Structure and Properties of ZnO Coatings Obtained by Atomic Layer Deposition (ALD) Method on a Cr-Ni-Mo Steel Substrate Type
Abstract
:1. Introduction
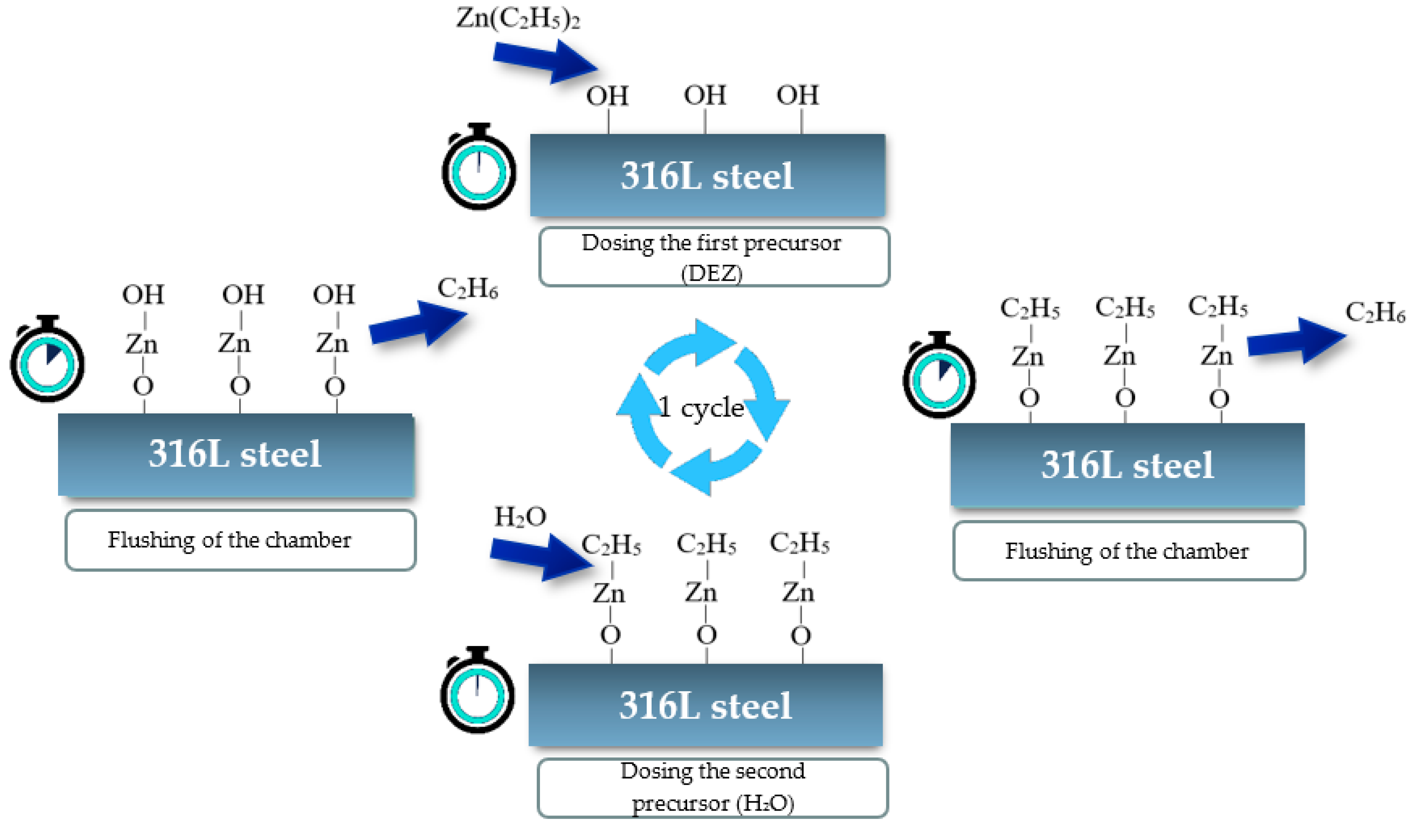
2. Materials and Methods
3. Results and Discussion
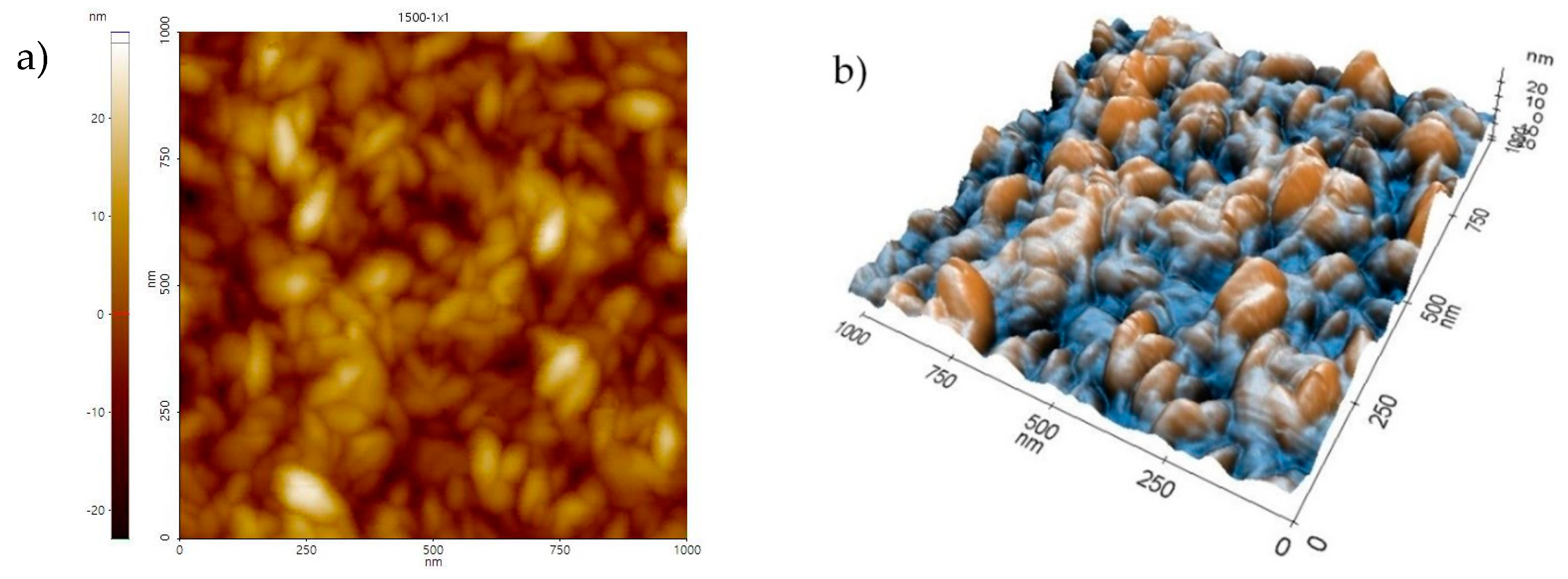
