Surface Characterization of Electro-Assisted Titanium Implants: A Multi-Technique Approach
Abstract
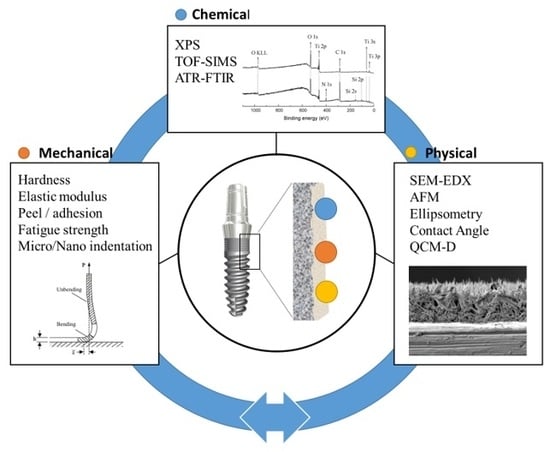
:1. Introduction
2. Surface Chemical Composition
2.1. X-ray Photoelectron Spectroscopy
2.2. Time-of-Flight Secondary Ion Mass Spectrometry
2.3. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy
3. Surface Topography/Morphology
3.1. Atomic Force Microscopy
3.2. Scanning Electron Microscopy with Energy-Dispersive X-ray Analysis
4. Surface Phenomena
4.1. Ellipsometry
4.2. Contact Angle
4.3. Quartz Crystal Microbalance Dissipation Monitoring
5. Mechanical Characterization
5.1. Nano- and Micro-Indentation
5.2. Peel (or Adhesion) Tests
5.3. Fatigue Tests
6. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| AgNPs | Silver nanoparticles |
| ASTM | American Society for Testing and Materials |
| ATR-FTIR | Attenuated total reflectance Fourier-transform infrared spectroscopy |
| BE | Binding energy |
| CA | Contact angle |
| CaP | Calcium phosphate |
| CH | Chitosan |
| Dex | Dexamethasone |
| DNA | Deoxyribonucleic Acid |
| ECTFE | Ethylene chlorotrifluoroethylene |
| EPD | Electro-phoretic deposition |
| E-QCM | Electrochemical quartz crystal microbalance |
| ESCA | Electron Spectroscopy for Chemical Analysis |
| ESEM | Environmental scanning electron microscopy |
| FESEM | Field emission scanning electron microscope |
| GelGO | Graphene Oxide cross-linked Gelatin Fillers |
| GelGOHA | Graphene Oxide cross-linked Gelatin Fillers dispersed in hydroxyapatite |
| GL | Gelatin |
| Gly | Glycine |
| GO | Graphene oxide |
| HA | HydroxyApatite |
| HTCS | N-Hexyltrichlorosilane |
| Lig | Lignin |
| MPC | 2-(Methacryloyloxy)ethyl Phosphoryl Choline |
| MWNT | Multi-walled nanotubes |
| NaPSS | Poly(Sodium-4-stryrensulfonate) |
| P(HEMA-MOEP) | Poly-2(Hydroxyethyl Methacrylate-2-Methacryloyloxyethyl Phosphate) |
| PCL | Poly(ε-Caprolactone) |
| PDA | Poly(Dopamine) |
| PEEK | Poly(Ether Ether Ketone) |
| PEG | Poly(Ethylene Glycol) |
| PEGDA | Poly(Ethylene Glycol Diacrylate) |
| PHEMA | Poly(2-Hydroxyethyl Methacrylate) |
| PLGA | Poly(D,L-Lactic-Co-Glycolic acid) |
| PPy | Poly(Pyrrole) |
| PVS | Poly(Vinylsulfonic acid, Sodium salt) |
| PyHTCS | 6-(1,0-Pyrrolyl)-N-Hexyltrichlorosilane |
| Py-PD | 4-Pyrrolyphenyldiazonium |
| QCM-D | Quartz Crystal Microbalance with Dissipation Monitoring |
| RGD | Arginyl-glycyl-aspartic Acid |
| SAMs | Self-assembled monolayers |
| SEM–EDX | Scanning Electron Microscopy with Energy-Dispersive X-ray analysis |
| SF | Silk fibroin |
| TEM | Transmission electron microscopy |
| TNs | Tio2-nanotubes |
| TNTA | Titania nanotube arrays |
| TOF-SIMS | Time-of-flight secondary ion mass spectrometry |
| XPS | X-ray photoelectron spectroscopy |
References
- Cometa, S.; Bonifacio, M.A.; Mattioli-Belmonte, M.; Sabbatini, L.; De Giglio, E. Electrochemical strategies for titanium implant polymeric coatings: The why and how. Coatings 2019, 9, 268–287. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.M.; Adesina, A.Y.; Hussein, M.A.; Ramakrishna, S.; Al-Aqeeli, N.; Akhtar, S.; Saravanan, S. PEDOT/FHA nanocomposite coatings on newly developed Ti-Nb-Zr implants: Biocompatibility and surface protection against corrosion and bacterial infections. Mater. Sci. Eng. C 2019, 98, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Yamaguchi, S. Simulated body fluid and the novel bioactive materials derived from it. J. Biomed. Mater. Res. Part A 2019, 107, 968–977. [Google Scholar] [CrossRef]
- Gil, F.J.; Rodriguez, A.; Espinar, E.; Llamas, J.M.; Padullés, E.; Juàrez, A. Effect of oral behavior of Titanium Dental Implants. Int. J. Oral Maxillofac. Implants 2012, 27, 64–68. [Google Scholar]
- Su, Y.; Cockerill, I.; Zheng, Y.; Tang, L.; Qin, Y.X.; Zhu, D. Biofunctionalization of metallic implants by calcium phosphate coatings. Bioact. Mater. 2019, 4, 196–206. [Google Scholar] [CrossRef]
- Civantos, A.; Martinez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium coatings and surface modifications: Toward clinically useful bioactive implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef]
- Zhang, N.; Kohn, D.H. Using polymeric materials to control stem cell behavior for tissue regeneration. Birth Defects Res. Part C Embryo Today Rev. 2012, 96, 63–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Zhang, X.; Yu, B.; Zhou, F. Brushing up functional materials. NPG Asia Mater. 2019, 11, 1–39. [Google Scholar] [CrossRef]
