Field Validation of Concrete Transport Property Measurement Methods
Abstract
:1. Introduction
2. Materials and Mixture Proportioning
3. Experimental Program and Testing
3.1. Rapid Chloride Permeability Test
3.2. Density, Absorption, and Void Content
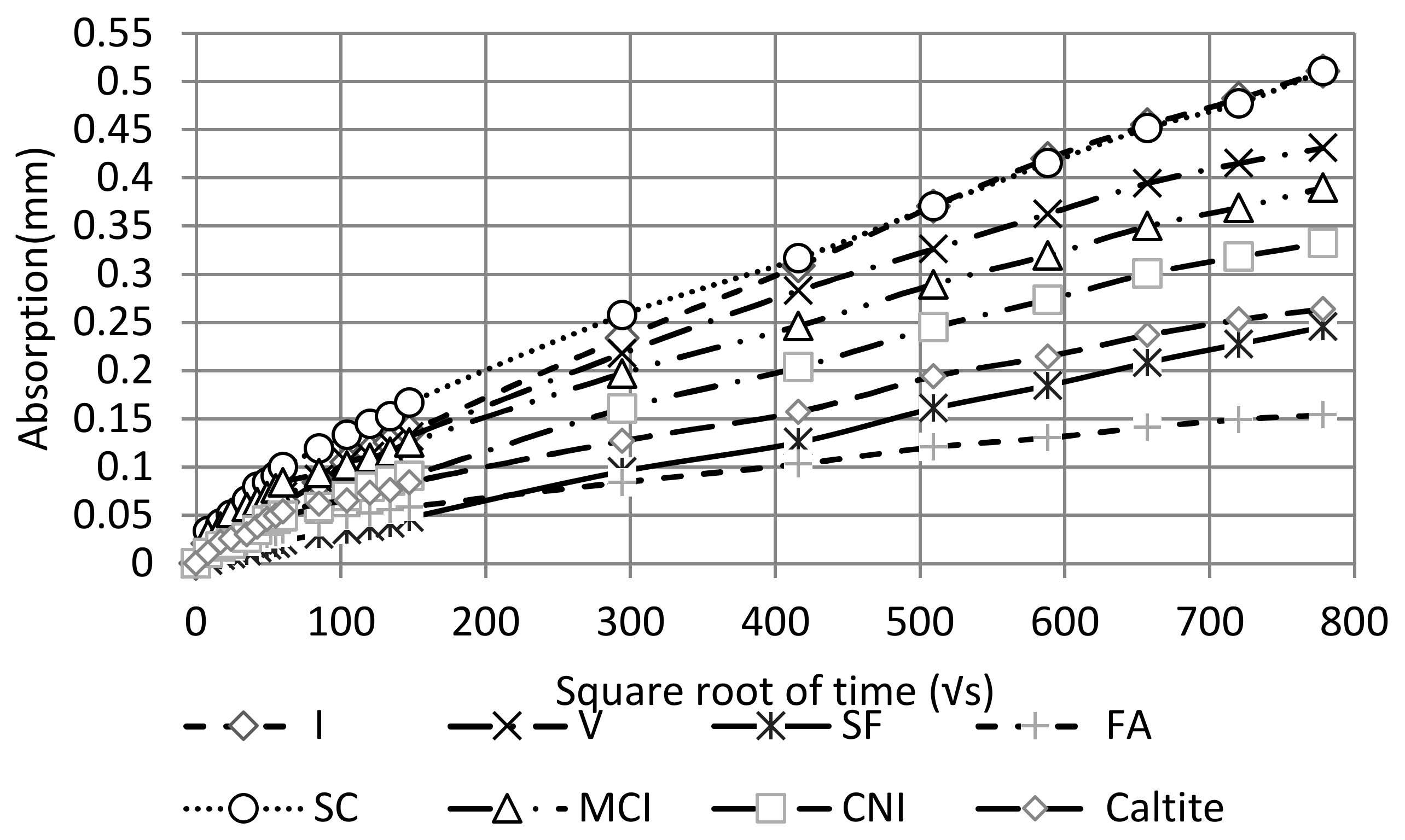
3.3. Sorptivity Test
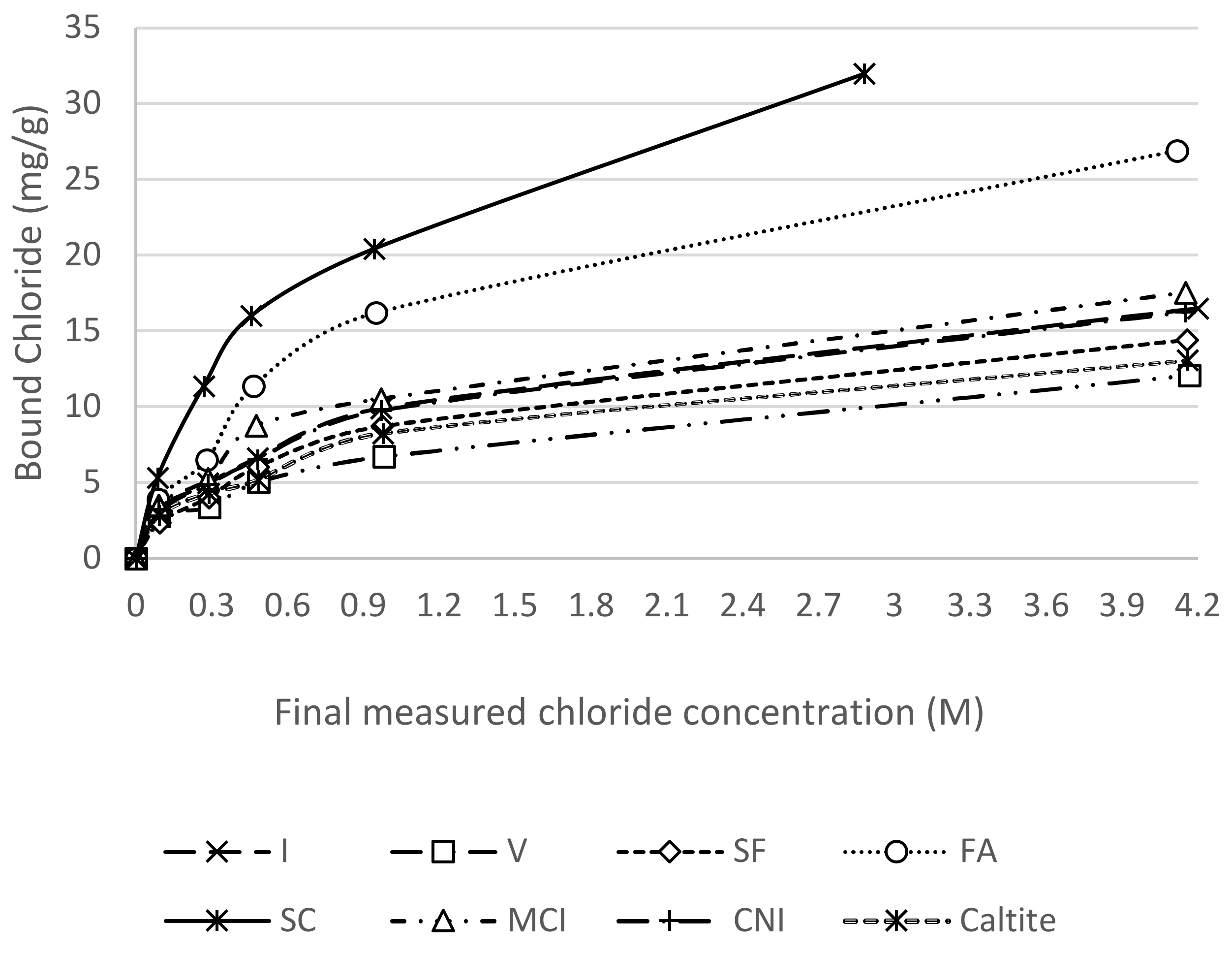
3.4. Chloride Binding Isotherms
3.5. Bulk Diffusion
3.6. Effective Chloride Diffusion Coefficient
4. Results and Discussion
4.1. Rapid Chloride Permeability Test
4.2. Density, Absorption, and Void Ratio
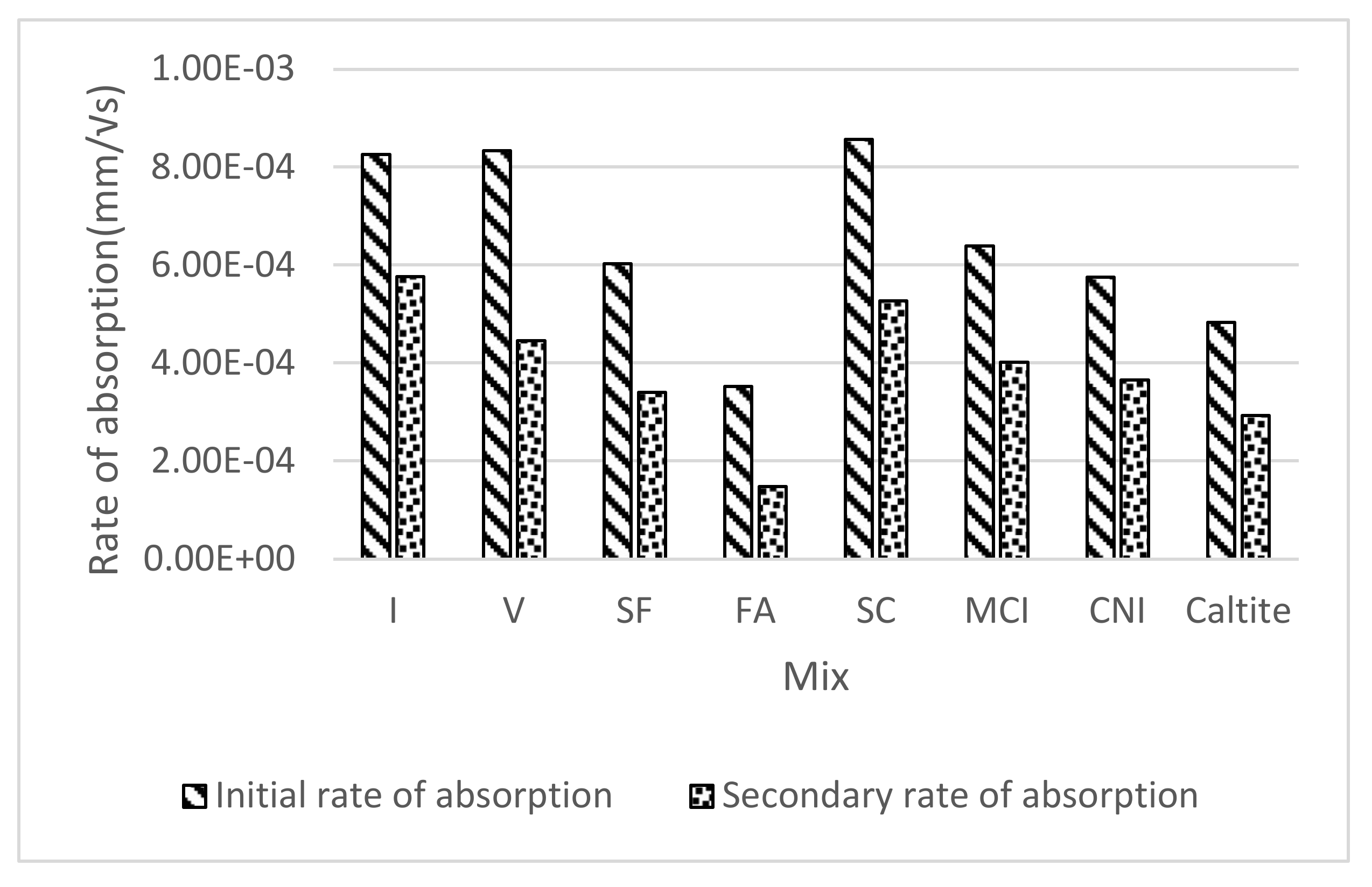
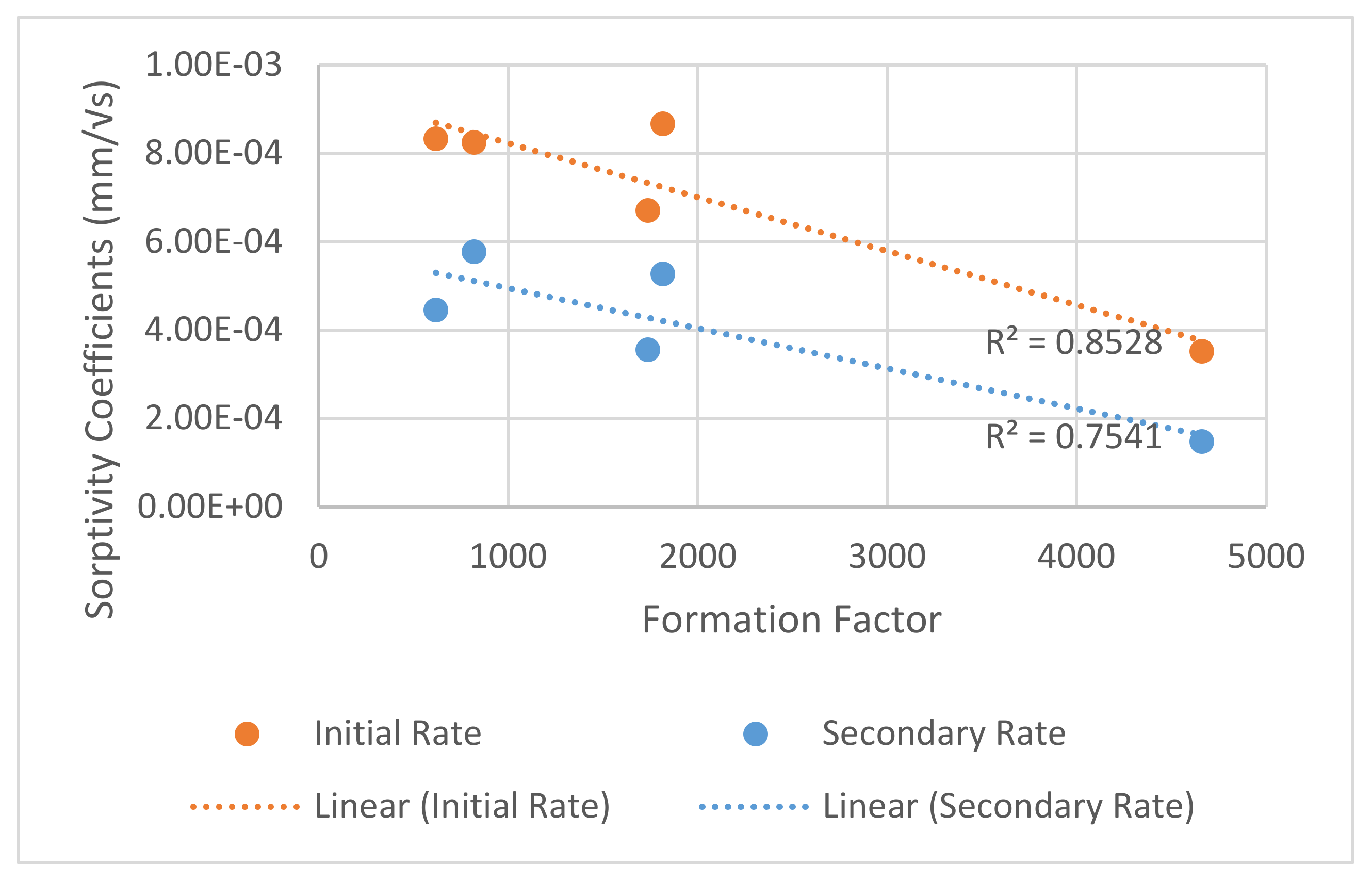
4.3. Sorptivity
4.4. Chloride Binding Isotherms
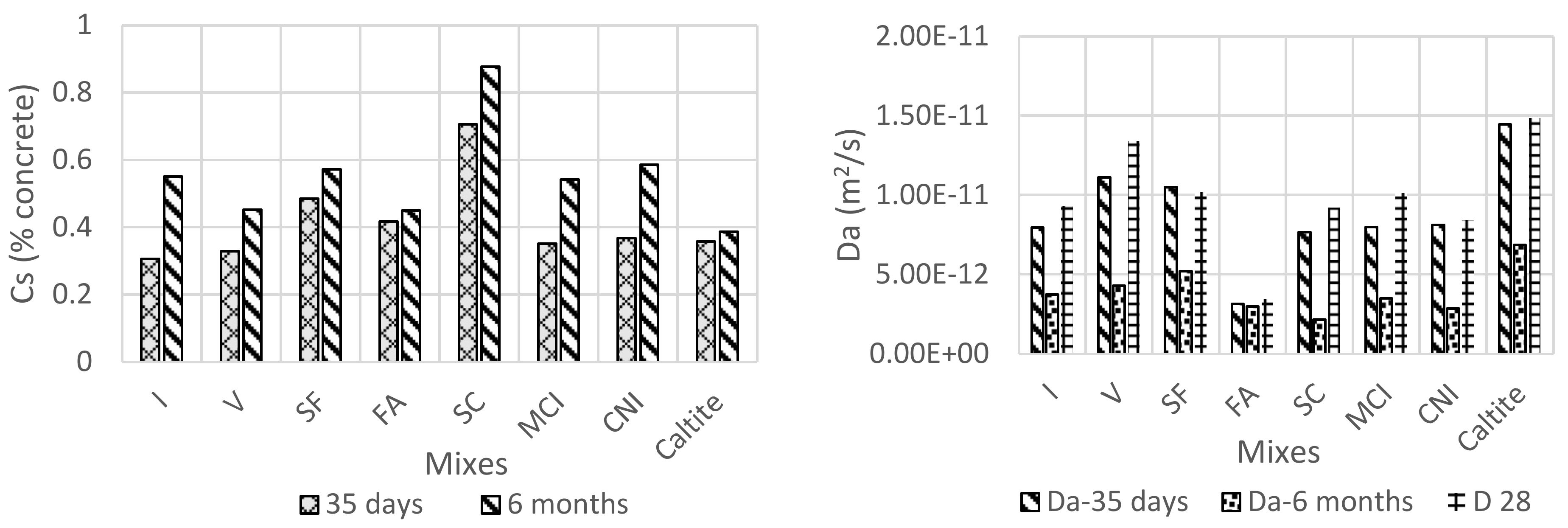
4.5. Bulk Diffusion
4.6. Effective Chloride Diffusion Coefficient
5. Conclusions
- Apparent diffusion coefficient calculated from concrete bulk diffusion tests using the error function and the effective diffusion coefficient at 28 days were similar. This implies that the simple approach used with the error function provides a good index of concrete quality.
- A good correlation was found between the concrete formation factor and effective diffusion coefficient as predicted by theory. Because the effective diffusion coefficient is a fit value that involves the use of several other parameters—such as chloride surface concentration, chloride binding isotherm, and concrete chloride profile—some error in the values was expected.
- The laboratory and field results showed that the cementitious materials outperformed the corrosion inhibitors. The use of SCMs provided the largest benefit in reducing chloride diffusion. The corrosion inhibitors showed better performance in the field, especially at larger depths, and after six months of exposure in the laboratory which could be attributed to the effectiveness of corrosion inhibitors at larger depths and leaching out decrease overtime.
- FA and SC mixtures performed the best compared to the other mixtures for the conducted experimental program in the laboratory and in the field, whereas I and V mixtures delivered the least quality performance as expected.
- Formation factor predicted the relative performance of concrete mixture in the field. The formation factor could be used as a reasonable surrogate for the effective diffusion coefficient in service life modeling and concrete durability applications. Since the formation factor can be estimated quickly and at a low cost, it could be used more frequently to measure durability than currently used tests. More work on the effect of curing on formation factor is needed.
