Fluoride Modification of Titanium Surfaces Enhance Complement Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Titanium Coin Production
2.2. Ethics
2.3. Buffy Coat Model
2.4. Levels of Biomarkers in Buffy Coat
2.5. Levels of C3a and C5
2.6. Statistical Analysis
3. Results
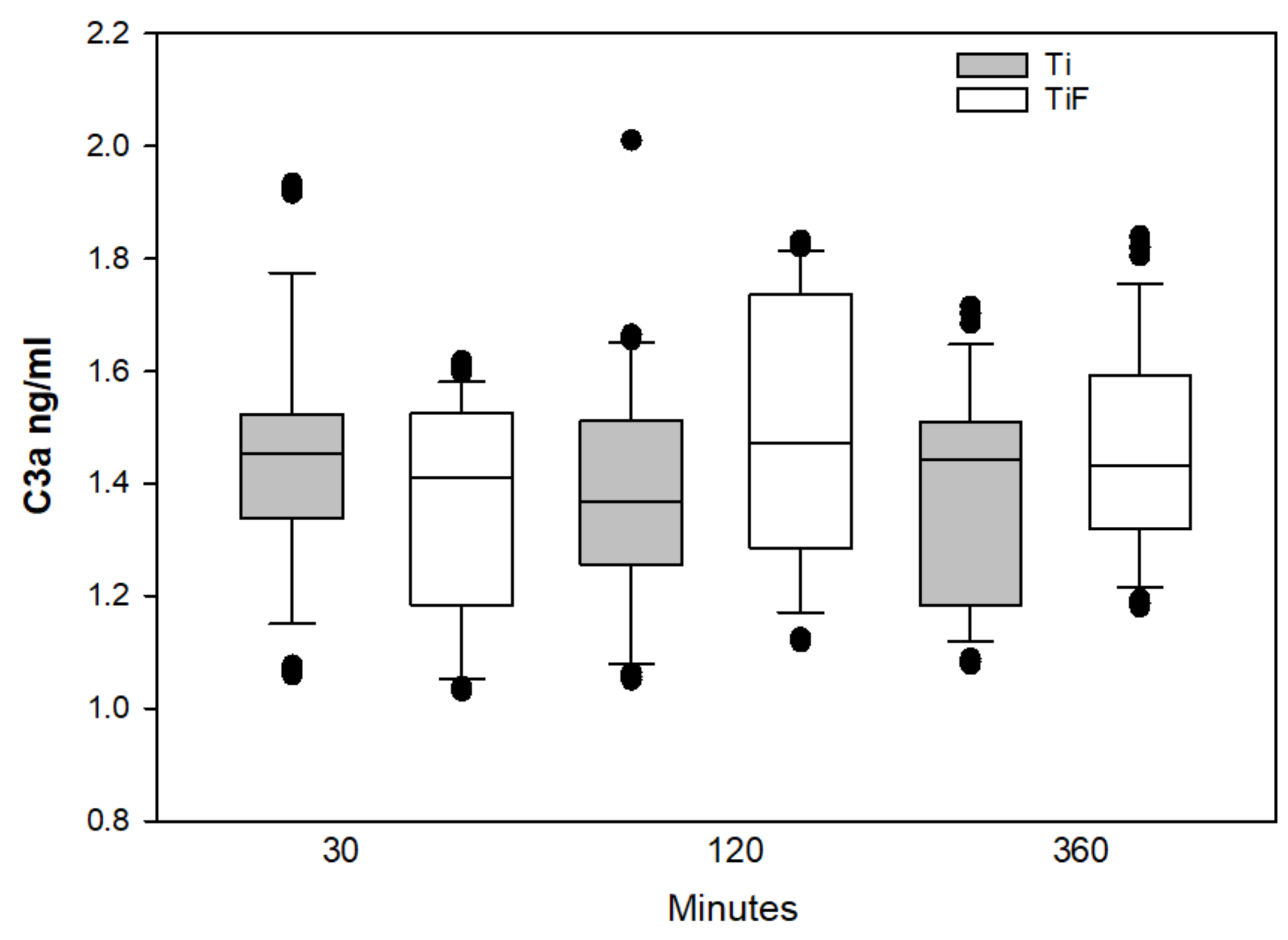
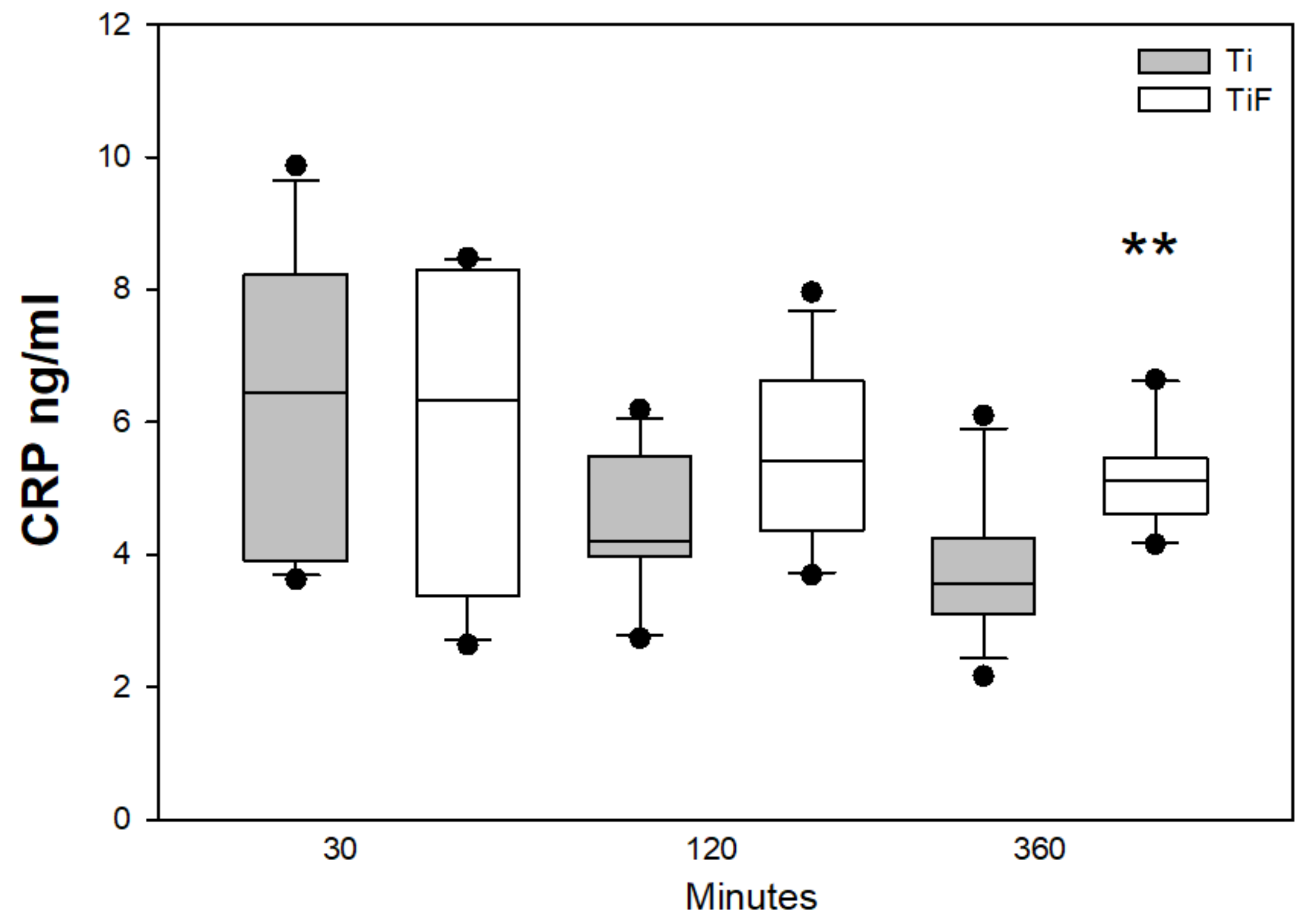
3.1. Complement Biomarkers
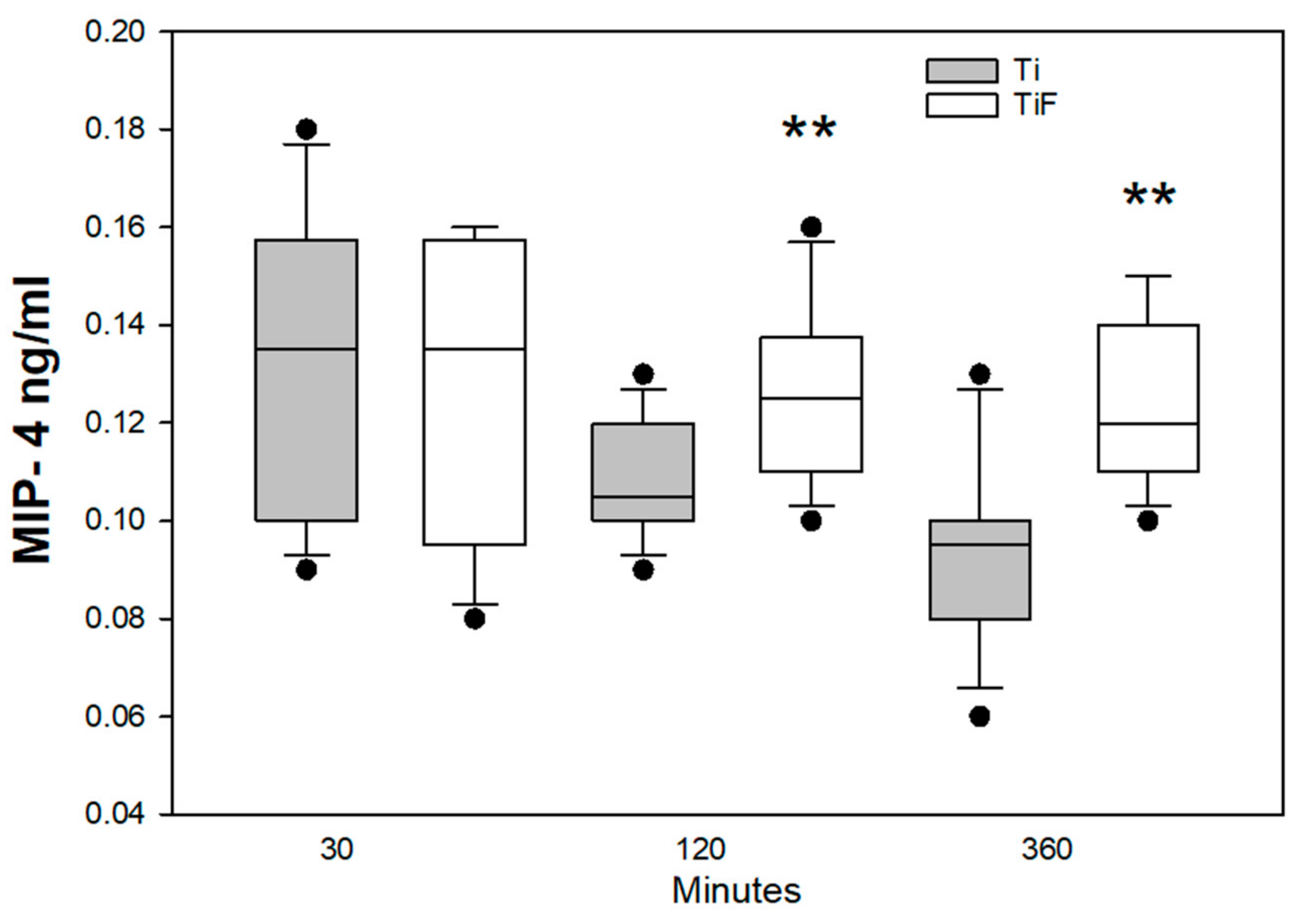
3.2. Angiogenetic Markers
3.3. Remodeling Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- MarketReport. iData Research, Europe Market Overview for Dental Bone Graft Substitutes and Other Biomaterials 2017—Research and Markets iData Research: 2017. Available online: https://idataresearch.com/product/dental-bone-graft-substitutes-market-analysis-europe-2017-2023-medcore/ (accessed on 15 January 2019).
- MarketReport. Millennium Research Group Inc., Dental Biomaterials Europe Market Analysis; Millennium Research Group Inc.: Toronto, ON, Canada, 2015; Available online: https://decisionresourcesgroup.com/report/572851-medtech-dental-biomaterials-medtech-360-market/ (accessed on 20 January 2019).
- Dental Implants Market Size, Share & Trends Analysis Report By Product (Titanium Implants, Zirconium Implants), By Region (North America, Europe, Asia Pacific, Latin America, MEA), And Segment Forecasts, 2018–2024. Report GVR-1-68038-566-3]. San Francisco: Grand View Research: 2018. Available online: https://www.grandviewresearch.com/industry-analysis/dental-implants-market (accessed on 22 January 2019).
