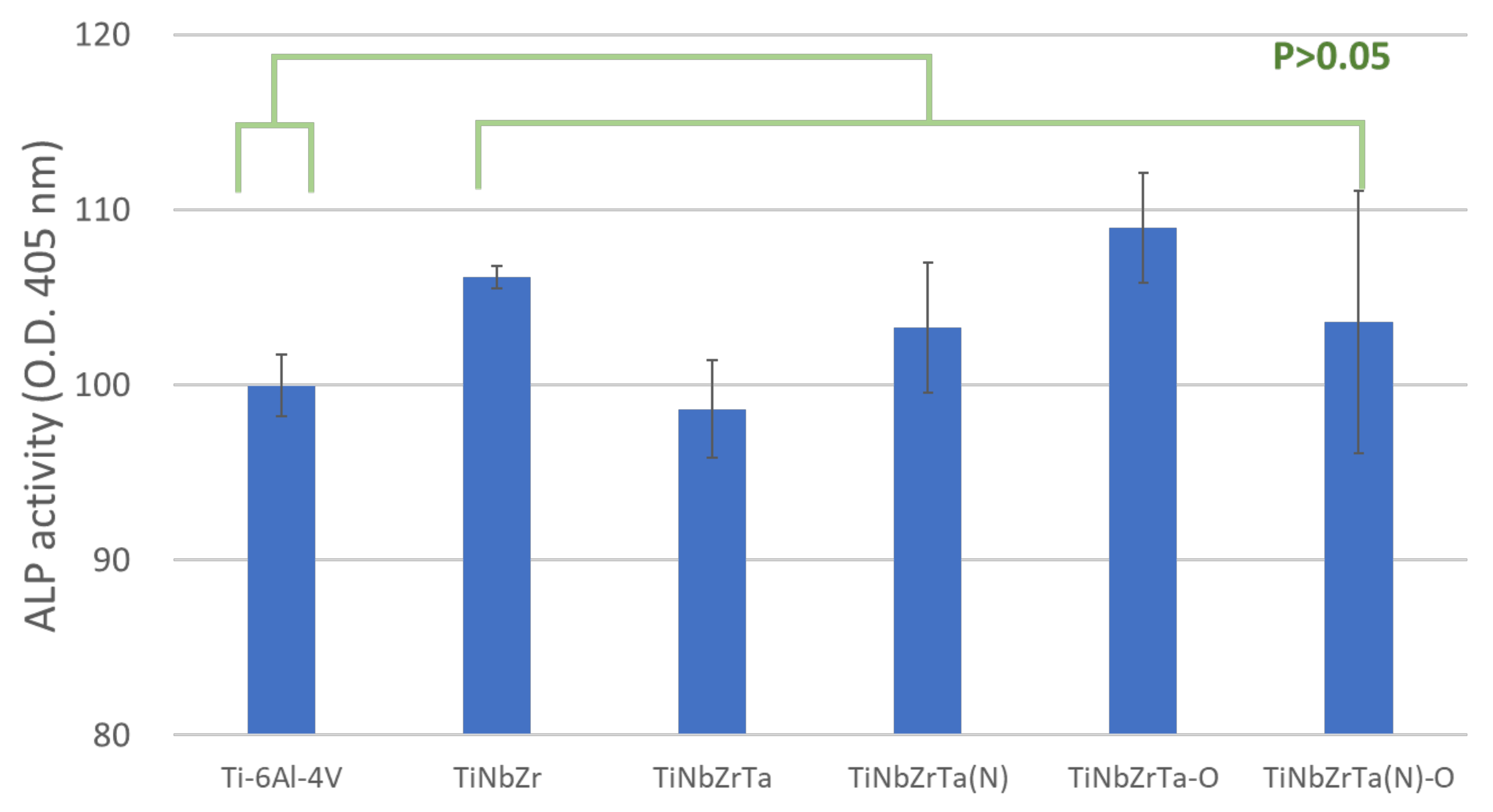
3.3. ALP Analyses
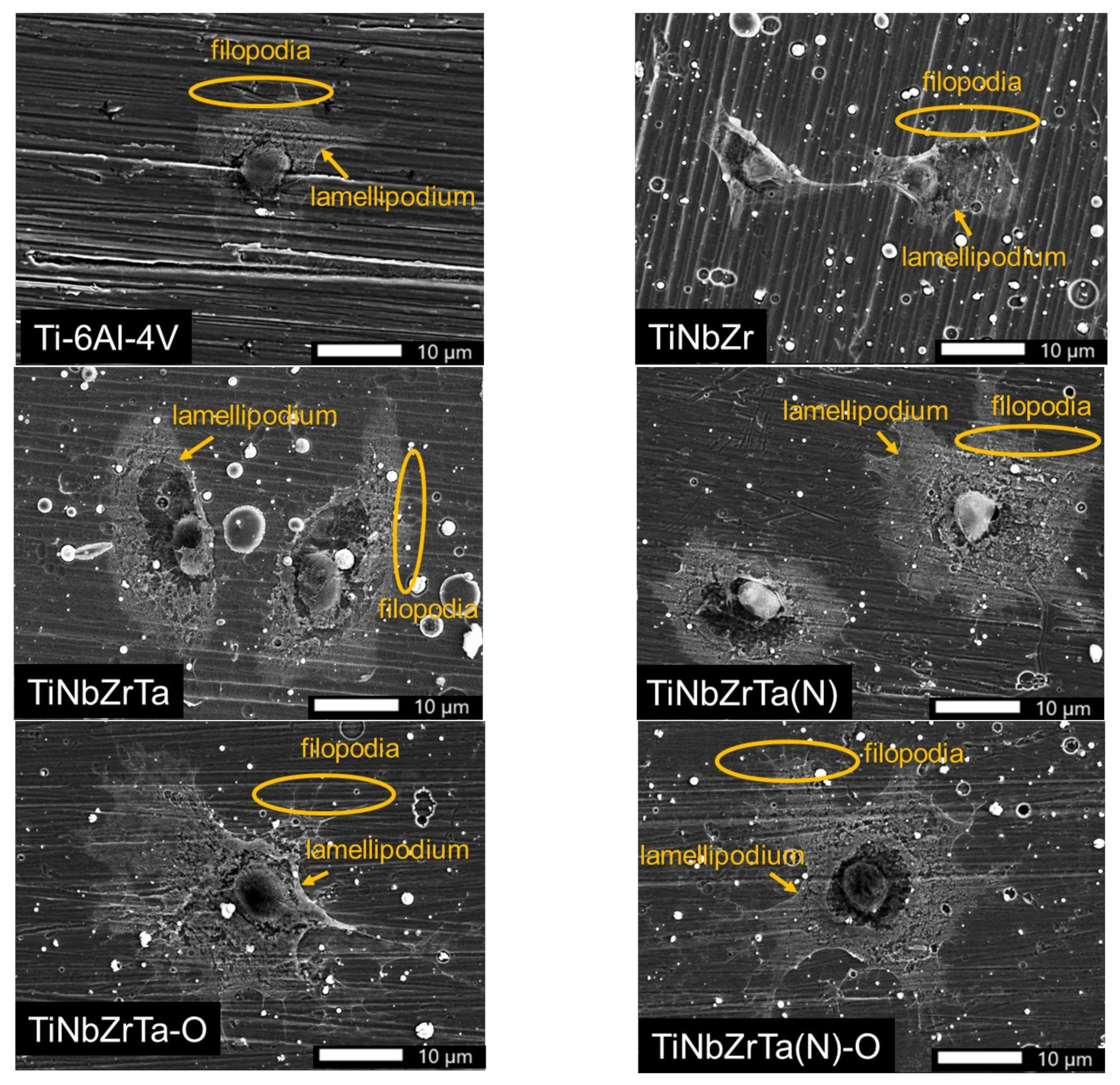
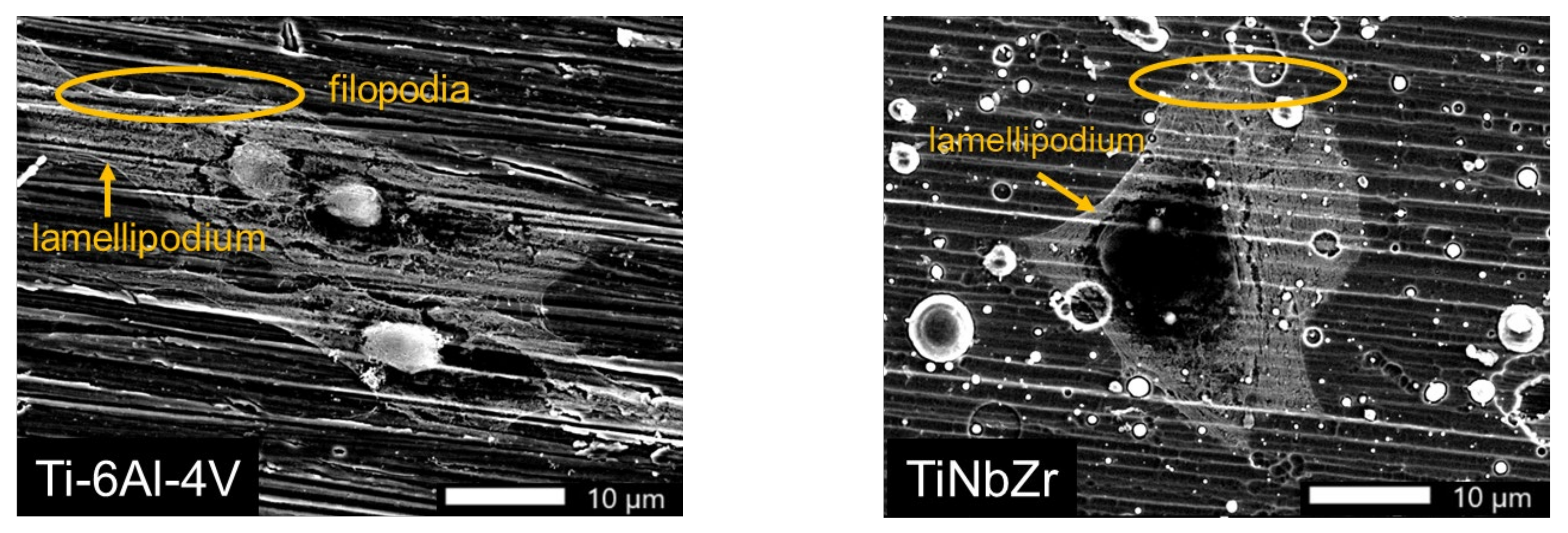
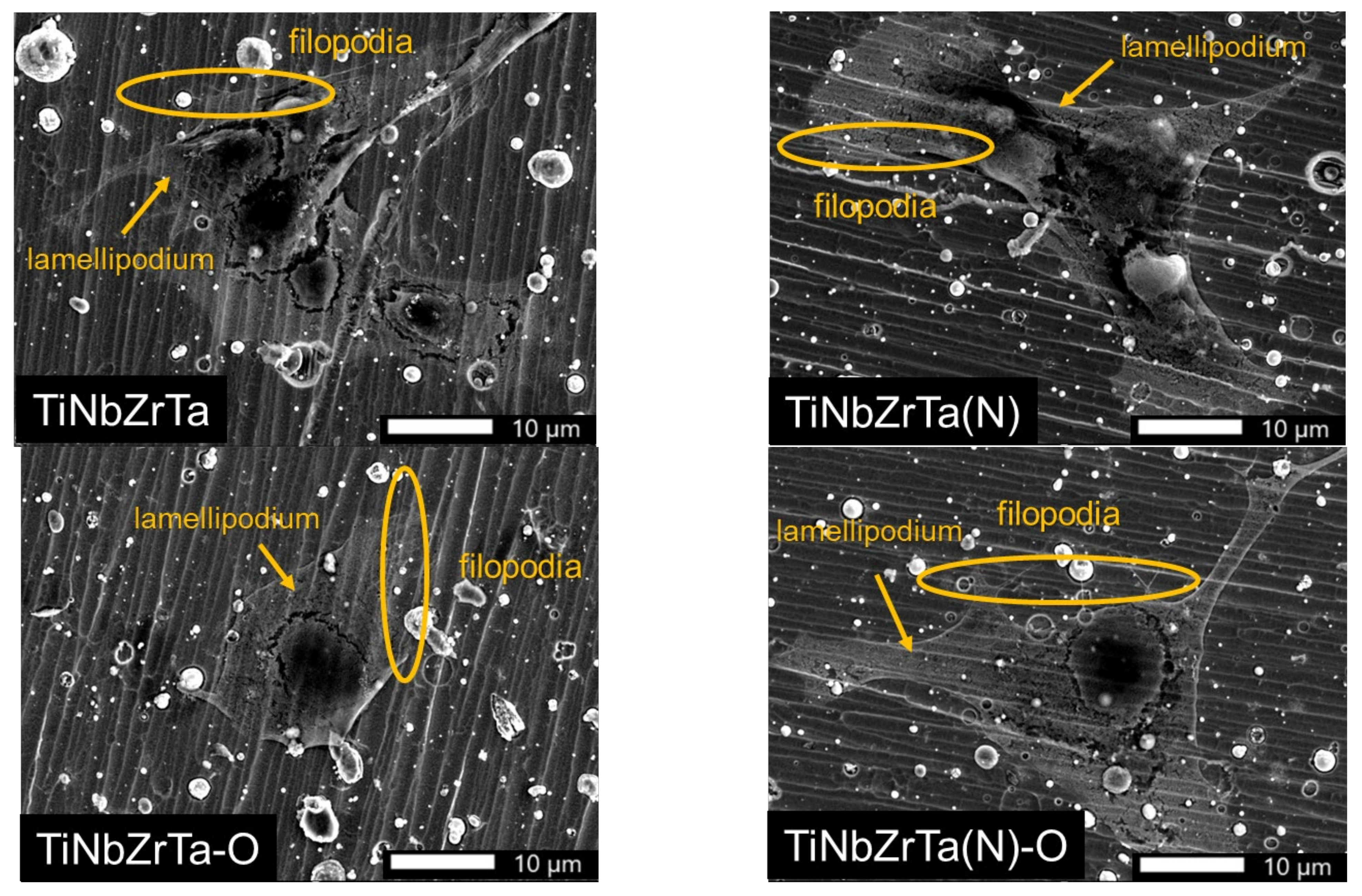
3.4. Cell Morphology Analyses
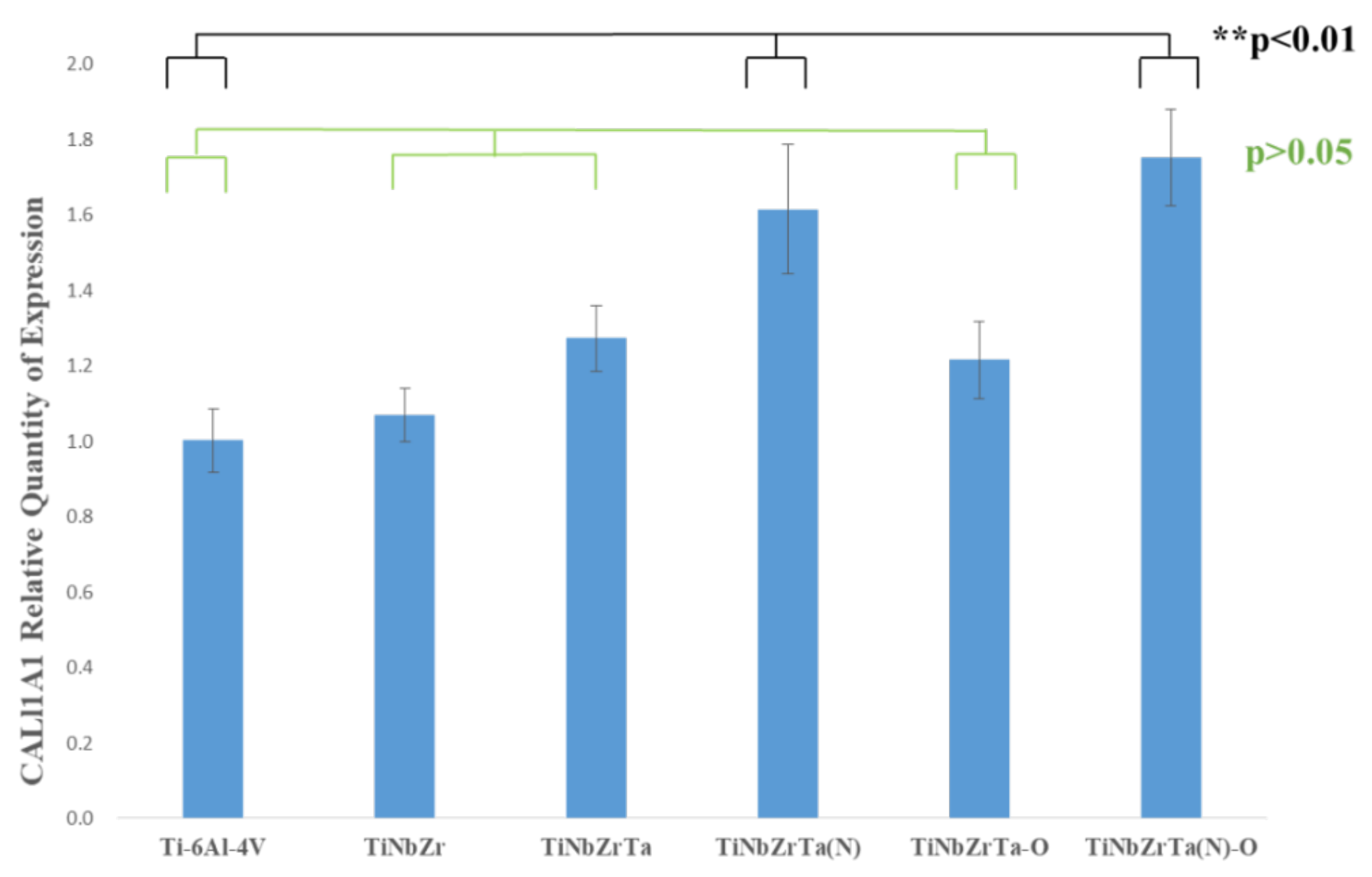
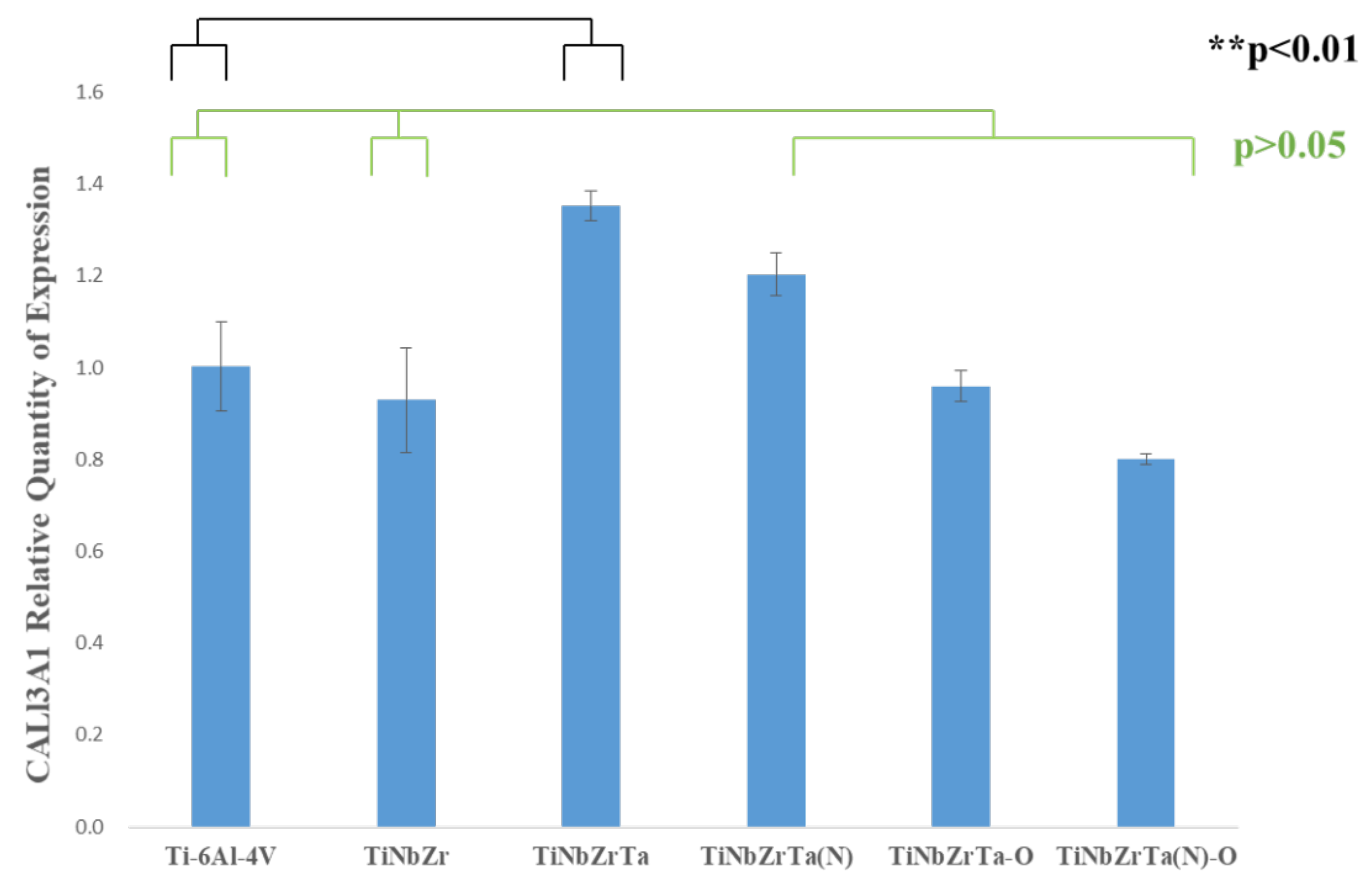
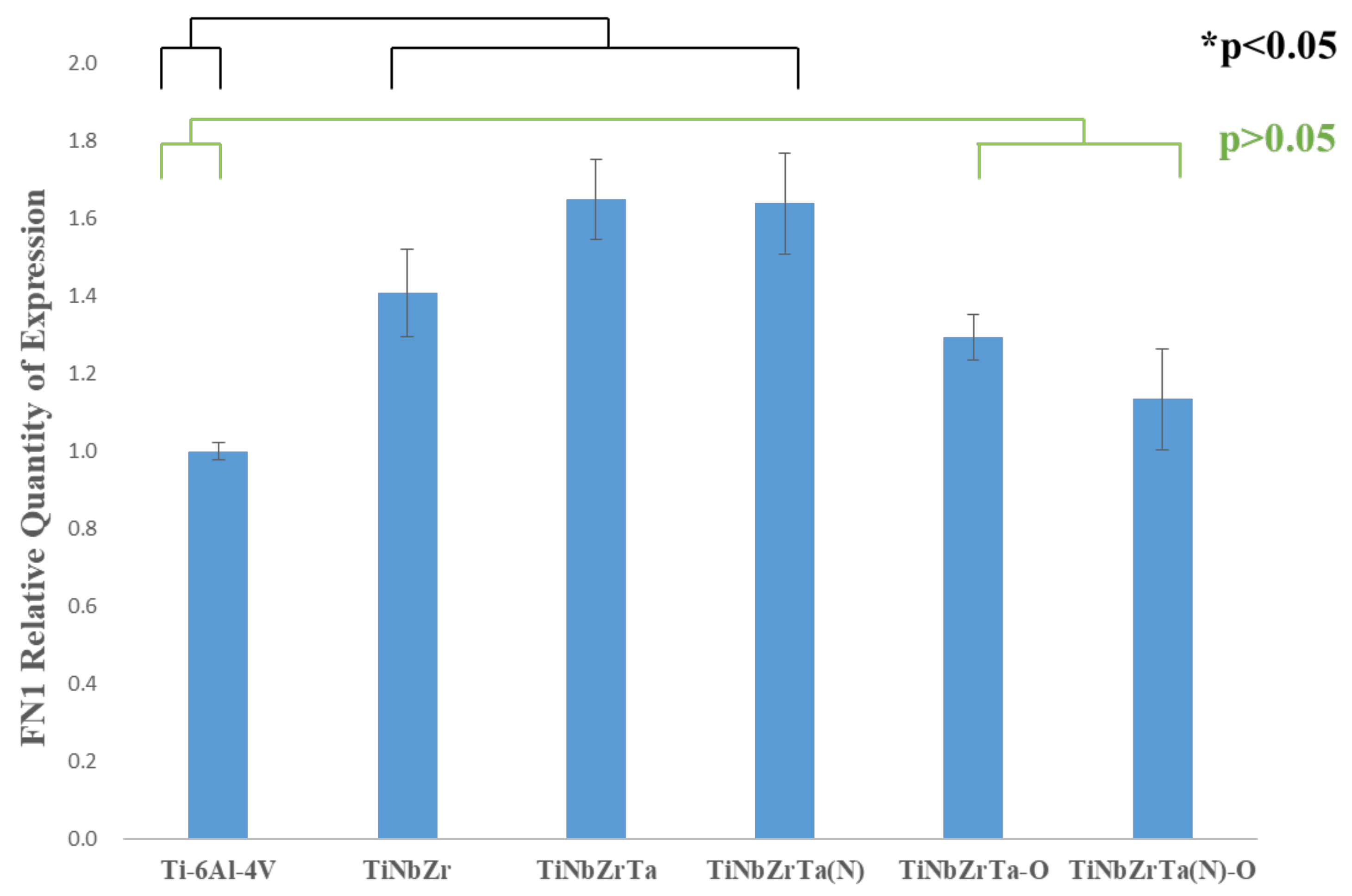
3.5. RT-qPCR
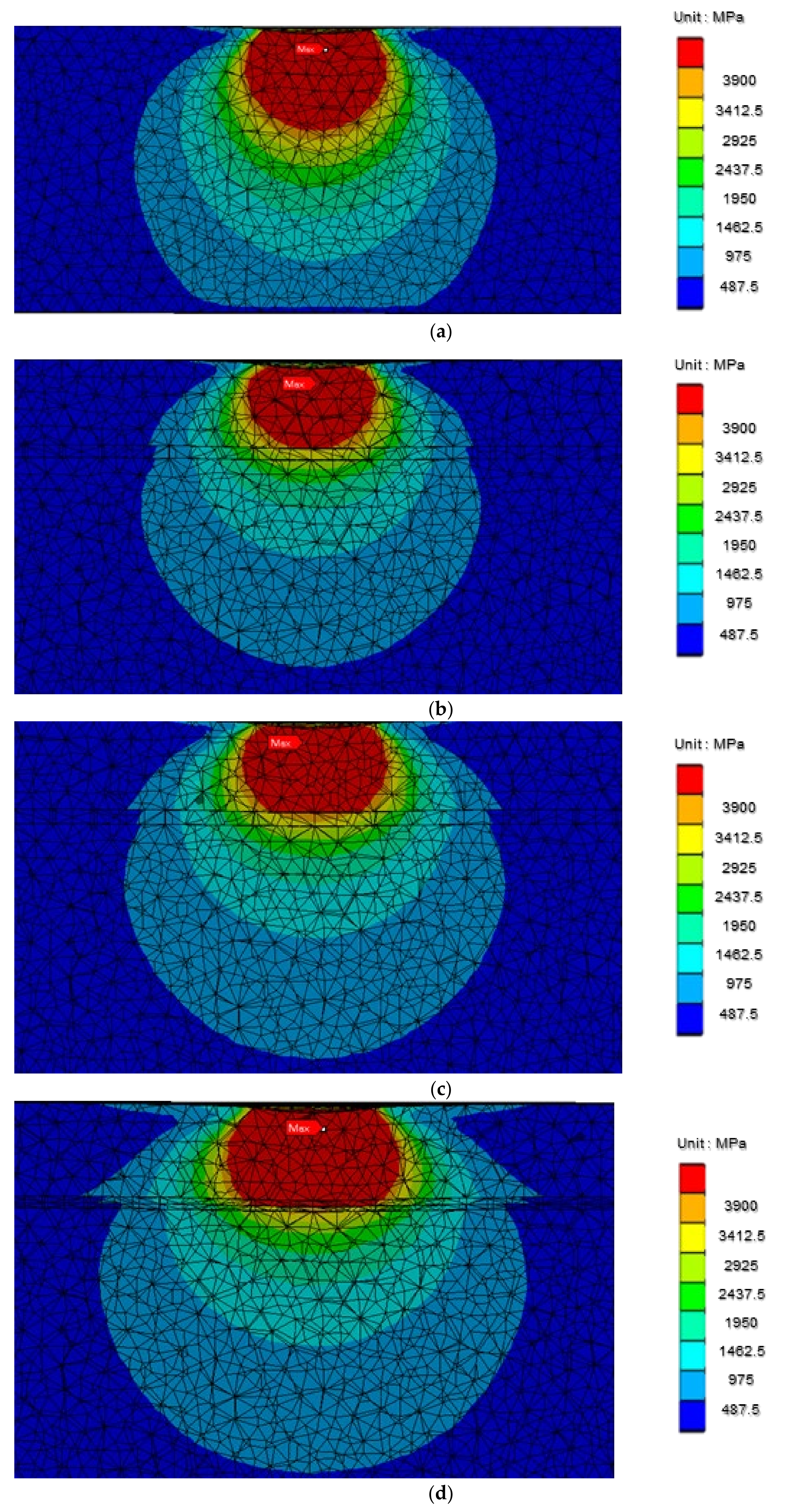
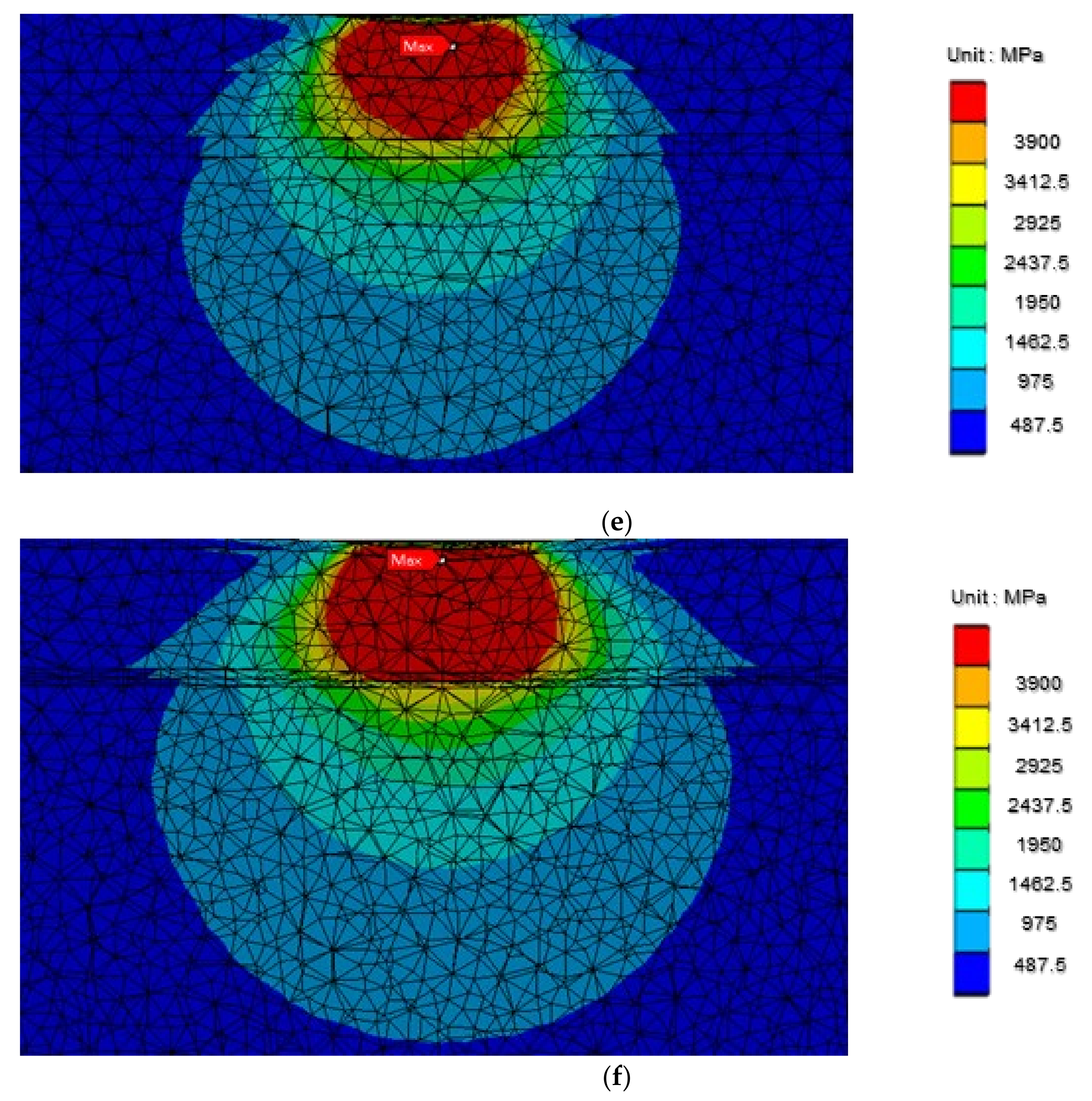
3.6. Microstructure Stress Analyses