- Absorption and binding capacity tests along with the formation factor could be indicative of long-term performance of concrete mixtures since their results agreed with the performance of the concrete in the marine exposure site.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- PCA, T. Causes of Concrete Deterioration; IS536 Portland Cement Association: Skokie, IL, USA, 2002. [Google Scholar]
- Hobbs, D.W. Concrete deterioration: Causes, diagnosis, and minimising risk. Int. Mater. Rev. 2001, 46, 117–144. [Google Scholar] [CrossRef]
- Ann, K.Y.; Song, H.W. Chloride threshold level for corrosion of steel in concrete. Corros. Sci. 2007, 49, 4113–4133. [Google Scholar] [CrossRef]
- Malikakkal, N.C. Chloride Diffusion in Concrete/prediction of the Onset of Corrosion in Reinforced Concrete Structures. Ph.D. Thesis, KFUPM, Saudi Arabia, 1994. [Google Scholar]
- Neville, A.M. Properties of Concrete; Longma: London, UK, 1995. [Google Scholar]
- Chalee, W.; Jaturapitakkul, C.A.; Chindaprasirt, P. Predicting the chloride penetration of fly ash concrete in seawater. Mar. Struct. 2009, 22, 341–353. [Google Scholar] [CrossRef]
- Costa, A.; Appleton, J. Chloride penetration into concrete in marine environment-Part II: Prediction of long term chloride penetration. Mater. Struct. 1999, 32, 354–359. [Google Scholar] [CrossRef]
- Costa, A.; Appleton, J. Chloride penetration into concrete in marine environment—Part I: Main parameters affecting chloride penetration. Mar. Struct. 1999, 32, 252. [Google Scholar] [CrossRef]
- Beaudoin, J.J.; Ramachandran, V.S.; Feldman, R.F. Interaction of chloride and C-S-H. Cem. Concr. Res. 1990, 20, 875–883. [Google Scholar] [CrossRef] [Green Version]
- Riding, K.; Thomas, M.; Folliard, K. Apparent diffusivity model for concrete containing supplementary cementitious materials. ACI Mater. J. 2013, 110, 705–714. [Google Scholar]
- Burris, L.E.; Riding, K.A. Diffusivity of binary and ternary concrete mixture blends. ACI Mater. J. 2014, 111, 373. [Google Scholar] [CrossRef]
- Mindess, S.; Young, F.J.; Darwin, D. Concrete, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Smith, J.L.; Virmani, Y.P. Materials and Methods for Corrosion Control of Reinforced and Prestressed Concrete Structures in New Construction (No. FHWA-RD-00-081); Federal Highway Administration: Washington, DC, USA, 2000. [Google Scholar]
- Richardson, I.G. The nature of the hydration products in hardened cement pastes. Cem. Concr. Compos. 2000, 22, 97–113. [Google Scholar] [CrossRef]
- Juenger, M.C.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- Thomas, M.D.; Hooton, R.D.; Scott, A.; Zibara, H. The effect of supplementary cementitious materials on chloride binding in hardened cement paste. Cem. Concr. Res. 2012, 42, 1–7. [Google Scholar] [CrossRef]