- Chen, L.; Yan, C.; Zheng, Z. Functional polymer surfaces for controlling cell behaviors. Mater. Today 2018, 21, 38–59. [Google Scholar] [CrossRef]
- Wischerhoff, E.; Uhlig, K.; Lankenau, A.; Börner, H.G.; Laschewsky, A.; Duschl, C.; Lutz, J.F. Controlled cell adhesion on PEG-based switchable surfaces. Angew. Chem. Int. Ed. 2008, 47, 5666–5668. [Google Scholar] [CrossRef]
- Mizutani, A.; Kikuchi, A.; Yamato, M.; Kanazawa, H.; Okano, T. Preparation of thermoresponsive polymer brush surfaces and their interaction with cells. Biomaterials 2008, 29, 2073–2081. [Google Scholar] [CrossRef]
- do Nascimento, C.; Pita, M.S.; de Souza Santos, E.; Monesi, N.; Pedrazzi, V.; de Albuquerque, R.F., Jr.; Ribeiro, R.F. Microbiome of titanium and zirconia dental implants abutments. Dent. Mater. 2016, 32, 93–101. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, E.; Ditaranto, N.; Sabbatini, L. Polymer Surface Chemistry: Characterization by XPS. In Polymer Surface Characterization; Sabbatini, L., Ed.; Walter de Gruyter GmbH: Berlin, Germany; Boston, MA, USA, 2014; Chapter 3; pp. 73–111. ISBN 978-3-11-027508-7. [Google Scholar]
- Aronsson, B.O.; Krozer, A.; Lausmaa, J.; Kasemo, B. Commercially Pure Titanium and Ti6Al4V: XPS Comparison Between Different Commercial Ti Dental Implants and Foils Prepared by Various Oxidation Procedures. Surf. Sci. Spectra 1996, 4, 42–89. [Google Scholar] [CrossRef]
- Arys, A.; Philippart, C.; Dourov, N.; He, Y.; Le, Q.T.; Pireaux, J.J. Analysis of titanium dental implants after failure of osseointegration: Combined histological, electron microscopy, and X-ray photoelectron spectroscopy approach. J. Biomed. Mater. Res. 1998, 43, 300–312. [Google Scholar] [CrossRef]
- Ameen, A.P.; Short, R.D.; Johns, R.; Schwach, G. The surface analysis of implant materials. The surface composition of a titanium dental implant material. Clin. Oral Implants Res. 1993, 4, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Cochis, A.; Azzimonti, B.; Della Valle, C.; De Giglio, E.; Bloise, N.; Visai, L.; Cometa, S.; Rimondini, L.; Chiesa, R. The effect of silver or gallium doped titanium against the multidrug resistant Acinetobacter baumannii. Biomaterials 2016, 80, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, M.D.; Kramer, E.J.; Hawker, C.J. Advanced techniques for the characterization of surface structure in polymer thin films and coatings. Arab. J. Sci. Eng. 2014, 39, 1–13. [Google Scholar] [CrossRef]
- Sabbatini, L.; Malitesta, C.; De Giglio, E.; Losito, I.; Torsi, L.; Zambonin, P.G. Electrosynthesised thin polymer films: The role of XPS in the design of application oriented innovative materials. J. Electron Spectrosc. Relat. Phenom. 1999, 100, 35–53. [Google Scholar] [CrossRef]
- De Giglio, E.; Losito, I.; Dagostino, F.; Sabbatini, L.; Zambonin, P.G.; Torrisi, A.; Licciardello, A. Analytical Characterization of Poly (Pyrrole-3-Carboxylic Acid) Films Electrosynthesised on Pt, Ti and Ti/Al/V Substrates. Ann. Chim. J. Anal. Environ. Cult. Herit. Chem. 2004, 94, 207–218. [Google Scholar] [CrossRef]
- De Giglio, E.; Calvano, C.D.; Losito, I.; Sabbatini, L.; Zambonin, P.G.; Torrisi, A.; Licciardello, A. Surface (XPS, SIMS) chemical investigation on poly (pyrrole-3-acetic acid) films electrosynthesized on Ti and TiAlV substrates for the development of new bioactive substrates. Surf. Interface Anal. 2005, 37, 580–586. [Google Scholar] [CrossRef]
- De Giglio, E.; Cometa, S.; Calvano, C.D.; Sabbatini, L.; Zambonin, P.G.; Colucci, S.; Di Benedetto, A.; Colaianni, G. A new titanium biofunctionalized interface based on poly (pyrrole-3-acetic acid) coating: Proliferation of osteoblast-like cells and future perspectives. J. Mater. Sci. Mater. Med. 2007, 18, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, E.; Cometa, S.; Sabbatini, L.; Zambonin, P.G.; Spoto, G. Electrosynthesis and analytical characterization of PMMA coatings on titanium substrates as barriers against ion release. Anal. Bioanal. Chem. 2005, 381, 626–633. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, E.; Cometa, S.; Cioffi, N.; Torsi, L.; Sabbatini, L. Analytical investigations of poly (acrylic acid) coatings electrodeposited on titanium-based implants: A versatile approach to biocompatibility enhancement. Anal. Bioanal. Chem. 2005, 389, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, E.; Cometa, S.; Satriano, C.; Sabbatini, L.; Zambonin, P.G. Electrosynthesis of hydrogel films on metal substrates for the development of coatings with tunable drug delivery performances. J. Biomed. Mater. Res. Part A 2009, 88, 1048–1057. [Google Scholar] [CrossRef]