3.7. Research Limitations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Avivi-Arber, L.; Zarb, G.A. Clinical effectiveness of implant-supported single-tooth replacement: The Toronto Study. Int. J. Oral Maxillofac. Implant. 1996, 11, 311–321. [Google Scholar]
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDP s) after a mean observation period of at least 5 years. Clin. Oral Implant. Res. 2012, 23, 22–38. [Google Scholar] [CrossRef]
- Jung, R.E.; Pjetursson, B.E.; Glauser, R.; Zembic, A.; Zwahlen, M.; Lang, N.P. A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clin. Oral Implant. Res. 2008, 19, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, P.; Larsson, C.; Ericson, L.; Sennerby, L.; Lausmaa, J.; Kasemo, B. Structure of the interface between rabbit cortical bone and implants of gold, zirconium and titanium. J. Mater. Sci. Mater. Med. 1997, 8, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Sidambe, A.T. Biocompatibility of advanced manufactured titanium implants—A review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef] [Green Version]
- López, M.F.; Gutiérrez, A.; Jiménez, J.A. Surface characterization of new non-toxic titanium alloys for use as biomaterials. Surf. Sci. 2001, 482, 300–305. [Google Scholar] [CrossRef]
- Musil, J. Hard and superhard nanocomposite coatings. Surf. Coat. Technol. 2000, 125, 322–330. [Google Scholar] [CrossRef]