- Berglundh, T.; Abrahamsson, I.; Albouy, J.-P.; Lindhe, J. Bone healing at implants with a fluoride-modified surface: An experimental study in dogs. Clin. Oral Implant. Res. 2007, 18, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.F.; Reside, G.; Stanford, C.; Barwacz, C.; Feine, J.; Nader, S.A.; Scheyer, E.T.; McGuire, M. A multicenter randomized comparative trial of implants with different abutment interfaces to replace anterior maxillary single teeth. Int. J. Oral Maxillofac. Implant. 2015, 30, 622–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansson, S.; Halldin, A. Alveolar ridge resorption after tooth extraction: A consequence of a fundamental principle of bone physiology. J. Dent. Biomech. 2012, 3, 1758736012456543. [Google Scholar] [CrossRef] [PubMed]
- Mertens, C.; Steveling, H.G. Early and immediate loading of titanium implants with fluoride-modified surfaces: Results of 5-year prospective study. Clin. Oral Implant. Res. 2011, 22, 1354–1360. [Google Scholar] [CrossRef]
- Schliephake, H.; Rodiger, M.; Phillips, K.; McGlumphy, E.A.; Chacon, G.E.; Larsen, P. Early loading of surface modified implants in the posterior mandible - 5 year results of an open prospective non-controlled study. J. Clin. Periodontol. 2012, 39, 188–195. [Google Scholar] [CrossRef]
- Masaki, C.; Schneider, G.B.; Zaharias, R.; Seabold, D.; Stanford, C. Effects of implant surface microtopography on osteoblast gene expression. Clin. Oral Implant. Res. 2005, 16, 650–656. [Google Scholar] [CrossRef]
- Ellingsen, J.E.; Johansson, C.B.; Wennerberg, A.; Holmen, A. Improved retention and bone-tolmplant contact with fluoride-modified titanium implants. Int. J. Oral Maxillofac. Implant. 2004, 19, 659–666. [Google Scholar]
- Cooper, L.F.; Zhou, Y.; Takebe, J.; Guo, J.; Abron, A.; Holmen, A.; Ellingsen, J.E. Fluoride modification effects on osteoblast behavior and bone formation at TiO2 grit-blasted c.p. titanium endosseous implants. Biomaterials 2006, 27, 926–936. [Google Scholar] [CrossRef]
- Noelken, R.; Oberhansl, F.; Kunkel, M.; Wagner, W. Maintenance of marginal hard and soft tissue support at immediately inserted and provisionalized osseospeed profile implants-3-year results. Clin. Oral Implant. Res. 2014, 25, 683. [Google Scholar] [CrossRef]
- Schiegnitz, E. Evaluation of the survival and soft tissue maintenance of an implant with a sloped configuration in the posterior mandible-2 years results of a prospective multi-center study. Clin. Oral Implant. Res. 2015, 26, 367. [Google Scholar]
- Pham, M.H.; Landin, M.A.; Tiainen, H.; Reseland, J.E.; Ellingsen, J.E.; Haugen, H.J. The effect of hydrofluoric acid treatment of titanium and titanium dioxide surface on primary human osteoblasts. Clin. Oral Implant. Res. 2014, 25, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.H.; Haugen, H.J.; Rinna, A.; Ellingsen, J.E.; Reseland, J.E. Hydrofluoric acid treatment of titanium surfaces enhances the proliferation of human gingival fibroblasts. J. Tissue Eng. 2019, 10, 2041731419828950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thor, A.; Rasmusson, L.; Wennerberg, A.; Thomsen, P.; Hirsch, J.M.; Nilsson, B.; Hong, J. The role of whole blood in thrombin generation in contact with various titanium surfaces. Biomaterials 2007, 28, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Castillo, A.B.; Turner, C.H. Biomechanical and molecular regulation of bone remodeling. Annu. Rev. Biomed. Eng. 2006, 8, 455–498. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Gruber, R.; Bosshardt, D.D. Soft tissue wound healing around teeth and dental implants. J. Clin. Periodontol. 2014, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geurs, N.C.; Vassilopoulos, P.J.; Reddy, M.S. Soft Tissue Considerations in Implant Site Development. Oral Maxillofac. Surg. Clin. North Am. 2010, 22, 387–405. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Mastellos, D.; Lambris, J.D. Complement: More than a ‘guard’ against invading pathogens? Trends Immunol. 2002, 23, 485–491. [Google Scholar] [CrossRef]
