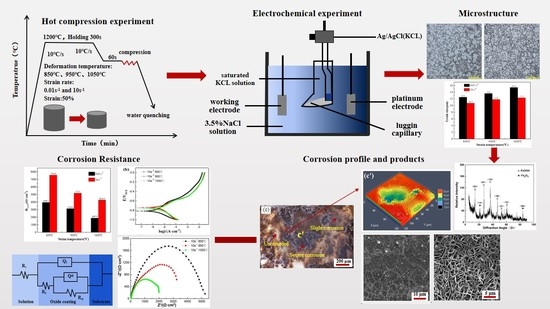
Effect of Hot Deformation Process Parameters on Microstructure and Corrosion Behavior of 35CrMoV Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microscopic Analysis
2.3. Electrochemical Measurements
2.4. Corrosion Surface Analysis
3. Results and Discussion
3.1. Microstructure
3.2. Electrochemical Analysis
3.2.1. Potentiodynamic Polarization Curve
3.2.2. Electrochemical Impedance Spectroscopy characteristics
3.3. Corrosion Products and Corrosion Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiang, X.; Zhou, Y.; Shi, C.; Mao, D. Effects of Ultrasonic-Aided Quenching on the Corrosion Resistance of GB 35CrMoV Steel in Seawater Environment. Metals 2018, 8, 104. [Google Scholar] [CrossRef]
- Niu, L.; Cheng, Y. Corrosion behavior of X-70 pipe steel in near-neutral pH solution. Appl. Surf. Sci. 2007, 253, 8626–8631. [Google Scholar] [CrossRef]
- Alizadeh, M.; Bordbar, S. The influence of microstructure on the protective properties of the corrosion product layer generated on the welded API X70 steel in chloride solution. Corros. Sci. 2013, 70, 170–179. [Google Scholar] [CrossRef]
- Shimura, T.; Aramaki, K. Prevention of passive film breakdown on iron by coverage with one-dimensional polymer films of a carboxylate ion self-assembled monolayer modified with alkyltriethoxysilanes. Corros. Sci. 2004, 46, 2563–2581. [Google Scholar]
- Zhang, B.; Zhang, B.; Ruan, X.; Zhang, Y. The Hot Deformation Behavior and Dynamic Recrystallization Model Of 35 crmo Steel. Acta. Metall. Sin. 2003, 16, 183–191. [Google Scholar]
- Wang, S.; Huang, Y.; Xiao, Z.; Liu, Y.; Liu, H. A Modified Johnson-Cook Model for Hot Deformation Behavior of 35CrMo Steel. Metals 2017, 7, 337. [Google Scholar] [CrossRef]
- Chen, X.-M.; Lin, Y.; Wen, D.-X.; Zhang, J.-L.; He, M. Dynamic recrystallization behavior of a typical nickel-based superalloy during hot deformation. Mater. Des. 2014, 57, 568–577. [Google Scholar] [CrossRef]
- He, D.-G.; Lin, Y.; Chen, J.; Chen, D.-D.; Huang, J.; Tang, Y.; Chen, M.-S. Microstructural evolution and support vector regression model for an aged Ni-based superalloy during two-stage hot forming with stepped strain rates. Mater. Des. 2018, 154, 51–62. [Google Scholar] [CrossRef]
- Ren, F.; Chen, F.; Chen, J.; Tang, X. Hot deformation behavior and processing maps of AISI 420 martensitic stainless steel. J. Manuf. Process. 2018, 31, 640–649. [Google Scholar] [CrossRef]
- Kingkam, W.; Zhao, C.-Z.; Li, H.; Zhang, H.-X.; Li, Z.-M. Hot Deformation and Corrosion Resistance of High-Strength Low-Alloy Steel. Acta Met. Sin. 2018, 32, 495–505. [Google Scholar] [CrossRef]
- Xiao, Z.-B.; Huang, Y.-C.; Liu, Y. Plastic Deformation Behavior and Processing Maps of 35CrMo Steel. J. Mater. Eng. Perform. 2016, 25, 1219–1227. [Google Scholar] [CrossRef]
- Xu, L.; Chen, L.; Chen, G.; Wang, M. Hot deformation behavior and microstructure analysis of 25Cr3Mo3NiNb steel during hot compression tests. Vacuum 2018, 147, 8–17. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, S.; Xiao, Z.; Liu, H. Critical Condition of Dynamic Recrystallization in 35CrMo Steel. Metals 2017, 7, 161. [Google Scholar] [CrossRef]