- Hauert, R.; Patscheider, J. From alloying to nanocomposites—Improved performance of hard coatings. Adv. Eng. Mater. 2000, 2, 247–259. [Google Scholar] [CrossRef]
- Bernhardt, A.; Schneider, J.; Schroeder, A.; Papadopoulous, K.; Lopez, E.; Brückner, F.; Botzenhart, U. Surface conditioning of additively manufactured titanium implants and its influence on materials properties and in vitro biocompatibility. Mater. Sci. Eng. C 2021, 119, 111631. [Google Scholar] [CrossRef]
- Asadi-Samani, M.; Rafieian-Kopaei, M.; Lorigooini, Z.; Shirzad, H. A screening of growth inhibitory activity of Iranian medicinal plants on prostate cancer cell lines. BioMedicine 2018, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Fan, Z.-Q.; Shi, X.-Q.; Wang, N.; Bo, Y.-Y.; Guo, H.-E. A novel silkworm pupae carboxymethyl chitosan inhibits mouse L929 fibroblast proliferation. SCIENCEASIA 2020, 46, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-Y.; Peng, C.-W.; Wu, W.-W. Fibrous hydrogel scaffolds with cells embedded in the fibers as a potential tissue scaffold for skin repair. J. Mater. Sci. Mater. Med. 2014, 25, 259–269. [Google Scholar] [CrossRef]
- Yu-Ju, Y. Mechanical Properties and Biocompatibilities of TiNbZrTa Coatings Synthesized by Cathodic Arc Evaporation, National Formosa University: Huwei Township, Yunlin County, Taiwan, July 2020.