- De Giglio, E.; Cometa, S.; Ricci, M.A.; Zizzi, A.; Cafagna, D.; Manzotti, S.; Sabbatini, L.; Mattioli-Belmonte, M. Development and characterization of rhVEGF-loaded poly (HEMA–MOEP) coatings electrosynthesized on titanium to enhance bone mineralization and angiogenesis. Acta Biomater. 2010, 6, 282–290. [Google Scholar] [CrossRef]
- Park, J.W.; Kurashima, K.; Tustusmi, Y.; An, C.H.; Suh, J.Y.; Doi, H.; Nomura, N.; Noda, K.; Hanawa, T. Bone healing of commercial oral implants with RGD immobilization through electrodeposited poly (ethylene glycol) in rabbit cancellous bone. Acta Biomater. 2011, 7, 3222–3229. [Google Scholar] [CrossRef]
- De Giglio, E.; Cafagna, D.; Cometa, S.; Allegretta, A.; Pedico, A.; Giannossa, L.C.; Sabbatini, L.; Mattioli-Belmonte, M.; Iatta, R. An innovative, easily fabricated, silver nanoparticle-based titanium implant coating: Development and analytical characterization. Anal. Bioanal. Chem. 2013, 405, 805–816. [Google Scholar] [CrossRef]
- Cometa, S.; Bonifacio, M.A.; Baruzzi, F.; de Candia, S.; Giangregorio, M.M.; Giannossa, L.C.; Dicarlo, M.; Mattioli-Belmonte, M.; Sabbatini, L.; De Giglio, E. Silver-loaded chitosan coating as an integrated approach to face titanium implant-associated infections: Analytical characterization and biological activity. Anal. Bioanal. Chem. 2017, 409, 7211–7221. [Google Scholar] [CrossRef]
- Bonifacio, M.A.; Cometa, S.; Dicarlo, M.; Baruzzi, F.; de Candia, S.; Gloria, A.; Giangregorio, M.M.; Mattioli-Belmonte, M.; De Giglio, E. Gallium-modified chitosan/poly(acrylic acid) bilayer coatings for improved titanium implant performances. Carb. Pol. 2017, 166, 348–357. [Google Scholar] [CrossRef]
- Dai, G.; Wan, W.; Chen, J.; Wu, J.; Shuai, X.; Wang, Y. Enhanced osteogenic differentiation of MC3T3-E1 on rhBMP-2 immobilized titanium surface through polymer-mediated electrostatic interaction. Appl. Surf. Sci. 2019, 471, 986–998. [Google Scholar] [CrossRef]
- Erakovic, S.; Veljovic, D.; Diouf, P.N.; Stevanovic, T.; Mitric, M.; Milonjic, S.; Miskovic-Stankovic, V.B. Electrophoretic Deposition of Biocomposite Lignin/Hydroxyapatite Coatings on Titanium. Int. J. Chem. React. Eng. 2009, 7, A62. [Google Scholar] [CrossRef]
- Suriano, R.; Oldani, V.; Bianchi, C.L.; Turri, S. AFM nanomechanical properties and durability of new hybrid fluorinated sol-gel coatings. Surf. Coat. Technol. 2015, 264, 87–96. [Google Scholar] [CrossRef]
- Jugowiec, D.; Łukaszczyk, A.; Cieniek, Ł.; Kowalski, K.; Rumian, Ł.; Pietryga, K.; Kot, M.; Pamuła, E.; Moskalewicz, T. Influence of the electrophoretic deposition route on the microstructure and properties of nano-hydroxyapatite/chitosan coatings on the Ti-13Nb-13Zr alloy. Surf. Coat. Technol. 2017, 324, 64–79. [Google Scholar] [CrossRef]
- Mekhalif, Z.; Cossement, D.; Hevesi, L.; Delhalle, J. Electropolymerization of pyrrole on silanized polycrystalline titanium substrates. Appl. Surf. Sci. 2008, 254, 4056–4062. [Google Scholar] [CrossRef]
- Jacques, A.; Chehimi, M.M.; Poleunis, C.; Delcorte, A.; Delhalle, J.; Mekhalif, Z. Grafting of 4-pyrrolyphenyldiazonium in situ generated on NiTi, an adhesion promoter for pyrrole electropolymerisation? Electrochim. Acta 2016, 211, 879–890. [Google Scholar] [CrossRef] [Green Version]
- Erakovic, S.; Jankovic, A.; Matic, I.Z.; Juranic, Z.D.; Vukasinovic-Sekulic, M.; Stevanovic, T.; Miskovic-Stankovic, V. Investigation of silver impact on hydroxyapatite/lignin coatings electrodeposited on titanium. Mater. Chem. Phys. 2013, 142, 521–530. [Google Scholar] [CrossRef]
- Sirivisoot, S.; Pareta, P.; Webster, T.J. Electrically controlled drug release from nanostructured polypyrrole coated on titanium. Nanotechnology 2011, 22, 085101. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Gu, J.; Yang, H.; Nie, J.; Ma, G. Electrodeposition of alginate/chitosan layer-by-layer composite coatings on titanium substrates. Carb. Pol. 2014, 103, 38–45. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X.; Mao, H.; Huang, Y.; Ding, Q.; Pang, X. Hydroxyapatite/gelatin functionalized graphene oxide composite coatings deposited on TiO2 nanotube by electrochemical deposition for biomedical applications. Appl. Surf. Sci. 2015, 329, 76–82. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Aguilar, L.E.; Ambade, R.B.; Lee, S.H.; Park, C.H.; Kim, C.S. Immobilization of silver nanoparticles on electropolymerized polydopamine fi lms for metal implant applications. Colloids Interface Sci. Commun. 2015, 6, 5–8. [Google Scholar] [CrossRef]