- Wen, D.; Lin, Y.; Zhou, Y. A new dynamic recrystallization kinetics model for a Nb containing Ni-Fe-Cr-base superalloy considering influences of initial δphase. Vacuum 2017, 141, 316–327. [Google Scholar] [CrossRef]
- Quan, G.; Mao, A.; Zou, Z.; Luo, G.; Liang, J. Description of Grain Refinement by Dynamic Recrystallization Under Hot Compressions for As-Extruded 3Cr20Ni10W2 Heat-Resistant Alloy. High Temp. Mater. Process. 2015, 34, 697–713. [Google Scholar]
- Song, D.; Ma, A.-B.; Jiang, J.-H.; Lin, P.-H.; Yang, D.-H. Corrosion behavior of ultra-fine grained industrial pure Al fabricated by ECAP. Trans. Nonferrous Met. Soc. 2009, 19, 1065–1070. [Google Scholar] [CrossRef]
- Alvarez-Lopez, M.; Pereda, M.D.; Del Valle, J.; Fernandez-Lorenzo, M.; Garcia-Alonso, M.C.; Ruano, O.; Escudero, M.L.; Ruano, O. Corrosion behaviour of AZ31 magnesium alloy with different grain sizes in simulated biological fluids. Biomaterials 2010, 6, 1763–1771. [Google Scholar] [CrossRef]
- Argade, G.; Panigrahi, S.; Mishra, R.; Mishra, R. Effects of grain size on the corrosion resistance of wrought magnesium alloys containing neodymium. Corros. Sci. 2012, 58, 145–151. [Google Scholar] [CrossRef]
- Balyanov, A.; Kutnyakova, J.; Amirkhanova, N.; Stolyarov, V.; Valiev, R.; Liao, X.; Zhao, Y.; Jiang, Y.; Xu, H.; Lowe, T.; et al. Corrosion resistance of ultra fine-grained Ti. Scr. Mater. 2004, 51, 225–229. [Google Scholar] [CrossRef]
- Aung, N.; Zhou, W. Effect of grain size and twins on corrosion behaviour of AZ31B magnesium alloy. Corros. Sci. 2010, 52, 589–594. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, G.; Jiang, J.; Wong, H.M.; Yeung, K.W.; Chu, P.K. Improved corrosion resistance and cytocompatibility of magnesium alloy by two-stage cooling in thermal treatment. Corros. Sci. 2012, 59, 360–365. [Google Scholar] [CrossRef]
- Song, G.-L.; Bowles, A.L.; StJohn, D.H. Corrosion resistance of aged die cast magnesium alloy AZ91D. Mater. Sci. Eng. A 2004, 366, 74–86. [Google Scholar] [CrossRef]
- Hsiao, H.; Tsai, W. Effect of heat treatment on anodization and electrochemical behavior of AZ91D m agnesium alloy. J. Mater. Res. 2005, 20, 2763–2771. [Google Scholar] [CrossRef]
- Yu, X.; Chen, S.; Liu, Y.; Ren, F. A study of intergranular corrosion of austenitic stainless steel by electrochemical potentiodynamic reactivation, electron back-scattering diffraction and cellular automaton. Corros. Sci. 2010, 52, 1939–1947. [Google Scholar] [CrossRef]
- Pradhan, S.; Bhuyan, P.; Mandal, S. Individual and synergistic influences of microstructural features on intergranular corrosion behavior in extra-low carbon type 304L austenitic stainless steel. Corros. Sci. 2018, 139, 319–332. [Google Scholar] [CrossRef]
- Zhang, T.; Shao, Y.; Meng, G.; Cui, Z.; Wang, F. Corrosion of hot extrusion AZ91 magnesium alloy: I-relation between the microstructure and corrosion behavior. Corros. Sci. 2011, 53, 1960–1968. [Google Scholar] [CrossRef]
- Qian, J.; Chen, C.; Yu, H.; Liu, F.; Yang, H.; Zhang, Z. The influence and the mechanism of the precipitate/austenite interfacial C-enrichment on the intergranular corrosion sensitivity in 310 S stainless steel. Corros. Sci. 2016, 111, 352–361. [Google Scholar] [CrossRef]