- Lollini, F.; Redaelli, E.; Bertolini, L. A study on the applicability of the efficiency factor of supplementary cementitious materials to durability properties. Constr. Build. Mater. 2016, 120, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Dhir, R.; El-Mohr, M.; Dyer, T. Chloride binding in GGBS concrete. Cem. Concr. Res. 1996, 26, 1767–1773. [Google Scholar] [CrossRef]
- Dhir, R.; El-Mohr, M.; Dyer, T. Developing chloride resisting concrete using PFA. Cem. Concr. Res. 1997, 27, 1633–1639. [Google Scholar] [CrossRef]
- Hooton, R.; Pun, P.; Kojundic, T.; Fidjestol, P. Influence of silica fume on chloride resistance of concrete. In Proceedings of the PCI/FHWA International Symposium on High Performance Concrete, Chicago, IL, USA, 20–22 October 1997; pp. 245–249. [Google Scholar]
- Kayali, O.; Zhu, B. Corrosion performance of medium-strength and silica fume high-strength reinforced concrete in a chloride solution. Cem. Concr. Compos. 2005, 27, 117–124. [Google Scholar] [CrossRef]
- Matsumura, T.; Shirai, K.; Saegusa, T. Verification method for durability of reinforced concrete structures subjected to salt attack under high temperature conditions. Nucl. Eng. Des. 2008, 238, 1181–1188. [Google Scholar] [CrossRef]
- Nokken, M.; Boddy, A.; Hooton, R.; Thomas, M. Time dependent diffusion in concrete—Three laboratory studies. Cem. Concr. Res. 2006, 36, 200–207. [Google Scholar] [CrossRef]
- Siddique, R. Compressive strength, water absorption, sorptivity, abrasion resistance and permeability of self-compacting concrete containing coal bottom ash. Constr. Build. Mater. 2013, 47, 1444–1450. [Google Scholar] [CrossRef]
- Song, H.; Kwon, S. Evaluation of chloride penetration in high performance concrete using neural network algorithm and micro pore structure. Cem. Concr. Res. 2009, 39, 814–824. [Google Scholar] [CrossRef]
- Stanish, K.; Thomas, M. The use of bulk diffusion tests to establish time-dependent concrete chloride diffusion coefficients. Cem. Concr. Res. 2003, 33, 55–62. [Google Scholar] [CrossRef]
- Tamimi, A.; Abdalla, J.; Sakka, Z. Prediction of long term chloride diffusion of concrete in harsh environment. Constr. Build. Mater. 2008, 22, 829–836. [Google Scholar] [CrossRef]
- Thomas, M.D.; Bamforth, P.B. Modelling chloride diffusion in concrete: Effect of fly ash and slag. Cem. Concr. Res. 1999, 29, 487–495. [Google Scholar] [CrossRef]
- Valipour, M.; Pargar, F.; Shekarchi, M.; Khani, S. Comparing a natural pozzolan, zeolite, to metakaolin and silica fume in terms of their effect on the durability characteristics of concrete: A laboratory study. Constr. Build. Mater. 2013, 41, 879–888. [Google Scholar] [CrossRef]