- Ðošic, M.; Eraković, S.; Janković, A.; Vukašinović-Sekulić, M.; Matić, I.Z.; Stojanović, J.; Rhee, K.Y.; Mišković-Stanković, V.; Park, S.J. In vitro investigation of electrophoretically deposited bioactive hydroxyapatite/chitosan coatings reinforced by graphene. J. Ind. Eng. Chem. 2017, 47, 336–347. [Google Scholar] [CrossRef]
- Simi, V.S.; Satish, A.; Korrapati, P.S.; Rajendran, N. In-vitro biocompatibility and corrosion resistance of electrochemically assembled PPy/TNTA hybrid material for biomedical applications. Appl. Surf. Sci. 2018, 445, 320–334. [Google Scholar] [CrossRef]
- Stevanović, M.; Đošić, M.; Janković, A.; Kojić, V.; Vukašinović-Sekulić, M.; Stojanović, J.; Odović, J.; Sakač, M.C.; Rhee, K.Y.; Mišković-Stanković, V. Gentamicin-Loaded Bioactive Hydroxyapatite/Chitosan Composite Coating Electrodeposited on Titanium. ACS Biomater. Sci. Eng. 2018, 4, 3994–4007. [Google Scholar] [CrossRef]
- Keller, B.A. Investigation of polymer surfaces by time-of-flight secondary mass spectrometry. In Polymer Surface Characterization; Sabbatini, L., Ed.; Walter de Gruyter GmbH: Berlin, Germany; Boston, MA, USA, 2014; Chapter 2; pp. 39–71. ISBN 978-3-11-027508-7. [Google Scholar]
- Kovač, J. Surface characterization of polymers by XPS and SIMS techniques. Mater. Technol. 2011, 45, 191–197. [Google Scholar]
- ErikssonK, C.; Börner, K.; Nygren, H.; Ohlson, K.; Bexell, U.; Billerdahl, N.; Johansson, M. Studies by imaging TOF-SIMS of bone mineralization on porous titanium implants after 1 week in bone. Appl. Surf. Sci. 2006, 252, 6757–6760. [Google Scholar] [CrossRef]
- Metoki, N.; Mandler, D.; Eliaz, N. Effect of Decorating Titanium with Different Self-Assembled Monolayers on the Electrodeposition of Calcium Phosphate. Cryst. Growth Des. 2016, 16, 2756–2764. [Google Scholar] [CrossRef]
- Ilda, K.; Johansson, L.S.; Campbell, J.M.; Inganas, O. XPS and SIMS study: Adhesion of polypyrrole film on titanium. Surf. Interface Anal. 2000, 30, 557–560. [Google Scholar]
- Teo, L.L.; Sin, S.L.; Chan, C.Y. ESCA and TOF-SIMS Study on Oxidised and Reduced Polypyrrole-Poly(Vinylsulfonic Acid, Sodium Salt) Films Synthesized on Ti Electrodes. J. Nanomater. 2010, 385617. [Google Scholar] [CrossRef]
- Pawlik, A.; Rehman, M.A.U.; Nawaz, Q.; Bastan, F.E.; Sulka, G.D.; Boccaccini, A.R. Fabrication and characterization of electrophoretically deposited chitosan-hydroxyapatite composite coatings on anodic titanium dioxide layers. Electrochim. Acta 2019, 307, 465–473. [Google Scholar] [CrossRef]
- Palla-Rubio, B.; Araújo-Gomes, N.; Fernández-Gutiérrez, M.; Rojo, L.; Suay, J.; Gurruchaga, M.; Goñi, I. Synthesis and characterization of silica-chitosan hybrid materials as antibacterial coatings for titanium implants. Carbohydr. Polym. 2019, 203, 331–341. [Google Scholar] [CrossRef]
- Cruz, M.A.E.; Ramos, A.P. Bioactive CaCO3/poly (acrylic acid)/chitosan hybrid coatings deposited on titanium. Surf. Coat. Technol. 2016, 294, 145–152. [Google Scholar] [CrossRef]
- Radda’a, N.S.; Goldmann, W.H.; Detsch, R.; Roether, J.A.; Cordero-Arias, L.; Virtanen, S.; Moskalewicz, T.; Boccaccini, A.R. Electrophoretic deposition of tetracycline hydrochloride loaded halloysite nanotubes chitosan/bioactive glass composite coatings for orthopedic implants. Surf. Coat. Technol. 2017, 327, 146–157. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Zych, A.; Kruk, A.; Kopia, A.; Zimowski, S.; Sitarz, M.; Cieniek, Ł. Electrophoretic deposition and microstructure development of Si3N4/polyetheretherketone coatings on titanium alloy. Surf. Coat. Technol. 2018, 350, 633–647. [Google Scholar] [CrossRef]
- Rehman, M.A.U.; Bastan, F.E.; Haider, B.; Boccaccini, A.R. Electrophoretic deposition of PEEK/bioactive glass composite coatings for orthopedic implants: A design of experiments (DoE) study. Mater. Des. 2017, 130, 223–230. [Google Scholar] [CrossRef]
- Avcu, E.; Avcu, Y.Y.; Baştan, F.E.; Rehman, M.A.U.; Üstel, F.; Boccaccini, A.R. Tailoring the surface characteristics of electrophoretically deposited chitosan-based bioactive glass composite coatings on titanium implants via grit blasting. Prog. Org. Coat. 2018, 123, 362–373. [Google Scholar] [CrossRef]
- Ning, C.; Jiajia, J.; Meng, L.; Hongfei, Q.; Xianglong, W.; Tingli, L. Electrophoretic deposition of GHK-Cu loaded MSN-chitosan coatings with pH-responsive release of copper and its bioactivity. Mater. Sci. Eng. C 2019, 104, 109746. [Google Scholar] [CrossRef]