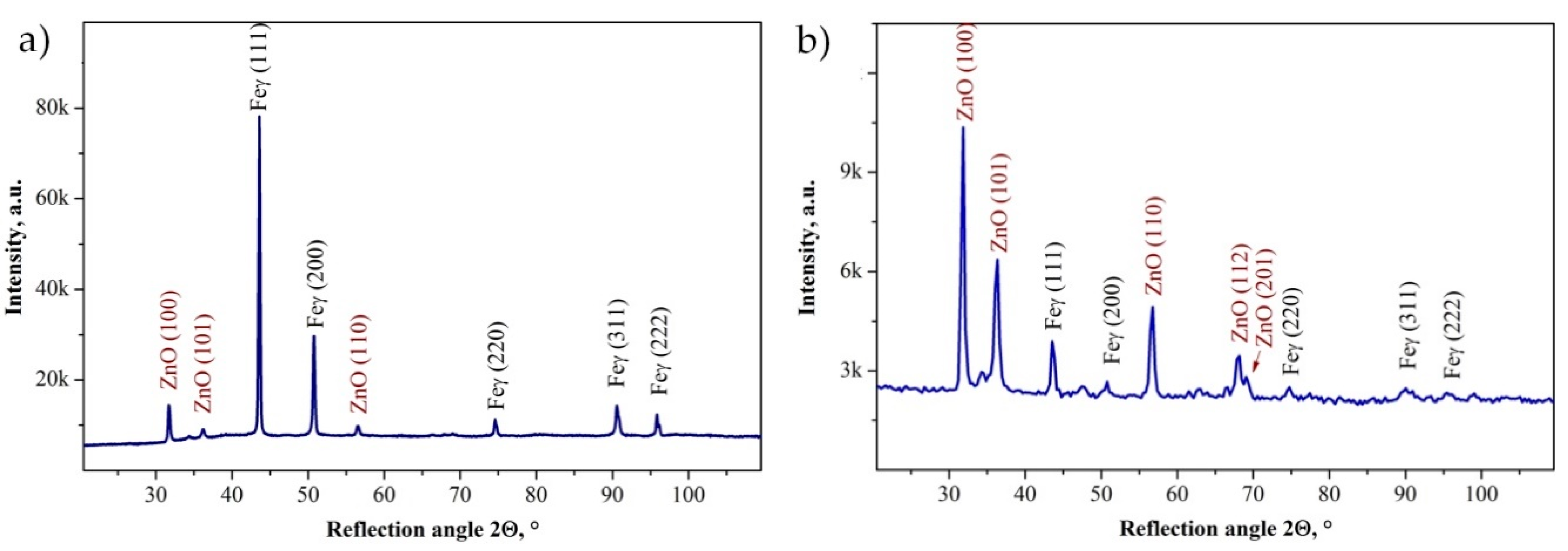
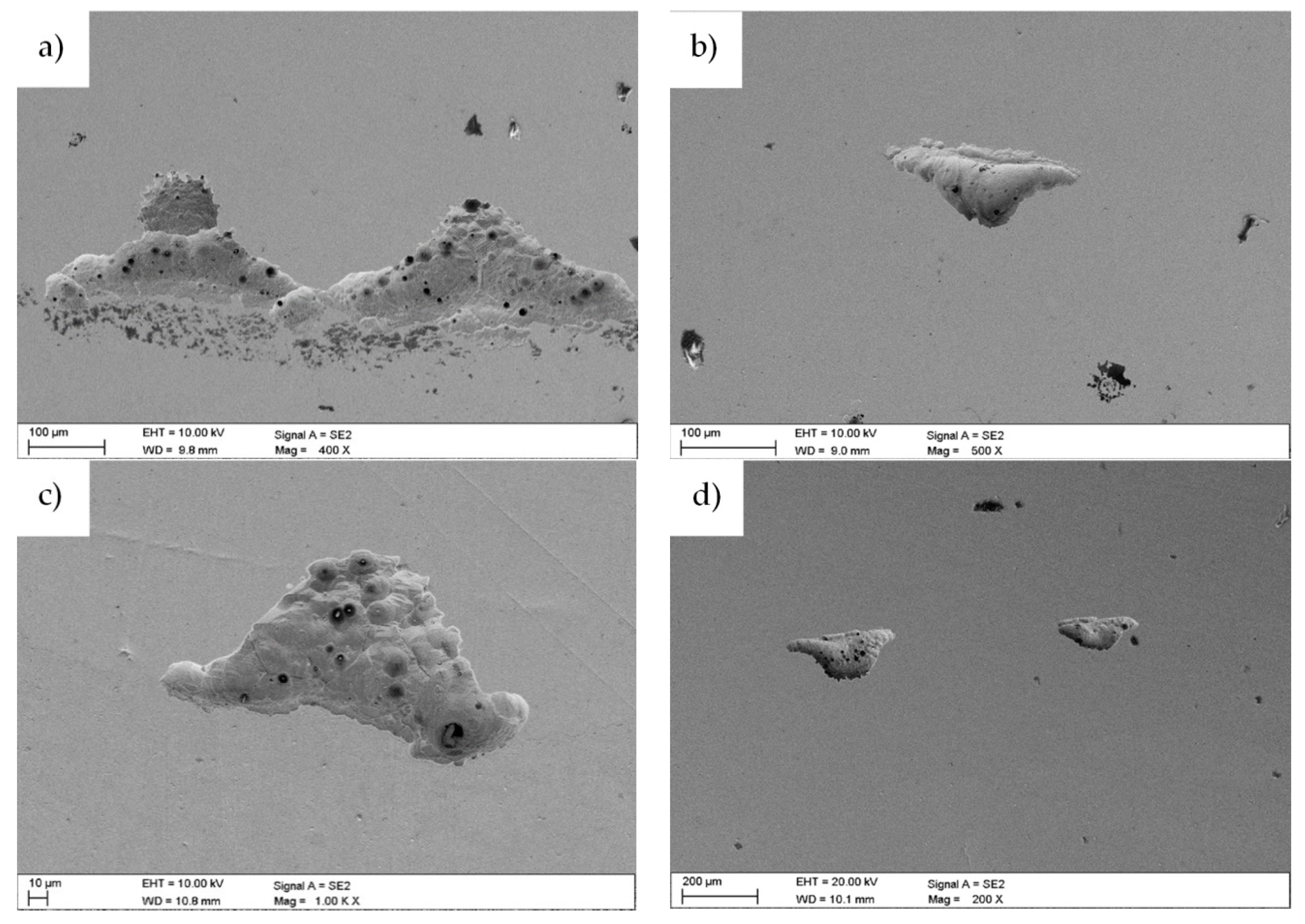
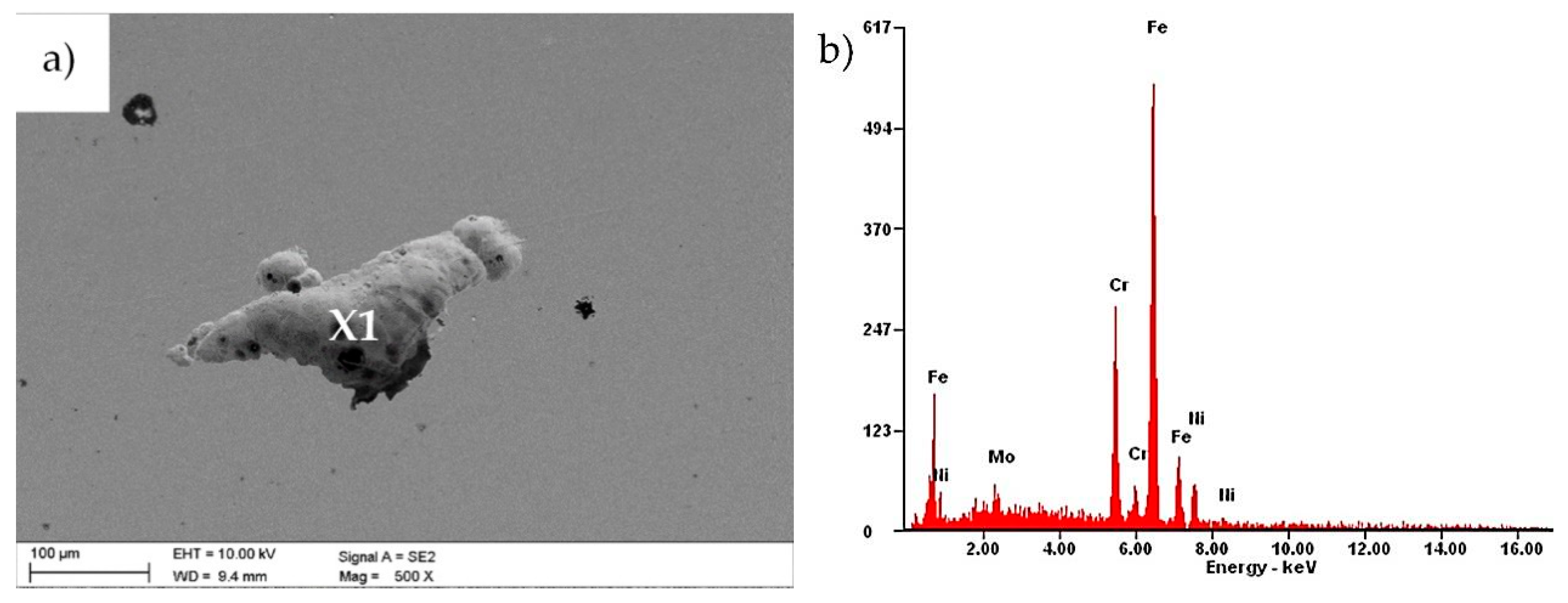
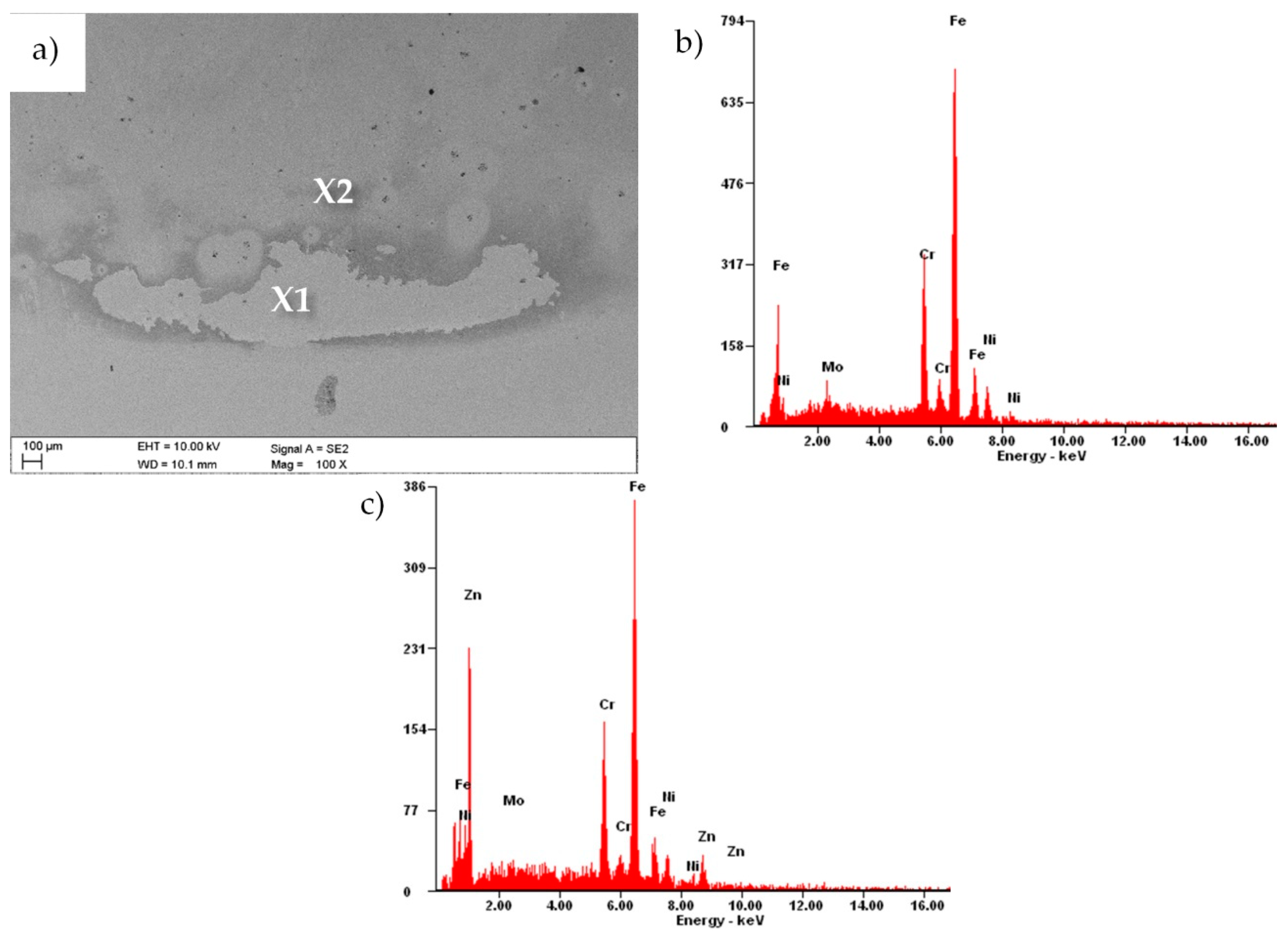
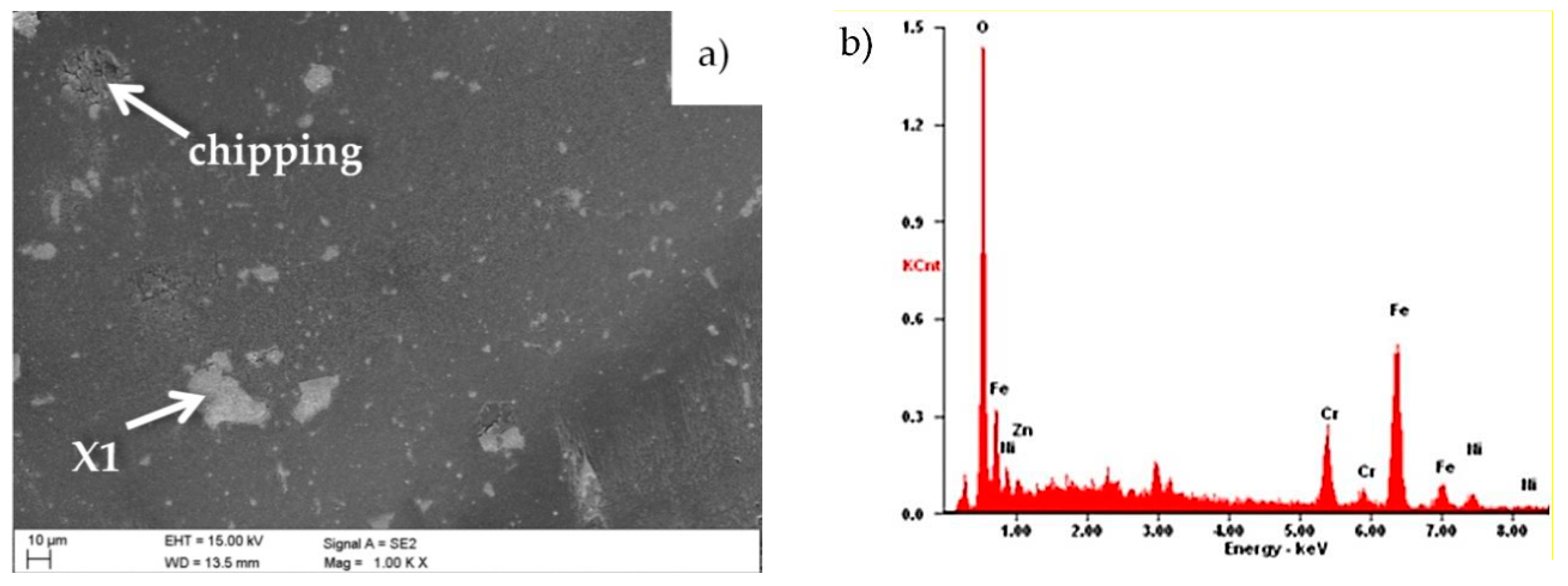
3.1. Morphology and Structure
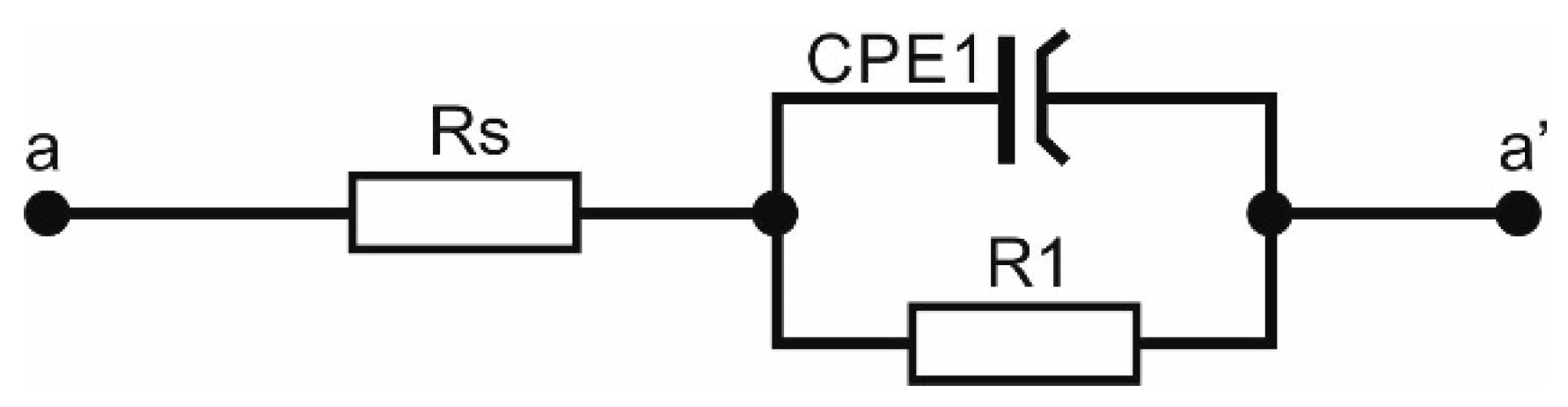
3.2. Electrochemical Properties