- Singh, R.; Chowdhury, S.G.; Kumar, B.R.; Das, S.K.; De, P.; Chattoraj, I. The importance of grain size relative to grain boundary character on the sensitization of metastable austenitic stainless steel. Scr. Mater. 2007, 57, 185–188. [Google Scholar] [CrossRef]
- Singh, R.; Chowdhury, S.; Chattoraj, I. Modification of Sensitization Resistance of AISI 304L Stainless Steel through Changes in Grain Size and Grain Boundary Character Distributions. Metal. Mater. Tran. A. 2008, 39, 2504–2512. [Google Scholar] [CrossRef]
- Ahmedabadi, P.M.; Kain, V.; Muralidhar, K.V.; Samajdar, I. On the role of residual strain in controlling sensitisation of twin-boundary engineered type 304 stainless steel. J. Mater. 2013, 432, 243–251. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, X.-Y.; Chen, X.-M.; Chen, J.; Wen, D.-X.; Zhang, J.-L.; Li, L.-T. EBSD study of a hot deformed nickel-based superalloy. J. Alloys Compd. 2015, 640, 101–113. [Google Scholar] [CrossRef]
- Zhao, M.-C.; Liu, M.; Song, G.-L.; Atrens, A. Influence of pH and chloride ion concentration on the corrosion of Mg alloy ZE41. Corros. Sci. 2008, 50, 3168–3178. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Shi, C.; Mao, D. Microscopic Analysis and Electrochemical Behavior of Fe-Based Coating Produced by Laser Cladding. Metals 2017, 7, 435. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, Y.; Zou, Z.; Wu, D. Microstructure and properties of Fe-based composite coating by laser cladding Fe–Ti–V–Cr–C–CeO2 powder. Opt. Laser Technol. 2015, 65, 119–125. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, G.; Chen, M.-S.; Zhang, J.-L.; Chen, Z.-G.; Jiang, Y.-Q.; Li, J. Corrosion resistance of a two-stage stress-aged Al–Cu–Mg alloy: Effects of external stress. J. Alloys Compd. 2016, 661, 221–230. [Google Scholar] [CrossRef]
- Lv, J. Effect of grain size on mechanical property and corrosion resistance of the Ni-based alloy 690. J. Mater. Sci. Technol. 2018, 34, 1685–1691. [Google Scholar] [CrossRef]
- Awasthi, S.; Pandey, S.K.; Juyal, A.; Pandey, C.P.; Balani, K. Synergistic effect of carbonaceous reinforcements on microstructural, electrochemical, magnetic and tribological properties of electrophoretically deposited nickel. J. Alloys Compd. 2017, 711, 424–433. [Google Scholar] [CrossRef]
- Jiang, K.; Li, J.; Liu, J. Electrochemical codeposition of graphene platelets and nickel for improved corrosion resistant properties. RSC Adv. 2014, 4, 36245. [Google Scholar] [CrossRef]
- Rai, P.K.; Shekhar, S.; Mondal, K. Development of gradient microstructure in mild steel and grain size dependence of its electrochemical response. Corros. Sci. 2018, 138, 85–95. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, G.; Chen, M.-S.; Huang, Y.-C.; Chen, Z.-G.; Ma, X.; Jiang, Y.-Q.; Li, J. Corrosion resistance of a two-stage stress-aged Al–Cu–Mg alloy: Effects of stress-aging temperature. J. Alloys Compd. 2016, 657, 855–865. [Google Scholar] [CrossRef]
- Cai, C.; Song, R.; Wang, L.; Li, J. Surface corrosion behavior and reaction product film deposition mechanism of Mg-Zn-Zr-Nd alloys during degradation process in Hank’s solution. Surf. Coatings Technol. 2018, 342, 57–68. [Google Scholar] [CrossRef]