- Rizzo, P.; Nasrollahi, A.; Deng, W.; Vandenbossche, J.M. Detecting the presence of high water-to-cement ratio in concrete surfaces using highly nonlinear solitary waves. Appl. Sci. 2016, 6, 104. [Google Scholar] [CrossRef]
- Zhutovsky, S.; Kovler, K. Influence of water to cement ratio on the efficiency of internal curing of high-performance concrete. Constr. Build. Mater. 2017, 144, 311–316. [Google Scholar] [CrossRef]
- Lotfi-Omran, O.; Sadrmomtazi, A.; Nikbin, I.M. A comprehensive study on the effect of water to cement ratio on the mechanical and radiation shielding properties of heavyweight concrete. Constr. Build. Mater. 2019, 229, 116905. [Google Scholar] [CrossRef]
- Delagrave, A.; Marchand, J.; Ollivier, J.P.; Julien, S.; Hazrati, K. Chloride binding capacity of various hydrated cement paste systems. Adv. Cem. Based Mater. 1997, 6, 28–35. [Google Scholar] [CrossRef]
- Luo, R.; Cai, Y.; Wang, C.; Huang, X. Study of chloride binding and diffusion in GGBS concrete. Cem. Concr. Res. 2003, 33, 1–7. [Google Scholar] [CrossRef]
- Yuan, Q.; Shi, C.; De Schutter, G.; Audenaert, K.; Deng, D. Chloride binding of cement-based materials subjected to external chloride environment—A review. Constr. Build. Mater. 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Fink, J. Petroleum Engineer’s Guide to Oil Field Chemicals and Fluids; Gulf Professional Publishing Oxford: Oxford, UK, 2015. [Google Scholar]
- Tritthart, J.; Banfill, P.F.G. Nitrite binding in cement. Cem. Concr. Res. 2001, 31, 1093–1100. [Google Scholar] [CrossRef]
- Sideris, K.K.; Savva, A.E. Durability of mixtures containing calcium nitrite based corrosion inhibitor. Cem. Concr. Compos. 2005, 27, 277–287. [Google Scholar] [CrossRef]
- Söylev, T.A.; Richardson, M.G. Corrosion inhibitors for steel in concrete: State-of-the-art report. Constr. Build. Mater. 2008, 22, 609–622. [Google Scholar] [CrossRef]
- Hewayde, E.; Nehdi, M.L.; Allouche, E.; Nakhla, G. Using concrete admixtures for sulphuric acid resistance. Proc. Inst. Civ. Eng. Constr. Mater. 2007, 160, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Nanukuttan, S.V.; Basheer, L.; McCarter, W.J.; Robinson, D.J.; Basheer, P.M. Full-Scale Marine Exposure Tests on Treated and Untreated Concretes-Initial 7-Year Results. ACI Mater. J. 2008, 105, 81. [Google Scholar]
- Weiss, W.J.; Ley, T.; Isgor, O.B.; Van Dam, T. Toward performance specifications for concrete durability: Using the formation factor for corrosion and critical saturation for freeze-thaw. In Proceedings of the 96th Annual Transportation Research Board, Washington, DC, USA, 8–12 January 2017. [Google Scholar]