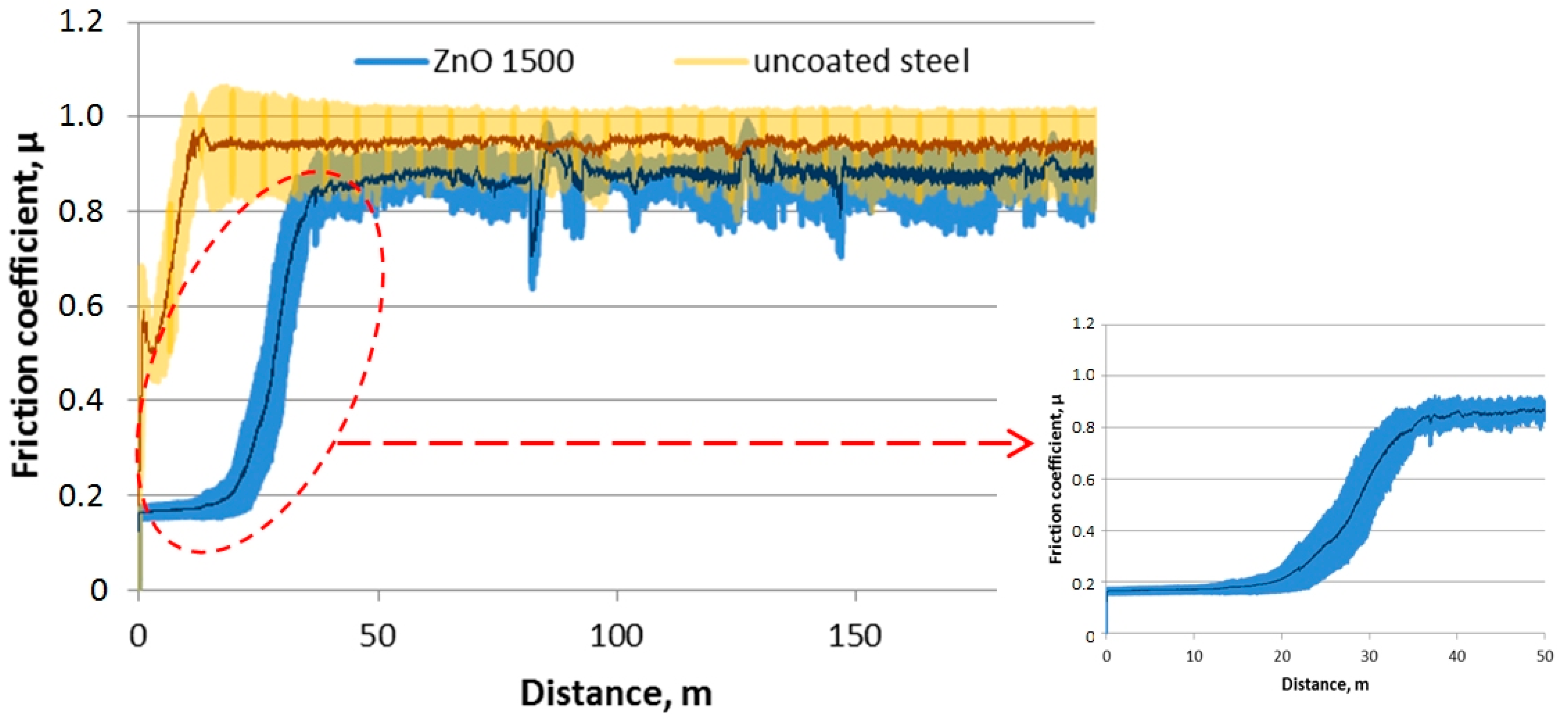
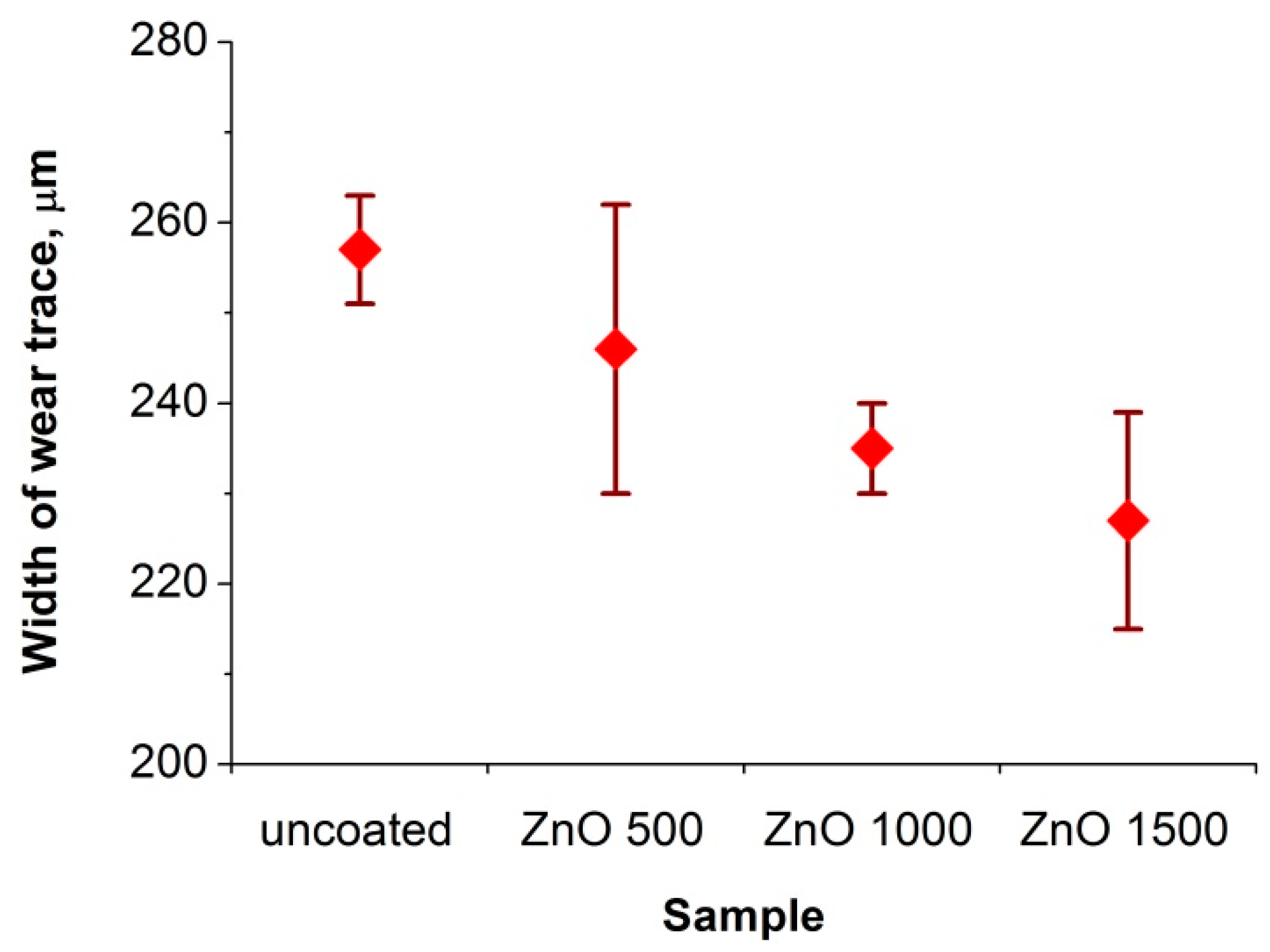
3.3. Tribologycal Properties
4. Summary
5. Conclusions
- The ZnO layer applied to the steel substrate improved the resistance to corrosion damage of the tested material, which was indicated by a decrease in the value of the corrosion current by one order of magnitude in relation to the uncoated sample and by an over 10-fold increase in the material resistance in the case of 1500 cycles. The increasing number of cycles by which the ZnO layer was obtained was conducive to obtaining better values for the electrochemical parameters of the investigated material, as evidenced by the increasing values of the corrosion potential and polarization resistance.
- The performed impedance spectroscopy tests confirmed the results obtained in the DC corrosion resistance tests, as indicated by the spectral characteristic of the impedance in the Nyquist plot and the increase of the curve slopes: there was an increase in the value of the impedance of materials with an increasing number of ZnO layer cycles, and an extension of the highest value of the phase shift angle in the Bode plot to an ever greater range of frequencies.