- Jin, W.; Wu, G.; Feng, H.; Wang, W.; Zhang, X.; Chu, P.K. Improvement of corrosion resistance and biocompatibility of rare-earth WE43 magnesium alloy by neodymium self-ion implantation. Corros. Sci. 2015, 94, 142–155. [Google Scholar] [CrossRef]
- Wu, P.-P.; Xu, F.-J.; Deng, K.-K.; Han, F.-Y.; Zhang, Z.-Z.; Gao, R. Effect of extrusion on corrosion properties of Mg-2Ca-χAl (χ = 0, 2, 3, 5) alloys. Corros. Sci. 2017, 127, 280–290. [Google Scholar] [CrossRef]
- Wu, G.; Feng, K.; Shanaghi, A.; Zhao, Y.; Xu, R.; Yuan, G.; Chu, P.K. Effects of surface alloying on electrochemical corrosion behavior of oxygen-plasma-modified biomedical magnesium alloy. Surf. Coatings Technol. 2012, 206, 3186–3195. [Google Scholar] [CrossRef]
- Xu, R.; Shen, Y.; Zheng, J.; Wen, Q.; Li, Z.; Yang, X.; Chu, P. Effects of one-step hydrothermal treatment on the surface morphology and corrosion resistance of ZK60 magnesium alloy. Surf. Coat. Technol. 2016, 206, 3186–3195. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Li, J.; Lu, J.; Wang, F. Long-term anticorrosion behaviour of polyaniline on mild Steel. Corros. Sci. 2007, 49, 3052–3063. [Google Scholar] [CrossRef]
- Jin, W.; Wang, G.; Lin, Z.; Feng, H.; Li, W.; Peng, X.; Qasim, A.M.; Chu, P.K. Corrosion resistance and cytocompatibility of tantalum-surface-functionalized biomedical ZK60 Mg alloy. Corros. Sci. 2017, 114, 45–56. [Google Scholar] [CrossRef]
- Park, J.; Lee, G.; Nishikata, A.; Tsuru, T. Anticorrosive behavior of hydroxyapatite as an environmentally friendly pigment. Corros. Sci. 2002, 44, 1087–1095. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Wang, F. Electrochemical Corrosion Behavior of Nanocrystalline Materials—A Review. J. Mater. Sci. Technol. 2010, 26, 1–14. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y. Effects of deep cryogenic treatment on wear resistance 4 and structure of GB 35CrMoV steel. Metals 2018, 8, 502. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Du, C.; Qi, H.; Huang, Y. Raman and IR spectroscopy study of corrosion products on the surface of the hot-dip galvanized steel with alkaline mud adhesion. J. Raman Spectrosc. 2009, 40, 656–660. [Google Scholar] [CrossRef]
- Eliyan, F.F.; Alfantazi, A. Corrosion of the Heat-Affected Zones (HAZs) of API-X100 pipeline steel in dilute bicarbonate solutions at 90 °C—An electrochemical evaluation. Corros. Sci. 2013, 74, 297–307. [Google Scholar] [CrossRef]
- Kimura, M.; Kihira, H.; Ohta, N.; Hashimoto, M.; Senuma, T. Control of Fe (O,OH)6 nano-network structures of rust for high atmospheric-corrosion resistance. Corros. Sci. 2005, 47, 2499–2509. [Google Scholar] [CrossRef]
- Hao, X.; Su, P.; Xiao, K. Effect of different NaCl concentration on corrosion products of weathering steel. Corros. Prot. 2009, 30, 297–299. [Google Scholar]
- Fu, A.; Cheng, Y. Characterization of corrosion of X65 pipeline steel under disbonded coating by scanning Kelvin probe. Corros. Sci. 2009, 51, 914–920. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Liu, G. Grain Size Effect on the Elect ro-chemical Corrosion Behavior of Surface Nanocrystallized Low-Carbon Steel. Corrosion 2004, 60, 891–896. [Google Scholar] [CrossRef]