- ASTM International (American Society for testing and materials). Standard Test Method for Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration. In ASTM C1202-19; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- ASTM International. (American Society for testing and materials) Standard Test Method for Bulk Electrical Conductivity of Hardened Concrete. In ASTM C1760-12; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar] [CrossRef]
- AASHTO, T. 358. Standard Method of Test for Surface Resistivity Indication of Concrete’s Ability to Resist Chloride Ion Penetration; AASHTO: Washington, DC, USA, 2011. [Google Scholar]
- Wong, S.F.; Wee, T.H.; Swaddiwudhipong, S.; Lee, S.L. Study of water movement in concrete. Mag. Concr. Res. 2001, 53, 205–220. [Google Scholar] [CrossRef]
- Chia, K.S.; Zhang, M.H. Water permeability and chloride penetrability of high-strength lightweight aggregate concrete. Cem. Concr. Res. 2002, 32, 639–645. [Google Scholar] [CrossRef]
- Liu, X.; Chia, K.S.; Zhang, M.H. Water absorption, permeability, and resistance to chloride-ion penetration of lightweight aggregate concrete. Constr. Build. Mater. 2011, 25, 335–343. [Google Scholar] [CrossRef]
- ASTM International (American Society for testing and materials). Standard Test Method for Bulk Electrical Resistivity or Bulk Conductivity of Concrete. In ASTM C1876-19; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar] [CrossRef]
- Azad, J.V.; Erbektas, A.R.; Qiao, C.; Isgor, O.B.; Weiss, W.J. Relating the Formation Factor and Chloride Binding Parameters to the Apparent Chloride Diffusion Coefficient of Concrete. J. Mater. Civ. Eng. 2018, 31, 04018392. [Google Scholar] [CrossRef]
- El Fattah, A.A.; Al-Duais, I.; Riding, K.; Thomas, M. Field evaluation of corrosion mitigation on reinforced concrete in marine exposure conditions. Constr. Build. Mater. 2018, 165, 663–674. [Google Scholar] [CrossRef]
- ASTM (American Society for testing and materials). Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. In ASTM C39/C39M-15a; ASTM: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM (American Society for testing and materials). Standard Test Method for Density, Absorption, and Voids in Hardened Concrete. In ASTM C 642; American Society for testing and materials: West Conshohocken, PA, USA, 1997. [Google Scholar]
- ASTM (American Society for testing and materials). Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic-Cement Concretes. In ASTM C 1585; ASTM: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Luping, T.; Nilsson, L. Chloride binding capacity and binding isotherms of OPC pastes and mortars. Cem. Concr. Res. 1993, 23, 247–253. [Google Scholar] [CrossRef]
- ASTM (American Society for testing and materials). Determining the Apparent Chloride Diffusion Coefficient of Cementitious Mixtures by Bulk Diffusion 1. In ASTM C 1556; ASTM: West Conshohocken, PA, USA, 2003. [Google Scholar]