- As a result of the tribological investigations of the ZnO coatings, a slight increase in the abrasion resistance was found. Therefore, these coatings can be used in applications exposed to low abrasion. Moreover, our study may contribute to the further development of these coatings so as to increase their abrasion resistance.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borysiewicz, M. ZnO as a Functional Material, a Review. Crystals 2019, 9, 505. [Google Scholar] [CrossRef] [Green Version]
- Boryło, P.; Matus, K.; Lukaszkowicz, K.; Kubacki, J.; Balin, K.; Basiaga, M.; Szindler, M.; Mikuła, J. The influence of atomic layer deposition process temperature on ZnO thin film structure. Appl. Surf. Sci. 2019, 474, 177–186. [Google Scholar] [CrossRef]
- Umar, A.; Akhtar, M.S.; Almas, T.; Ibrahim, A.A.; Al-Assiri, M.S.; Masuda, Y.; Rahman, Q.I.; Baskoutas, S. Direct Growth of Flower-Shaped ZnO Nanostructures on FTO Substrate for Dye-Sensitized Solar Cells. Crystals 2019, 9, 405. [Google Scholar] [CrossRef] [Green Version]
- Popescu, M.; Ungureanu, C.; Buse, E.; Nastase, F.; Țucureanu, V.; Suchea, M.; Draga, S. Antibacterial efficiency of cellulose-based fibers covered with ZnO and Al2O3 by Atomic Layer Deposition. Appl. Surf. Sci. 2019, 481, 1287–1298. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ma, J.; Lee, S.; Jo, S.; Kim, C.S. Effect of Ultraviolet-Ozone Treatment on the Properties and Antibacterial Activity of Zinc Oxide Sol-Gel Film. Materials 2019, 12, 2422. [Google Scholar] [CrossRef] [Green Version]
- Zinnatullin, A.L.; Gumarov, A.I.; Gilmutdinov, I.F.; Kiiamov, A.; Vagizov, F.G.; Nuzhdin, V.I.; Валеев, В.; Khaibullin, R. Magnetic phase composition of ZnO film heavily implanted with Fe ions. Appl. Surf. Sci. 2019, 489, 220–225. [Google Scholar] [CrossRef]
- Kerasidou, A.; Bardakas, A.; Botzakaki, M.; Georga, S.; Krontiras, C.; Mergia, K.; Psycharis, V.; Tsamis, C. Growth of ZnO nanowires on seeding layers deposited by ALD: The influence of process parameters. Microelectron. Eng. 2019, 217, 111091. [Google Scholar] [CrossRef]
- Graniel, O.; Fedorenko, V.; Viter, R.; Iatsunskyi, I.; Nowaczyk, G.; Weber, M.; Zaleski, K.; Jurga, S.; Smyntyna, V.; Miele, P.; et al. Optical properties of ZnO deposited by atomic layer deposition (ALD) on Si nanowires. Mater. Sci. Eng. B 2018, 236–237, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Chang, M.; Cho, S.; Kim, M.; Kim, H.; Choi, E.; Ko, H.; Hwang, J.-H.; Yang, H. Formation of a functional homo-junction interface through ZnO atomic layer passivation: Enhancement of carrier mobility and threshold voltage in a ZnO nanocrystal field effect transistor. J. Alloys Compd. 2019, 804, 213–219. [Google Scholar] [CrossRef]
- Matysiak, W.; Tański, T.; Zaborowska, M. Manufacturing process and characterization of electrospun PVP/ZnO NPs nanofibers. Bull. Pol. Acad. Sci. Tech. Sci. 2019, 67, 193–200. [Google Scholar] [CrossRef]
- Matysiak, W.; Tański, T. Novel bimodal ZnO (amorphous)/ZnO NPs (crystalline) electrospun 1D nanostructure and their optical characteristic. Appl. Surf. Sci. 2019, 474, 232–242. [Google Scholar] [CrossRef]
- Nouri, A.; Wen, C. Introduction to surface coating and modification for metallic biomaterials. In Surface Coating and Modification of Metallic Biomaterials, 1st ed.; Wen, C., Ed.; Woodhead Publishing-Elsevier: Amsterdam, The Netherlands, 2015; Volume 1, pp. 3–60. [Google Scholar]
- Hanawa, T. Surface treatment and modification of metals to add biofunction. Dent. Mater. J. 2017, 36, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.-Y.; Chen, C.; Wang, X.-M.; Lee, I.-S. Advances in the surface modification techniques of bone-related implants for last 10 years. Regen. Biomater. 2014, 1, 67–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, C.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Nayak, T.R.; Hong, H.; Cai, W. Biomedical applications of zinc oxide nanomaterials. Curr. Mol. Med. 2013, 13, 1633–1645. [Google Scholar] [CrossRef] [Green Version]
- Laurenti, M.; Cauda, V. Porous Zinc Oxide Thin Films: Synthesis Approaches and Applications. Coatings 2018, 8, 67. [Google Scholar] [CrossRef] [Green Version]
- Wojcik, A.; Godlewski, M.; Guziewicz, E.; Minikayev, R.; Paszkowicz, W. Controlling of preferential growth mode of ZnO thin films grown by atomic layer deposition. J. Cryst. Growth 2008, 310, 284–289. [Google Scholar] [CrossRef]
- Yuan, N.-Y.; Wang, S.; Tan, C.; Wang, X.Q.; Chen, G.; Ding, J. The influence of deposition temperature on growth mode, optical and mechanical properties of ZnO films prepared by the ALD method. J. Cryst. Growth 2013, 366, 43–46. [Google Scholar] [CrossRef]
- Kaszewski, J.; Kielbik, P.; Wolska, E.; Witkowski, B.; Wachnicki, L.; Gajewski, Z.; Godlewski, M.; Godlewski, M.M. Tuning the luminescence of ZnO:Eu nanoparticles for applications in biology and medicine. Opt. Mater. 2018, 80, 77–86. [Google Scholar] [CrossRef]
- Pietruszka, R.; Witkowski, B.; Zimowski, S.; Stapinski, T.; Godlewski, M. Abrasion resistance of ZnO and ZnO:Al films on glass substrates by atomic layer deposition. Surf. Coat. Technol. 2017, 319, 164–169. [Google Scholar] [CrossRef]