- Ungureanu, C.; Pirvu, C.; Mindroiu, M.; Demetrescu, I. Antibacterial polymeric coating based on polypyrrole and polyethylene glycol on a new alloy TiAlZr. Prog. Org. Coat. 2012, 75, 349–355. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.-W.; Cai, W.-B. Recent applications of in situ ATR-IR spectroscopy in interfacial electrochemistry. Curr. Opin. Electrochem. 2017, 1, 73–79. [Google Scholar] [CrossRef]
- Bosh, N.; Deggelmann, L.; Blattert, C.; Mozaffari, H.; Müller, C. Synthesis and characterization of Halar® polymer coating deposited on titanium substrate by electrophoretic deposition process. Surf. Coat. Technol. 2018, 347, 369–378. [Google Scholar] [CrossRef]
- Farrokhi-Rad, M.; Fateh, A.; Shahrabi, T. Electrophoretic deposition of vancomycin loaded halloysite nanotubes-chitosan nanocomposite coatings. Surf. Coat. Technol. 2018, 349, 144–156. [Google Scholar] [CrossRef]
- Javadi, A.; Solouk, A.; Nazarpak, M.H.; Bagheri, F. Surface engineering of titanium-based implants using electrospraying and dip coating methods. Mater. Sci. Eng. C 2019, 99, 620–630. [Google Scholar] [CrossRef]
- Sinha, R.K.; Morris, F.; Shah, S.A.; Tuan, R.S. Surface Composition of Orthopaedic Implant Metals Regulates Cell Attachment, Spreading, and Cytoskeletal Organization of Primary Human Osteoblasts In Vitro. Clin. Orthop. Relat. Res. 1994, 305, 258–272. [Google Scholar] [CrossRef]
- Martin, J.Y.; Schwartz, Z.; Hummert, T.W.; Schraub, D.M.; Simpson, J.; Lankford, J.; Dean, D.D., Jr.; Cochran, D.L.; Boyan, B.D. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J. Biomed. Mater. Res. 1995, 29, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, C.T.; Branemark, P.I.; Hansson, H.A.; Lindstrom, J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliaz, N.; Eliyahu, M. Electrochemical processes of nucleation and growth of hydroxyapatite on titanium supported by real-time electrochemical atomic force microscopy. J. Biomed. Mater. Res. Part A 2007, 80A, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, C.; Drob, P.; Vasilescu, E.; Demetrescu, I.; Ionita, D.; Prodana, M.; Drob, S.I. Characterisation and corrosion resistance of the electrodeposited hydroxyapatite and bovine serum albumin/hydroxyapatite films on Ti–6Al–4V–1Zr alloy surface. Corros. Sci. 2011, 53, 992–999. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, K.; Huang, B.; Li, Z.; Zhu, S.; Wang, G. Preparation of an electrodeposited hydroxyapatite coating on titanium substrate suitable for in-vivo applications. J. Mater. Sci. Mater. Med. 2008, 19, 437–442. [Google Scholar] [CrossRef]
- Manara, S.; Paolucci, F.; Palazzo, B.; Marcaccio, M.; Foresti, E.; Tosi, G.; Sabbatini, S.; Sabatino, P.; Altankov, G.; Roveri, N. Electrochemically-assisted deposition of biomimetic hydroxyapatite–collagen coatings on titanium plate. Inorg. Chim. Acta 2008, 361, 1634–1645. [Google Scholar] [CrossRef]
- Pirvu, C.; Mindroiu, M.; Popescu, S.; Demetrescu, I. Electrodeposition of Polypyrrole/Poly(Styrene Sulphonate) Composite Coatings on Ti6Al7Nb Alloy. Mol. Cryst. Liq. Cryst. 2010, 521, 126–139. [Google Scholar] [CrossRef]
- Popescu, S.; Pirvu, C.; Mindroiu, M.; Manole, C.; Demetrescu, I. Electrochemical Synthesis and Characterization of Ti Modified Electrodes with Polypyrrole—Polyethylene Glycol Hybrid Coating. Rev. Chim. Buchar. Orig. Ed. 2010, 61, 245–248. [Google Scholar]
- Popescu, S.; Ungureanu, C.; Albu, A.M.; Pirvu, C. Poly(dopamine) assisted deposition of adherent PPy film on Ti Substrate. Prog. Org. Coat. 2014, 77, 1890–1900. [Google Scholar] [CrossRef]
- Mindroiu, M.; Ion, R.; Pirvu, C.; Cimpean, A. Surfactant-dependent macrophage response to polypyrrole-based coatings electrodeposited on Ti6Al7Nb alloy. Mater. Sci. Eng. C 2013, 33, 3353–3361. [Google Scholar] [CrossRef] [PubMed]
- Ordikhani, F.; Tamjid, E.; Simchi, A. Characterization and antibacterial performance of electrodeposited chitosan– vancomycin composite coatings for prevention of implant-associated infections. Mater. Sci. Eng. C 2014, 41, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Ordikhani, F.; Farani, M.R.; Dehghani, M.; Tamjid, E.; Simchi, A. Physicochemical and biological properties of electrodeposited graphene oxide/chitosan films with drug-eluting capacity. Carbon 2015, 84, 91–102. [Google Scholar] [CrossRef]
- Battistoni, C.; Casaletto, M.P.; Ingo, G.M.; Kaciulis, S.; Mattogno, G.; Pandolfi, L. Surface characterization of biocompatible hydroxyapatite coatings. Surf. Interface Anal. 2000, 29, 773. [Google Scholar] [CrossRef]