- ASTM (American Society for testing and materials). Standard Test Method for Acid-Soluble Chloride in Mortar and Concrete. In ASTM C1152; ASTM International: West Conshohocken, PA, USA, 2006; pp. 3–5. [Google Scholar]
- Martın-Pérez, B. Service life modelling of RC higway structures exposed to chlorides. PhD Thesis, University of Toronto, Toronto, Canada, 1999. [Google Scholar]
- O’Reilly, M.; Sperry, J.; Browning, J.; Darwin, D. Evaluation of Effects of Casting and Curing Conditions and Specimen Type on Concrete Strength and Permeability; University of Kansas Center for Research, Inc.: Lawrence, KS, USA, 2017. [Google Scholar]
- Haque, M.N.; Kayali, O. Properties of high-strength concrete using a fine fly ash. Cem. Concr. Res. 1998, 28, 1445–1452. [Google Scholar] [CrossRef]
- Benli, A.; Karataş, M.; Bakir, Y. An experimental study of different curing regimes on the mechanical properties and sorptivity of self-compacting mortars with fly ash and silica fume. Constr. Build. Mater. 2017, 144, 552–562. [Google Scholar] [CrossRef]
- Song, S.; Jennings, H.M. Pore solution chemistry of alkali-activated ground granulated blast-furnace slag. Cem. Concr. Res. 1999, 29, 159–170. [Google Scholar] [CrossRef]
- De la Varga, I.; Spragg, R.P.; Di Bella, C.; Castro, J.; Bentz, D.P.; Weiss, J. Fluid transport in high volume fly ash mixtures with and without internal curing. Cem. Concr. Compos. 2014, 45, 102–110. [Google Scholar] [CrossRef]
- Cheewaket, T.; Jaturapitakkul, C.; Chalee, W. Long term performance of chloride binding capacity in fly ash concrete in a marine environment. Constr. Build. Mater. 2010, 24, 1352–1357. [Google Scholar] [CrossRef]
- Dousti, A.; Shekarchi, M.; Alizadeh, R.; Taheri-Motlagh, A. Binding of externally supplied chlorides in micro silica concrete under field exposure conditions. Cem. Concr. Compos. 2011, 33, 1071–1079. [Google Scholar] [CrossRef]
- Osborne, G.J. Durability of Portland blast-furnace slag cement concrete. Cem. Concr. Compos. 1999, 21, 11–21. [Google Scholar] [CrossRef]
- Xie, X.; Feng, Q.; Chen, Z.; Lu, W. Effect of the Electric Field on the Distribution Law of Chloride Ions and Microstructure in Concrete with the Addition of Mineral Admixtures. Materials 2019, 12, 1380. [Google Scholar] [CrossRef] [Green Version]






| Chemical and Physical Analysis (%) | OPC | Cement Type V | Silica Fume | Fly Ash | Slag |
|---|---|---|---|---|---|
| SiO2 | 20.8 | 20.97 | 91 | 51.47 | 34.8 |
| Al2O3 | 5.37 | 3.91 | 24.31 | 13.4 | |
| Fe2O3 | 3.32 | 4.8 | 8.87 | 0.62 | |
| TiO2 | 1.02 | ||||
| CaO | 63.77 | 64.27 | 5.15 | 43.4 | |
| MgO | 2.08 | 1.97 | 3.50 | 5.44 | |
| SO3 | 2.63 | 1.86 | 0.23 | 0.34 | |