- Verbič, A.; Gorjanc, M.; Simončič, B. Zinc Oxide for Functional Textile Coatings: Recent Advances. Coatings 2019, 9, 550. [Google Scholar] [CrossRef] [Green Version]
- Viorica, G.P.; Bujoreanu, V.M.; Pimentel, A.; Calmeiro, T.R.; Carlos, E.; Baroiu, L.; Martins, R.; Fortunato, E. Hybrid (Ag)ZnO/Cs/PMMA nanocomposite thin films. J. Alloys Compd. 2019, 803, 922–933. [Google Scholar] [CrossRef]
- Staszuk, M.; Pakula, D.; Tanski, T. Investigation studies involving wear-resistant ALD/PVD hybrid coatings on sintered tool substrates. Mater. Tehnol. 2016, 50, 755–759. [Google Scholar] [CrossRef]
- Basiaga, M.; Walke, W.; Staszuk, M.; Kajzer, W.; Kajzer, W.; Nowińska, K. Influence of ALD process parameters on the physical and chemical properties of the surface of vascular stents. Arch. Civ. Mech. Eng. 2017, 17, 32–42. [Google Scholar] [CrossRef]
- Basiaga, M.; Walke, W.; Staszuk, M.; Kajzer, W. Study of the Electrochemical Properties of 316LVM Steel with TiO2 Layer Deposited by Means of the ALD Method. Prop. Charact. Mod. Mater. Adv. Struct. Mater. 2017, 33, 297–308. [Google Scholar] [CrossRef]
- Staszuk, M. Application of PVD and ALD Methods for Surface Treatment of Al-Si-Cu Alloys. Solid State Phenom. 2019, 293, 97–109. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Pakula, D.; Staszuk, M. Chemical Vapor Deposition in Manufacturing. In Handbook of Manufacturing Engineering and Technology; Springer: London, UK, 2013. [Google Scholar]
- Yildirimer, L.; Thanh, N.T.K.; Loizidou, M.; Seifalian, A.M. Toxicology and clinical potential of nanoparticles. Nano Today 2011, 6, 585–607. [Google Scholar] [CrossRef] [Green Version]
- Hanley, C.; Layne, J.; Punnoose, A.; Reddy, K.M.; Coombs, I.; Coombs, A.; Feris, K.; Wingett, D.G. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 2008, 19, 295103. [Google Scholar] [CrossRef] [Green Version]
- Garino, N.; Limongi, T.; Dumontel, B.; Canta, M.; Racca, L.; Laurenti, M.; Castellino, M.; Casu, A.; Falqui, A.; Cauda, V. A Microwave-Assisted Synthesis of Zinc Oxide Nanocrystals Finely Tuned for Biological Applications. Nanoparticles 2019, 9, 212. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Kim, H.-J.; Go, M.-R.; Bae, S.-H.; Choi, S.-J. ZnO Interactions with Biomatrices: Effect of Particle Size on ZnO-Protein Corona. Nanomaterials 2017, 7, 377. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Ji, Y.; Liu, F.; Li, J.; Cao, Y. Cytotoxicity, oxidative stress and inflammation induced by ZnO nanoparticles in endothelial cells: Interaction with palmitate or lipopolysaccharide. J. Appl. Toxicol. 2016, 37, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Racca, L.; Canta, M.; Dumontel, B.; Ancona, A.; Limongi, T.; Garino, N.; Laurenti, M.; Canavese, G.; Cauda, V. Zinc Oxide Nanostructures in Biomedicine. Smart Nanopart. Biomed. 2018, 171–187. [Google Scholar] [CrossRef]
- Weckman, T.; Laasonen, K. Atomic Layer Deposition of Zinc Oxide: Diethyl Zinc Reactions and Surface Saturation from First-Principles. J. Phys. Chem. C 2016, 120, 21460–21471. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, J.; Jilani, A.; Hassan, P.Z.; Rafique, S.; Jafer, R.; Alghamdi, A.A. ALD grown nanostructured ZnO thin films: Effect of substrate temperature on thickness and energy band gap. J. King Saud Univ. Sci. 2016, 28, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Park, H.K.; Yang, B.S.; Park, S.; Kim, M.S.; Shin, J.C.; Heo, J. Purge-time-dependent growth of ZnO thin films by atomic layer deposition. J. Alloys Compd. 2014, 605, 124–130. [Google Scholar] [CrossRef]






| Sample | Ecorr (mV) | Icorr (µA/cm2) | Rp (kΩ∙cm2) |
|---|---|---|---|
| uncoated | −67.39 | 0.015 | 766.50 |
| ZnO 500 | −202.67 | 0.002 | 1486.95 |
| ZnO 1000 | −174.57 | 0.006 | 2752.55 |
| ZnO 1500 | −97.12 | 0.001 | 8317.38 |
| Sample | Rs (Ω∙cm2) | CPE1 (µF/cm2) | N1 | R1 (kΩ∙cm2) |
|---|---|---|---|---|
| uncoated | 0.01 | 0.12 | 0.001 | 586 |
| ZnO 500 | 0.3 | 0.03 | 0.002 | 197 |
| ZnO 1000 | 0.5 | 0.02 | 0.003 | 676 |
| ZnO 1500 | 0.4 | 0.009 | 0.002 | 2350 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staszuk, M.; Pakuła, D.; Reimann, Ł.; Król, M.; Basiaga, M.; Mysłek, D.; Kříž, A. Structure and Properties of ZnO Coatings Obtained by Atomic Layer Deposition (ALD) Method on a Cr-Ni-Mo Steel Substrate Type. Materials 2020, 13, 4223. https://doi.org/10.3390/ma13194223
Staszuk M, Pakuła D, Reimann Ł, Król M, Basiaga M, Mysłek D, Kříž A. Structure and Properties of ZnO Coatings Obtained by Atomic Layer Deposition (ALD) Method on a Cr-Ni-Mo Steel Substrate Type. Materials. 2020; 13(19):4223. https://doi.org/10.3390/ma13194223
Chicago/Turabian StyleStaszuk, Marcin, Daniel Pakuła, Łukasz Reimann, Mariusz Król, Marcin Basiaga, Dominika Mysłek, and Antonín Kříž. 2020. "Structure and Properties of ZnO Coatings Obtained by Atomic Layer Deposition (ALD) Method on a Cr-Ni-Mo Steel Substrate Type" Materials 13, no. 19: 4223. https://doi.org/10.3390/ma13194223