- Fang, Y.; Yu, H.; Chen, L.; Chen, S. Facile glycerol-assisted synthesis of N-vinyl pyrrolidinone-based thermosensitive hydrogels via frontal polymerization. Chem. Mater. 2009, 21, 4711–4718. [Google Scholar] [CrossRef]
- Tian, H.; Deng, C.; Lin, H.; Sun, J.; Deng, M.; Chen, X.; Jing, X. Biodegradable cationic PEG–PEI–PBLG hyperbranched block copolymer: Synthesis and micelle characterization. Biomaterials 2005, 26, 4209–4217. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, X.S.; Lu, T.C.; Jing, X.B. The immobilization of proteins on biodegradable polymer fibers via click chemistry. Biomaterials 2008, 29, 1118–1126. [Google Scholar] [CrossRef]
- Qi, H.; Chen, Q.; Ren, H.; Wu, X.; Liu, X.; Lu, T. Electrophoretic deposition of dexamethasone-loaded gelatin nanospheres/chitosan coating and its dual function in anti-inflammation and osteogenesis. Colloids Surf. B Biointerfaces 2018, 169, 249–256. [Google Scholar] [CrossRef]
- Keddie, L.J. Structural analysis of organic interfacial layers by Ellipsometry. Curr. Opin. Colloid Interface Sci. 2001, 6, 102–110. [Google Scholar] [CrossRef]
- Ogieglo, W.; Wormeester, H.; Eichhorn, K.J.; Wessling, M.; Benes, N.E. In situ ellipsometry studies on swelling of thin polymer films: A review. Prog. Polym. Sci. 2015, 42, 42–78. [Google Scholar] [CrossRef]
- Silva-Bermudez, P.; Rodil, S.E. An overview of protein adsorption on metal oxide coatings for biomedical implants. Surf. Coat. Technol. 2013, 233, 147–158. [Google Scholar] [CrossRef]
- Stanfield, J.R.; Bamberg, S. Durability evaluation of biopolymer coating on titanium alloy substrate. J. Mech. Behav. Biomed. Mater. 2014, 35, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Doi, H.; Iwasaki, Y.; Hiromoto, S.; Yoneyama, T.; Asami, K.; Imai, H.; Hanawa, T. Electrodeposition of amine-terminated poly(ethylene glycol) to titanium surface. Mater. Sci. Eng. C 2007, 27, 206–212. [Google Scholar] [CrossRef]
- Tanaka, Y.; Doi, H.; Kobayashi, E.; Yoneyama, T.; Hanawa, T. Determination of the Immobilization Manner of Amine-Terminated Poly(Ethylene Glycol) Electrodeposited on a Titanium Surface with XPS and GD-OES. Mater. Trans. 2007, 48, 287–292. [Google Scholar] [CrossRef]
- Oya, K.; Tanaka, Y.; Saito, H.; Kurashima, K.; Nogi, K.; Tsutsumi, H.; Doi, H.; Nomura, N.; Hanawa, T. Calcification by MC3T3-E1 cells on RGD peptide immobilized on titanium through electrodeposited PEG. Biomaterials 2009, 30, 1281–1286. [Google Scholar] [CrossRef]
- Kawabe, A.; Nakagawa, I.; Kanno, Z.; Tsutsumi, Y.; Hanawa, T.; Ono, T. Evaluation of biofim formation in the presence of saliva on poly(ethylene glycol)-deposited titanium. Dent. Mater. J. 2014, 33, 638–647. [Google Scholar] [CrossRef]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A review on the wettability of dental implant surfaces I: Theoretical and experimental aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Igarashi, T.; Okumori, N.; Igarashi, T.; Maetani, T.; Liu, B.; Yoshinari, M. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed. Mater. 2009, 4, 045002. [Google Scholar] [CrossRef]
- Eriksson, C.; Nygren, H.; Ohlson, K. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: Cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials 2004, 25, 4759–4766. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Valderrama, P.; Jones, A.A.; Wilson, T.G.; Seibl, R.; Cochran, D.L. Bone apposition around two different sandblasted and acid-etched titanium implant surfaces: A histomorphometric study in canine mandibles. Clin. Oral Implants Res. 2008, 19, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.B.; Washburn, N.R.; Simon, C.G., Jr.; Amis, E.J. Combinatorial screen of the effect of surface energy on fibronectin-mediated osteoblast adhesion, spreading and proliferation. Biomaterials 2006, 27, 3817–3824. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Sayer, M.; Kawaja, M.; Shen, X.; Davies, J.E. Attachment, morphology, and protein expression of rat marrow stromal cells cultured on charged substrate surfaces. J. Biomed. Mater. Res. 1998, 42, 117–127. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, N.; Kharaziha, M.; Raeissi, K. Electrophoretic deposition of chitosan reinforced graphene oxide-hydroxyapatite on the anodized titanium to improve biological and electrochemical characteristics. Mater. Sci. Eng. C 2019, 98, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Cometa, S.; Iatta, R.; Ricci, M.A.; Ferretti, C.; De Giglio, E. Analytical characterization and antimicrobial properties of novel copper nanoparticle–loaded electrosynthesized hydrogel coatings. J. Bioact. Compat. Polym. 2013, 28, 508–522. [Google Scholar] [CrossRef]