- Koval, N.E.; Juaristi, J.I.; Muiño, R.D.; Alducin, M. Elastic properties of the TiZrNbTaMo multi-principal element alloy studied from first principles. Intermetallics 2019, 106, 130–140. [Google Scholar] [CrossRef] [Green Version]
- Nabrdalik, M.; Sobociński, M. The finite element method in the analysis of the stress and strain distribution in polyethylene elements of hip and knee joints endoprostheses. MATEC Web Conf. 2019, 254, 02025. [Google Scholar] [CrossRef]
- Biesiekierski, A.; Lin, J.; Munir, K.; Ozan, S.; Li, Y.; Wen, C. An investigation of the mechanical and microstructural evolution of a TiNbZr alloy with varied ageing time. Sci. Rep. 2018, 8, 5737. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-T.; Chang, Y.-Y.; Huang, H.-L.; Wu, Y.-H.; Shieh, T.-M. Micro-arc oxidation treatment enhanced the biological performance of human osteosarcoma cell line and human skin fibroblasts cultured on titanium–zirconium films. Surf. Coat. Technol. 2016, 303, 268–276. [Google Scholar] [CrossRef]
- Borisy, G.G.; Svitkina, T.M. Actin machinery: Pushing the envelope. Curr. Opin. Cell Biol. 2000, 12, 104–112. [Google Scholar] [CrossRef]
- Small, J.V. Lamellipodia architecture: Actin filament turnover and the lateral flow of actin filaments during motility. Semin. Cell Biol. 1994, 5, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Bordji, K.; Jouzeau, J.; Mainard, D.; Payan, E.; Netter, P.; Rie, K.; Stucky, T.; Hage-Ali, M. Cytocompatibility of Ti-6Al-4V and Ti-5Al-2.5 Fe alloys according to three surface treatments, using human fibroblasts and osteoblasts. Biomaterials 1996, 17, 929–940. [Google Scholar] [CrossRef]
- Faix, J.; Rottner, K. The making of filopodia. Curr. Opin. Cell Biol. 2006, 18, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Crawford, R.C.; Wang, J.H. Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. J. Biomech. 2004, 37, 1543–1550. [Google Scholar] [CrossRef]
- Siwik, D.A.; Pagano, P.J.; Colucci, W.S. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am. J. Physiol. -Cell Physiol. 2001, 280, C53–C60. [Google Scholar] [CrossRef] [PubMed]
- Röhlecke, C.; Witt, M.; Kasper, M.; Schulze, E.; Wolf, C.; Hofer, A.; Funk, R.H. Synergistic effect of titanium alloy and collagen type I on cell adhesion, proliferation and differentiation of osteoblast-like cells. Cells Tissues Organs 2001, 168, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Keselowsky, B.G.; Collard, D.M.; García, A.J. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J. Biomed. Mater. Res. Part A: Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2003, 66, 247–259. [Google Scholar] [CrossRef]
- Grill, V.; Sandrucci, M.A.; Di Lenarda, R.; Cadenaro, M.; Narducci, P.; Bareggi, R.; Martelli, A.M. Biocompatibility evaluation of dental metal alloys in vitro: Expression of extracellular matrix molecules and its relationship to cell proliferation rates. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2000, 52, 479–487. [Google Scholar]
- Zhao, X.; Xie, Z.; Munroe, P. Nanoindentation of hard multilayer coatings: Finite element modelling. Mater. Sci. Eng. A 2011, 528, 1111–1116. [Google Scholar] [CrossRef]
- Wo, P.; Zhao, X.; Munroe, P.; Zhou, Z.; Li, K.; Habibi, D.; Xie, Z. Extremely hard, damage-tolerant ceramic coatings with functionally graded, periodically varying architecture. Acta Mater. 2013, 61, 193–204. [Google Scholar] [CrossRef]
- Hélary, G.; Noirclère, F.; Mayingi, J.; Migonney, V. A new approach to graft bioactive polymer on titanium implants: Improvement of MG 63 cell differentiation onto this coating. Acta Biomater. 2009, 5, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; McQuillan, A.J.; Sharma, L.A.; Waddell, J.N.; Shibata, Y.; Duncan, W.J. Spark anodization of titanium–zirconium alloy: Surface characterization and bioactivity assessment. J. Mater. Sci. Mater. Med. 2015, 26, 221. [Google Scholar] [CrossRef] [PubMed]




| Gene | Sequence of Primers (5’-3’) | bps |
|---|---|---|
| Col1α1 | F-5’-ACTGTCTTGCCCCAAGTTCC-3’ | 124 |
| R-5’-TGGGCATCTGGTTTAGCCTT-3’ | ||
| Col3α1 | F-5’-ACGTAGATGAATTGGGATGCAG-3’ | 154 |
| R-5’-GGGTTGGGGCAGTCTAGTG-3’ | ||
| Fn1 | F-5’-CCGGGTGTCCTGATCGTT-3’ | 120 |
| R-5’-GGGGAGACCTGGGAAAAG-3’ | ||
| GAPDH | F-5’-TATGTCGTGGAGTCTACTGGT-3’ | 129 |
| R’-5-GAGTTGTCATATTTCTCGTGG-3’ |
| Material | Young’s Modulus (GPa) | Poisson’s Ratio |
|---|---|---|
| Ti-6Al-4V | 144.4 | 0.34 |
| TiNb | 120.2 | 0.36 |
| TiNb(N) | 206.6 | 0.36 |
| TiNbZr | 111.1 | 0.34 |
| TiNbZrTa | 130.8 | 0.36 |
| TiNbZrTa(N) | 180.6 | 0.36 |
| TiNbZrTa(N) | 124.5 | 0.36 |
| TiNbZrTa(N)-O | 176.4 | 0.36 |
| Inderter | 1140 | 0.07 |
| Ti-6Al-4V | 0.0004 |
|---|---|
| Film | 0.0004 |
| Indenter | 0.0004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, B.-W.; Chang, Y.-Y.; Shieh, T.-M.; Huang, H.-L. Biocompatibility and Microstructure-Based Stress Analyses of TiNbZrTa Composite Films. Materials 2022, 15, 29. https://doi.org/10.3390/ma15010029
Lai B-W, Chang Y-Y, Shieh T-M, Huang H-L. Biocompatibility and Microstructure-Based Stress Analyses of TiNbZrTa Composite Films. Materials. 2022; 15(1):29. https://doi.org/10.3390/ma15010029
Chicago/Turabian StyleLai, Bo-Wei, Yin-Yu Chang, Tzong-Ming Shieh, and Heng-Li Huang. 2022. "Biocompatibility and Microstructure-Based Stress Analyses of TiNbZrTa Composite Films" Materials 15, no. 1: 29. https://doi.org/10.3390/ma15010029