| Loss on Ignition (L.O.I) | 1.34 | 2.16 | 6.00 | 0.53 | |
| Insoluble residue (I.R.) | 0.39 | 0.60 | 0.34 | ||
| Na2Oeq | 0.52 | 0.48 | 0.56 | ||
| K2O | 1.47 | ||||
| P2O5 | 0.257 | ||||
| C3S | 53.20 | 63.84 | |||
| C2S | 19.50 | 11.96 | |||
| C3A | 8.61 | 2.24 | |||
| C4AF | 10.10 | 14.61 | |||
| C3AF + 2C3A | 27.33 | 19.09 | |||
| Fineness, Air permeability Test (m2/Kg) | 323 | 315 |
| Mix | W/C | Cement (Kg/m3) | Coarse Aggregate (Kg/m3) | Sand (Kg/m3) | Water (Kg/m3) | Silica Fume (Kg/m3) | Fly Ash (Kg/m3) | Slag Cement (Kg/m3) | Notes |
|---|---|---|---|---|---|---|---|---|---|
| I | 0.4 | 340 | 1070 | 775 | 136 | - | - | - | Type OP/CEM 1 |
| V | 340 | 1070 | 775 | 136 | - | - | - | Type V/ high sulfate-resistant Portland cement | |
| SF | 320 | 1100 | 735 | 136 | 21 | - | - | OP + SF | |
| FA | 255 | 1090 | 735 | 136 | - | 85 | - | OP + FA | |
| SC | 100 | 1095 | 735 | 136 | - | - | 240 | OP + SC | |
| MCI | 340 | 1070 | 775 | 136 | - | - | - | OP + MCI at 0.6 L/m3 of concrete | |
| CNI | 340 | 1070 | 775 | 136 | - | - | - | OP + CNI at 20 L/m3 of concrete | |
| Caltite | 340 | 1070 | 775 | 136 | - | - | - | Type I + Caltite at 30 L/m3 of concrete |
| Mix | Slump mm | Strength after 28 days-MPa |
|---|---|---|
| Type I | 30 | 28.1 |
| Type V | 32 | 28.6 |
| SF | 26 | 30.3 |
| FA | 34 | 39.1 |
| SC | 42 | 28.0 |
| MCI | 29 | 29.2 |
| CNI | 31 | 32.4 |
| Caltite | 34 | 28.1 |
| Time | 60 s | 5 min | 10 min | 20 min | 30 min | 60 min | Every Hour up to 6 h | Once a Day up to 7 days | Day 7 to 9, 1 Measurement |
|---|---|---|---|---|---|---|---|---|---|
| Tolerance | 2 s | 10 s | 2 min | 2 min | 2 min | 2 min | 5 min | 2 h | 2 h |
| Mix | RCPT | RCPT Permeability Classification | Resistivity (kΩ-cm) | Formation Factor |
|---|---|---|---|---|
| I | 3226 | Moderate | 8.17 | 819 |
| V | 4008 | High | 6.63 | 618 |
| SF | 1522 | Low | 21.57 | 1738 |
| FA | 1140 | Low | 21.66 | 4662 |
| SC | 1013 | Low | 20.4 | 1816 |
| MCI | 2491 | Moderate | - | - |
| CNI | 4094 | High | - | - |
| Caltite | 3509 | Moderate | - | - |
| Sample | Mass (g) | Absorption (%) | Dry Bulk Dens. (g/g) | Bulk Dens. (g/g) | Apparent Dens. (g/g) | Void % | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OD | B.SSD | Suspended | AVG OD | AVG B.SSD | AVG Susp. | ||||||
| Letter Des. | - | - | - | A | C | D | (C-A/A)*100 | A/(C-D) | C/(C-D) | A/(A-D) | (g2-g1)/g2*100 |
| I-1 | 722.3 | 768.2 | 434.4 | 700.5 | 745.6 | 421.6 | 6.44 | 2.16 | 2.30 | 2.51 | 13.92 |
| I-2 | 678.7 | 723 | 408.8 | ||||||||
| V-1 | 714.7 | 762.6 | 421 | 712.1 | 758.4 | 421.2 | 6.49 | 2.11 | 2.25 | 2.45 | 13.72 |
| V-2 | 709.5 | 754.1 | 421.4 | ||||||||
| SF-1 | 759.2 | 803 | 451.9 | 734.4 | 775.6 | 439.6 | 5.61 | 2.18 | 2.31 | 2.49 | 12.26 |