- Fukuhara, Y.; Kyuzo, M.; Tsutsumi, Y.; Nagai, A.; Chen, P.; Hanawa, T. Phospholipid polymer electrodeposited on titanium inhibits platelet adhesion. J. Biomed. Mater. Res. Part B 2016, 104B, 554–560. [Google Scholar] [CrossRef]
- Buxadera-Palomero, J.; Calvo, C.; Torrent-Camarero, S.; Gil, F.J.; Mas-Moruno, C.; Canal, C.; Rodríguez, D. Biofunctional polyethylene glycol coatings on titanium: An in vitro-based comparison of functionalization methods. Colloids Surf. B Biointerfaces 2017, 152, 367–375. [Google Scholar] [CrossRef]
- Höök, F.; Kasemo, B. The QCM-D Technique for Probing Biomacromolecular Recognition Reactions. In Piezoelectric Sensors. Springer Series on Chemical Sensors and Biosensors (Methods and Applications); Janshoff, A., Steinem, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 425–447. ISBN 3-540-33918-3. [Google Scholar]
- Deakin, M.R.; Buttry, D.A. Electrochemical applications of the quartz crystal microbalance. Anal. Chem. 1989, 61, 1147A–1154A. [Google Scholar] [CrossRef]
- Kasemo, B.; Gold, J. Implant Surfaces and Interface Processes. Adv. Dent. Res. 1999, 13, 8–20. [Google Scholar] [CrossRef]
- Höök, F.; Vörös, J.; Rodahl, M.; Kurrat, R.; Böni, P.; Ramsden, J.J.; Textor, M.; Spencer, N.D.; Tengvall, P.; Gold, J.; et al. A comparative study of protein adsorption on titanium oxide surfaces using in situ ellipsometry, optical waveguide lightmode spectroscopy, and quartz crystal microbalance/dissipation. Colloids Surf. B Biointerfaces 2002, 24, 155–170. [Google Scholar] [CrossRef]
- Monkawa, A.; Ikoma, T.; Yunoki, S.; Yoshioka, T.; Tanaka, J.; Chakarov, D.; Kasemo, B. Fabrication of hydroxyapatite ultra-thin layer on gold surface and its application for quartz crystal microbalance technique. Biomaterials 2006, 27, 5748–5754. [Google Scholar] [CrossRef] [PubMed]
- Lassalle, N.; Roget, A.; Livache, T.; Mailley, P.; Vieil, E. Electropolymerisable pyrrole-oligonucleotide: Synthesis and analysis of ODN hybridisation by fluorescence and QCM. Talanta 2001, 55, 993–1004. [Google Scholar] [CrossRef]
- De Giglio, E.; Cafagna, D.; Giangregorio, M.M.; Domingos, M.; Mattioli-Belmonte, M.; Cometa, S. PHEMA-based thin hydrogel films for biomedical applications. J. Bioact. Compat. Polym. 2011, 26, 420–434. [Google Scholar] [CrossRef]
- Sumitomo, N.; Noritake, K.; Hattori, T.; Morikawa, K.; Niwa, S.; Sato, K.; Niinomi, N. Experiment study on fracture fixation with low rigidity titanium alloy: Plate fixation of tibia fracture model in rabbit. J. Mater. Sci. Mater. Med. 2008, 19, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Noyama, Y.; MiuraIshimoto, T.; Itaya, T.; Niinomi, M.; Nakano, T. Bone loss and reduced bone quality of the human femur after total hip arthroplasty under stress-shielding effects by titanium-based implant. Mater. Trans. 2012, 53, 565–570. [Google Scholar] [CrossRef] [Green Version]
- Evans, S.L.; Gregson, P.J. Composite technology in load-bearing orthopaedic implants. Biomaterials 1998, 19, 1329–1342. [Google Scholar] [CrossRef]
- Bougherara, H.; Bureau, M.; Campbell, M.; Vadean, A.; Yahia, L.H. Design of a biomimetic polymer-composite hip prosthesis. J. Biomed. Mater. Res. A 2007, 82, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Anguiano-Sanchez, J.; Martinez-Romero, O.; Siller, H.R.; Diaz-Elizondo, J.A.; Flores Villalba, E.; Rodriguez, C.A. Influence of PEEK coating on hip implant stress shielding: A finite element analysis. Comput. Math. Methods Med. 2016, 2016, 6183679. [Google Scholar] [CrossRef] [Green Version]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Kruk, A.; Zimowski, S.; Lukaszczyk, A.; Cieniek, L.; Moskalewicz, T. The influence of heat treatment on the microstructure, surface topography and selected properties of PEEK coatings electrophoretically deposited on the Ti-6Al-4V alloy. Prog. Org. Coat. 2019, 133, 180–190. [Google Scholar] [CrossRef]
- Novitskaya, E.; Chen, P.Y.; Hamed, E.; Jun, L.; Lubarda, V.A.; Jasiuk, I.; McKittrick, J. Recent advances on the measurement and calculation of the elastic moduli of cortical and trabecular bone: A review. Theor. Appl. Mech. 2011, 38, 209–297. [Google Scholar] [CrossRef]
- Halar® ECTFE Electrostatic Powder Coating Processing Guide. Available online: https://www.solvay.com/en/brands/halar-ectfe/literature (accessed on 11 November 2019).