- Yamashitaa, M.; Miyukia, H.; Matsudaa, Y.; Naganoa, H.; Misawa, T. The long Term Grouth of The Protective Rust Layer Formed on Weathering Steel by Atmospheric Corrosion during a Quarter of a Century. Corros. Sci. 1994, 2, 283. [Google Scholar] [CrossRef]
- Misawa, T.; Asami, K.; Hashimoto, K.; Shimodaira, S. The mechanism of atmospheric rusting and the protective amorphous rust on low alloy steel. Corros. Sci. 1974, 14, 279–289. [Google Scholar] [CrossRef]
- Wang, X. Effect of ultrafine grain boundary on corrosion resistance of metals. Corros. Pro. 2015, 36, 695–699. [Google Scholar]
- Lin, Y.; Jiang, Y.-Q.; Zhang, X.-C.; Deng, J.; Chen, X.-M. Effect of creep-aging processing on corrosion resistance of an Al–Zn–Mg–Cu alloy. Mater. Des. 2014, 61, 228–238. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, Y.; Xia, Y.; Zhang, X.; Zhou, H.; Deng, J. Effects of creep-aging processing on the corrosion resistance and mechanical properties of a typical Al-Cu-Mg alloy. Mater. Sci. Eng. A 2014, 605, 192–202. [Google Scholar] [CrossRef]















| Chemical Composition | C | Si | Mn | Mo | S | P | Cr | V | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Measured | 0.36 | 0.23 | 0.29 | 0.26 | 0.009 | 0.035 | 1.19 | 0.14 | Bal |
| Sample | Ecorr/VSCE | Rp/(Ω·cm2) | Icorr/(μA/cm2) | Ba/(mV/dec) | Bc/(mV/dec) | |
|---|---|---|---|---|---|---|
| 0.01 s−1 | 850 °C | −0.720 | 12,290.8 | 2.441 | 87.73 | 57.20 |
| 950 °C | −0.747 | 6418.7 | 4.143 | 105.11 | 58.40 | |
| 1050 °C | −0.704 | 4083.5 | 5.393 | 145.06 | 52.38 | |
| 10 s−1 | 850 °C | −0.815 | 12,927.8 | 2.332 | 55.69 | 88.50 |
| 950 °C | −0.739 | 6795.4 | 4.354 | 90.24 | 56.71 | |
| 1050 °C | −0.767 | 6072.5 | 5.256 | 69.64 | 66.59 | |
| Sample | Rs (Ω·cm2) | Yf (Ω−1·cm−2·sn) | n | Rf (Ω·cm2) | Ydl (Ω−1·cm−2·sn) | n | Rct (Ω·cm2) | |
|---|---|---|---|---|---|---|---|---|
| 0.01 s−1 | 850 °C | 15.66 | 2.316 × 10−4 | 0.7438 | 3469.00 | 2.081 × 10−2 | 1.000 | 489.3 |
| 950 °C | 15.21 | 1.544 × 10−4 | 0.7619 | 2768.00 | 1.585 × 10−2 | 0.9732 | 360.6 | |
| 1050 °C | 12.84 | 1.637 × 10−4 | 0.7451 | 961.30 | 5.139 × 10−4 | 0.3708 | 926.6 | |
| 10 s−1 | 850 °C | 17.85 | 3.779 × 10−4 | 0.6669 | 26.83 | 1.88 × 10−4 | 0.8759 | 7530.0 |
| 950 °C | 14.42 | 1.236 × 10−4 | 0.7733 | 3713.00 | 3.016 × 10−4 | 0.8368 | 1460.0 | |
| 1050 °C | 36.56 | 4.464 × 10−5 | 1.0000 | 189.40 | 3.157 × 10−4 | 0.5485 | 4067.0 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Zhou, Y.; Li, Z.; Mao, D. Effect of Hot Deformation Process Parameters on Microstructure and Corrosion Behavior of 35CrMoV Steel. Materials 2019, 12, 1455. https://doi.org/10.3390/ma12091455
Yang Q, Zhou Y, Li Z, Mao D. Effect of Hot Deformation Process Parameters on Microstructure and Corrosion Behavior of 35CrMoV Steel. Materials. 2019; 12(9):1455. https://doi.org/10.3390/ma12091455
Chicago/Turabian StyleYang, Qiumei, Yajun Zhou, Zheng Li, and Daheng Mao. 2019. "Effect of Hot Deformation Process Parameters on Microstructure and Corrosion Behavior of 35CrMoV Steel" Materials 12, no. 9: 1455. https://doi.org/10.3390/ma12091455