- Dhurat, R.; Sukesh, M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J. Cutan. Aesthetic Surg. 2014, 7, 189–197. [Google Scholar] [CrossRef]
- Rønold, H.J.; Ellingsen, J.E. Effect of micro-roughness produced by TiO2 blasting--tensile testing of bone attachment by using coin-shaped implants. Biomaterials 2002, 23, 4211–4219. [Google Scholar] [CrossRef]
- Lamolle, S.F.; Monjo, M.; Rubert, M.; Haugen, H.J.; Lyngstadaas, S.P.; Ellingsen, J.E. The effect of hydrofluoric acid treatment of titanium surface on nanostructural and chemical changes and the growth of MC3T3-E1 cells. Biomaterials 2009, 30, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.M.H.; Rinna, A.; Haugen, H.J.; Ellingsen, J.E.; Reseland, J.E. Enhanced In Vitro HGF Proliferation and Differentiation on Surface Modified Hydrofluoric Acid treated TiO2. In Proceedings of the 37th Congress of Scandinavian Association of Oral and Maxillofacial Surgeons (SFOMK), Copenhagen, Denmark, 3–6 June 2015. [Google Scholar]
- Rokstad, A.M.; Brekke, O.-L.; Steinkjer, B.; Ryan, L.; Kolláriková, G.; Strand, B.L.; Skjåk-Bræk, G.; Lacík, I.; Espevik, T.; Mollnes, T.E. Alginate microbeads are complement compatible, in contrast to polycation containing microcapsules, as revealed in a human whole blood model. Acta Biomater. 2011, 7, 2566–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjoberg, A.P.; Manderson, G.A.; Morgelin, M.; Day, A.J.; Heinegard, D.; Blom, A.M. Short leucine-rich glycoproteins of the extracellular matrix display diverse patterns of complement interaction and activation. Mol. Immunol. 2009, 46, 830–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.; Andersson, J.; Ekdahl, K.N.; Elgue, G.; Axen, N.; Larsson, R.; Nilsson, B. Titanium is a highly thrombogenic biomaterial: Possible implications for osteogenesis. Thromb. Haemost. 1999, 82, 58–64. [Google Scholar] [CrossRef]
- Hong, J.; Azens, A.; Ekdahl, K.N.; Granqvist, C.G.; Nilsson, B. Material-specific thrombin generation following contact between metal surfaces and whole blood. Biomaterials 2005, 26, 1397–1403. [Google Scholar] [CrossRef]
- Ekdahl, K.N.; Lambris, J.D.; Elwing, H.; Ricklin, D.; Nilsson, P.H.; Teramura, Y.; Nicholls, I.A.; Nilsson, B. Innate immunity activation on biomaterial surfaces: A mechanistic model and coping strategies. Adv. Drug Deliv. Rev. 2011, 63, 1042–1050. [Google Scholar] [CrossRef] [Green Version]
- Sellborn, A.; Andersson, M.; Fant, C.; Gretzer, C.; Elwing, H. Methods for research on immune complement activation on modified sensor surfaces. Colloids Surf. B Biointerfaces 2003, 27, 295–301. [Google Scholar] [CrossRef]
- Lim, Y.J.; Oshida, Y. Initial contact angle measurements on variously treated dental/medical titanium materials. Bio Med. Mater. Eng. 2001, 11, 325–341. [Google Scholar]
- Ma, Q.-L.; Zhao, L.-Z.; Liu, R.-R.; Jin, B.-Q.; Song, W.; Wang, Y.; Zhang, Y.-S.; Chen, L.-H.; Zhang, Y.-M. Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization. Biomaterials 2014, 35, 9853–9867. [Google Scholar] [CrossRef]
- Park, J.Y.; Gemmell, C.H.; E Davies, J. Platelet interactions with titanium: Modulation of platelet activity by surface topography. Biomaterials 2001, 22, 2671–2682. [Google Scholar] [CrossRef]
- Soskolne, W.A.; Cohen, S.; Shapira, L.; Sennerby, L.; Wennerberg, A. The effect of titanium surface roughness on the adhesion of monocytes and their secretion of TNF-alpha and PGE2. Clin. Oral Implant. Res. 2002, 13, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Tunalı, M.; Özdemir, H.; Küçükodacı, Z.; Akman, S.; Yaprak, E.; Toker, H.; Fıratlı, E. A Novel Platelet Concentrate: Titanium-Prepared Platelet-Rich Fibrin. BioMed Res. Int. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wiegner, R.; Chakraborty, S.; Huber-Lang, M. Complement-coagulation crosstalk on cellular and artificial surfaces. Immunobiology 2016, 221, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Elwing, H. Complement activation on solid surfaces as determined by C3 deposition and hemolytic consumption. J. Biomed. Mater. Res. 1994, 28, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part I – Molecular Mechanisms of Activation and Regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, B.; Ekdahl, K.N.; Mollnes, T.E.; Lambris, J.D. The role of complement in biomaterial-induced inflammation. Mol. Immunol. 2007, 44, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Hilborn, J.; Bjursten, L.M. A new and evolving paradigm for biocompatibility. J. Tissue Eng. Regen. Med. 2007, 1, 110–119. [Google Scholar] [CrossRef]