- Sak, A.; Moskalewicz, T.; Zimowski, S.; Cieniek, L.; Dubiel, B.; Radziszewska, A.; Kot, M.; Lukaszczyk, A. Influence of polyetheretherketone coatings on the Ti-13Nb-13Zr titanium alloy’s bio-tribological properties and corrosion resistance. Mater. Sci. Eng. C 2016, 63, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Farokhi, M.; Mottaghitalab, F.; Samani, S.; Shokrgozar, M.A.; Kundu, S.C.; Reis, R.L.; Fatahi, Y.; Kaplan, D. Silk fibroin/hydroxyapatite composites for bone tissue engineering. Biotechnol. Adv. 2018, 36, 68–91. [Google Scholar] [CrossRef]
- Norowski, P.A.; Courtney, H.S.; Babu, J.; Haggard, W.O.; Bumgardner, J.D. Chitosan coatings deliver antimicrobials from titanium implants: A preliminary study. Implant Dent. 2011, 20, 56–67. [Google Scholar] [CrossRef]
- Guo, Y.; Guan, J.; Peng, H.; Shu, X.; Chen, L.; Guo, H. Tightly adhered silk fibroin coatings on Ti6Al4V biometals for improved wettability and compatible mechanical properties. Mater. Des. 2019, 175, 107825. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Vanderleyden, E.; Boterberg, V.; Dubruel, P. Gelatin functionalization of biomaterial surfaces: Strategies for immobilization and visualization. Polymers 2011, 3, 114–130. [Google Scholar] [CrossRef]
- Murugan, N.; Murugan, C.; Sundramoorthy, A.K. In vitro and in vivo characterization of mineralized hydroxyapatite/polycaprolactone-graphene oxide based bioactive multifunctional coating on Ti alloy for bone implant applications. Arab. J. Chem. 2018, 11, 959–969. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Cheng, C.M. Relationships between initial unloading slope, contact depth, and mechanical properties for conical indentation in linear viscoelastic solids. J. Mater. Res. 2005, 20, 1046–1053. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.M.; Li, M.; Zhang, T.; Cheng, Y.T.; Cheng, C.M. Influence of indenter tip roundness on hardness behavior in nanoindentation. Mater. Sci. Eng. A 2007, 445, 323–327. [Google Scholar] [CrossRef]
- Williams, J.G.; Hadavinia, H.; Cotterell, B. Anisotropic elastic and elastic–plastic bending solutions for edge constrained beams. Int. J. Solids Struct. 2005, 42, 4927–4946. [Google Scholar] [CrossRef]
- Ebnesajjad, S.; Ebnesajjad, C. Surface Treatment of Materials for Adhesive Bonding; William Andrew: Den Haag, The Netherlands, 2013. [Google Scholar]
- Rosentritt, M.; Siavikis, G.; Behr, M.; Kolbeck, C.; Handel, G. Approach for valuating the significance of laboratory simulation. J. Dent. 2008, 36, 1048–1053. [Google Scholar] [CrossRef]
- Zahran, M.; El-Mowafy, O.; Tam, L.; Watson, P.A.; Finer, Y. Fracture strength and fatigue resistance of all-ceramic molar crowns manufactured with CAD/CAM technology. J. Prosthodont. 2008, 17, 370–377. [Google Scholar] [CrossRef]









| Technique | Measured Parameter/s | Sampling Depth/Height | Information Obtainable | Limitations | References |
|---|---|---|---|---|---|
| XPS | Binding energy of electrons | 5–10 nm | Elemental composition (qualitative and quantitative), chemical bonds, or oxidation states | Extra-dry state, need for ultra-high vacuum, sensitivity to contamination, lack of hydrogen and helium detection | [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44] |
| TOF-SIMS | Mass/charge | 1–2 nm | Type of atoms, molecules, and pendant groups on the surface | Dry state, need for vacuum, sensitivity to contamination, difficult quantification | [20,21,46,47,48,49,50] |
| ATR-FTIR | Transmittance | 500 nm–2 µm | Organic (and some inorganic) material identification, both in liquid and solid state | Need for maximal optical contact between the sample and the IRE (flat surfaces) | [31,37,38,39,41,43,51,52,53,54,55,56,57,58,59,60] |
| AFM | Force between the probe and the sample | Atomic–few µm | Topography, coverage | Artefacts, contamination. | [41,59,67,68,69,70,71,72,73,74,75,76] |
| SEM–EDX | Interaction of electron beam with atoms | 0.2–2 µm | Detailed high-resolution images, with elemental identification and quantitative compositional information of the analyzed spots | Artifacts due to sample preparation, limited to solid and small samples, need for vacuum | [32,34,37,39,40,41,43,53,63,72,81] |
| Ellipsometry | Change in polarization of incident radiation interacting with sample | 300 nm | Coating thickness, absorption kinetics | Highly model-dependent, need of refractive indices of all layers, assumption of homogenous surfaces | [84,85,86,87,88,89] |
| CA | Angle between liquid and surface of solid sample | <1 nm | Surface free energy, wettability | Contamination | [31,38,43,54,57,59,63,71,73,74,81,97,98,99,100] |
| QCM-D | Change in resonance frequency (Δf) and energy dissipation factor (D) | Not applicable | Real-time, nanoscale analysis of surface phenomena (thin film formation, interactions, and reactions) | Simplifying assumptions in use of Sauerbray or other models, difficulty in interpretation | [23,25,106,107] |
| Nano- and micro- indentation | Hardness (H) and effective elastic modulus (Eeff) | a/R*< 0.3 | Surface hardness and elastic modulus | Conventional calculation of elastic modulus is limited to linear and isotropic materials | [34,61,70,116,117,122] |
| Peel (or adhesion) test | Adhesion strength | Depends on the peel angle (90° or 180°) | Adhesion strength between the coating and the substrate | Reliable only for tough, flexible coatings | [36,43,61,122] |
| Three-point bend fatigue test | Maximum stress | L > 4 W, W > 2 B # | Fatigue strength versus cycles’ number | Sensitive to specimen, loading geometry, and strain rate | [61] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cometa, S.; Bonifacio, M.A.; Ferreira, A.M.; Gentile, P.; De Giglio, E. Surface Characterization of Electro-Assisted Titanium Implants: A Multi-Technique Approach. Materials 2020, 13, 705. https://doi.org/10.3390/ma13030705
Cometa S, Bonifacio MA, Ferreira AM, Gentile P, De Giglio E. Surface Characterization of Electro-Assisted Titanium Implants: A Multi-Technique Approach. Materials. 2020; 13(3):705. https://doi.org/10.3390/ma13030705
Chicago/Turabian StyleCometa, Stefania, Maria A. Bonifacio, Ana M. Ferreira, Piergiorgio Gentile, and Elvira De Giglio. 2020. "Surface Characterization of Electro-Assisted Titanium Implants: A Multi-Technique Approach" Materials 13, no. 3: 705. https://doi.org/10.3390/ma13030705