| SF-2 | 738.2 | 779 | 434.7 | ||||||||
| FA-1 | 639.2 | 679 | 384.2 | 700.3 | 737 | 419.6 | 5.25 | 2.21 | 2.32 | 2.49 | 11.58 |
| FA-2 | 761.4 | 795.1 | 455 | ||||||||
| SC-1 | 676.6 | 731.1 | 409.6 | 689.9 | 746.1 | 417.8 | 8.15 | 2.10 | 2.27 | 2.54 | 9.02 |
| SC-2 | 703.1 | 761.1 | 426.1 | ||||||||
| MCI-1 | 785.9 | 863 | 473.1 | 789.6 | 852.4 | 476.2 | 7.95 | 2.10 | 2.27 | 2.52 | 16.7 |
| MCI-2 | 793.2 | 841.7 | 479.3 | ||||||||
| CNI-1 | 770.9 | 811.7 | 463 | 740.2 | 779.9 | 445.2 | 5.36 | 2.21 | 2.33 | 2.51 | 11.86 |
| CNI-2 | 709.5 | 748.1 | 427.3 | ||||||||
| Caltite-1 | 759.2 | 803 | 451.9 | 748.7 | 791 | 443.3 | 5.65 | 2.15 | 2.27 | 2.45 | 12.17 |
| Caltite-2 | 738.2 | 779 | 434.7 | ||||||||
| Mixture | α (mg Cl/ g paste) | β |
|---|---|---|
| I | 2.0844 | 0.4159 |
| V | 1.4750 | 0.4210 |
| SF | 1.6984 | 0.4320 |
| FA | 2.9867 | 0.4455 |
| SC | 4.3609 | 0.4338 |
| MCI | 2.5519 | 0.3891 |
| CNI | 2.0412 | 0.4180 |
| Caltite | 1.8011 | 0.3991 |
| Test | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| RCPT | FA | SC | SF | MCI | I | Caltite | V | CNI |
| Absorption | FA | SF | CNI | Caltite | I | V | MCI | SC |
| Void ratio | SC | FA | CNI | Caltite | SF | V | I | MCI |
| Sorptivity | FA | SF | Caltite | CNI | MCI | I | V | SC |
| Binding capacity | SC | FA | MCI | CNI | I | SF | Caltite | V |
| Bulk diffusion | FA | SC | SF | CNI | Caltite | MCI | I | V |
| Overall performance | FA | SC | SF | CNI | Caltite | MCI | I | V |
| Rank | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Steel corrosion | FA | SC | Caltite | MCI | CNI | SF | I | V |
| Diffusion coefficient | FA | CNI | I | SF | SC | Caltite | MCI | V |
| Overall performance | FA | SC | CNI | Caltite | MCI | SF | I | V |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El Fattah, A.; Al-Duais, I.; Riding, K.; Thomas, M.; Al-Dulaijan, S.; Al-Zahrani, M. Field Validation of Concrete Transport Property Measurement Methods. Materials 2020, 13, 1166. https://doi.org/10.3390/ma13051166
Abd El Fattah A, Al-Duais I, Riding K, Thomas M, Al-Dulaijan S, Al-Zahrani M. Field Validation of Concrete Transport Property Measurement Methods. Materials. 2020; 13(5):1166. https://doi.org/10.3390/ma13051166
Chicago/Turabian StyleAbd El Fattah, Ahmed, Ibrahim Al-Duais, Kyle Riding, Michael Thomas, Salah Al-Dulaijan, and Mesfer Al-Zahrani. 2020. "Field Validation of Concrete Transport Property Measurement Methods" Materials 13, no. 5: 1166. https://doi.org/10.3390/ma13051166
APA StyleAbd El Fattah, A., Al-Duais, I., Riding, K., Thomas, M., Al-Dulaijan, S., & Al-Zahrani, M. (2020). Field Validation of Concrete Transport Property Measurement Methods. Materials, 13(5), 1166. https://doi.org/10.3390/ma13051166