- Jung, S.; Kleinheinz, J. Influence of Angiogenesis on Osteogenesis. In Advances in Regenerative Medicine; InTech: London, UK, 2011. [Google Scholar]
- Ekdahl, K.N.; Huang, S.; Nilsson, B.; Teramura, Y. Complement inhibition in biomaterial- and biosurface-induced thromboinflammation. Semin. Immunol. 2016, 28, 268–277. [Google Scholar] [CrossRef]
- Osmand, A.; Mortensen, R.; Siegel, J.; Gewurz, H. Interactions of C-reactive protein with the complement system. III. Complement-dependent passive hemolysis initiated by CRP. J. Exp. Med. 1975, 142, 1065–1077. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, M.H.; E Volanakis, J. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J. Immunol. 1974, 112, 2135–2147. [Google Scholar]
- Atkinson, J.P.; Du Clos, T.W.; Mold, C.; Kulkarni, H.; Hourcade, D.; Wu, X. The Human Complement System: Basic Concepts and Clinical Relevance. In Clinical Immunology, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 299–317. [Google Scholar]
- Löbler, M.; Saß, M.; Kunze, C.; Schmitz, K.P.; Hopt, U.T. Biomaterial implants induce the inflammation marker CRP at the site of implantation. J. Biomed. Mater. Res. 2002, 61, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Goel, S. Infection following implant surgery. Indian J. Orthop. 2006, 40, 133. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Shirai, T.; Nishida, H.; Murakami, H.; Kabata, T.; Yamamoto, N.; Watanabe, K.; Nakase, J. Innovative antimicrobial coating of titanium implants with iodine. J. Orthop. Sci. 2012, 17, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Penton-Arias, E.; Haines, D.D. Natural Products: Immuno-Rebalancing Therapeutic Approaches. In Immune Rebalancing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 229–249. [Google Scholar]
- Xia, Z.; Triffitt, J.T. A review on macrophage responses to biomaterials. Biomed. Mater. 2006, 1, R1–R9. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, A.; Khaldoyanidi, S.K.; Judex, M.; Serobyan, N.; Discipio, R.G.; Schraufstatter, I.U. CCL18/PARC stimulates hematopoiesis in long-term bone marrow cultures indirectly through its effect on monocytes. Blood 2006, 108, 3722–3729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rokstad, A.M.; Brekke, O.-L.; Steinkjer, B.; Ryan, L.; Kolláriková, G.; Strand, B.L.; Skjåk-Bræk, G.; Lambris, J.D.; Lacík, I.; Mollnes, T.E.; et al. The induction of cytokines by polycation containing microspheres by a complement dependent mechanism. Biomaterials 2013, 34, 621–630. [Google Scholar] [CrossRef]
- Monsinjon, T.; Gasque, P.; Chan, P.; Ischenko, A.; Brady, J.J.; Fontaine, M.C. Regulation by complement C3a and C5a anaphylatoxins of cytokine production in human umbilical vein endothelial cells. FASEB J. 2003, 17, 1003–1014. [Google Scholar] [CrossRef]
- Kolev, M.; Le Friec, G.; Kemper, C. Complement—Tapping into new sites and effector systems. Nat. Rev. Immunol. 2014, 14, 811–820. [Google Scholar] [CrossRef]
- Lovell, D.P. Biological Importance and Statistical Significance. J. Agric. Food Chem. 2013, 61, 8340–8348. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, M.H.; Haugen, H.J.; Reseland, J.E. Fluoride Modification of Titanium Surfaces Enhance Complement Activation. Materials 2020, 13, 684. https://doi.org/10.3390/ma13030684
Pham MH, Haugen HJ, Reseland JE. Fluoride Modification of Titanium Surfaces Enhance Complement Activation. Materials. 2020; 13(3):684. https://doi.org/10.3390/ma13030684
Chicago/Turabian StylePham, Maria H., Håvard J. Haugen, and Janne E. Reseland. 2020. "Fluoride Modification of Titanium Surfaces Enhance Complement Activation" Materials 13, no. 3: 684. https://doi.org/10.3390/ma13030684





