Influence of Porous Dressings Based on Butyric-Acetic Chitin Co-Polymer on Biological Processes In Vitro and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Medisorb R and Medisorb Ag Dressing Materials
2.2. In Vitro Tests
2.3. Examination of Antibacterial Properties of the Medisorb Ag Dressing
2.4. In Vivo Tests
3. Results and Discussion
3.1. Test on Mass Loss and Possible Product of Degradation of Medisorb R in Plasma
- frozen plasma (for checking the influence of freezing/de-freezing process and impact of EDTA for enzyme activity)
- fresh plasma (sole effect of the presence of EDTA for enzyme activity)
- fresh serum with full enzymatic activity.
3.2. Studies of Skin Irritation and Sensitisation Effects of the Medisorb R Dressing
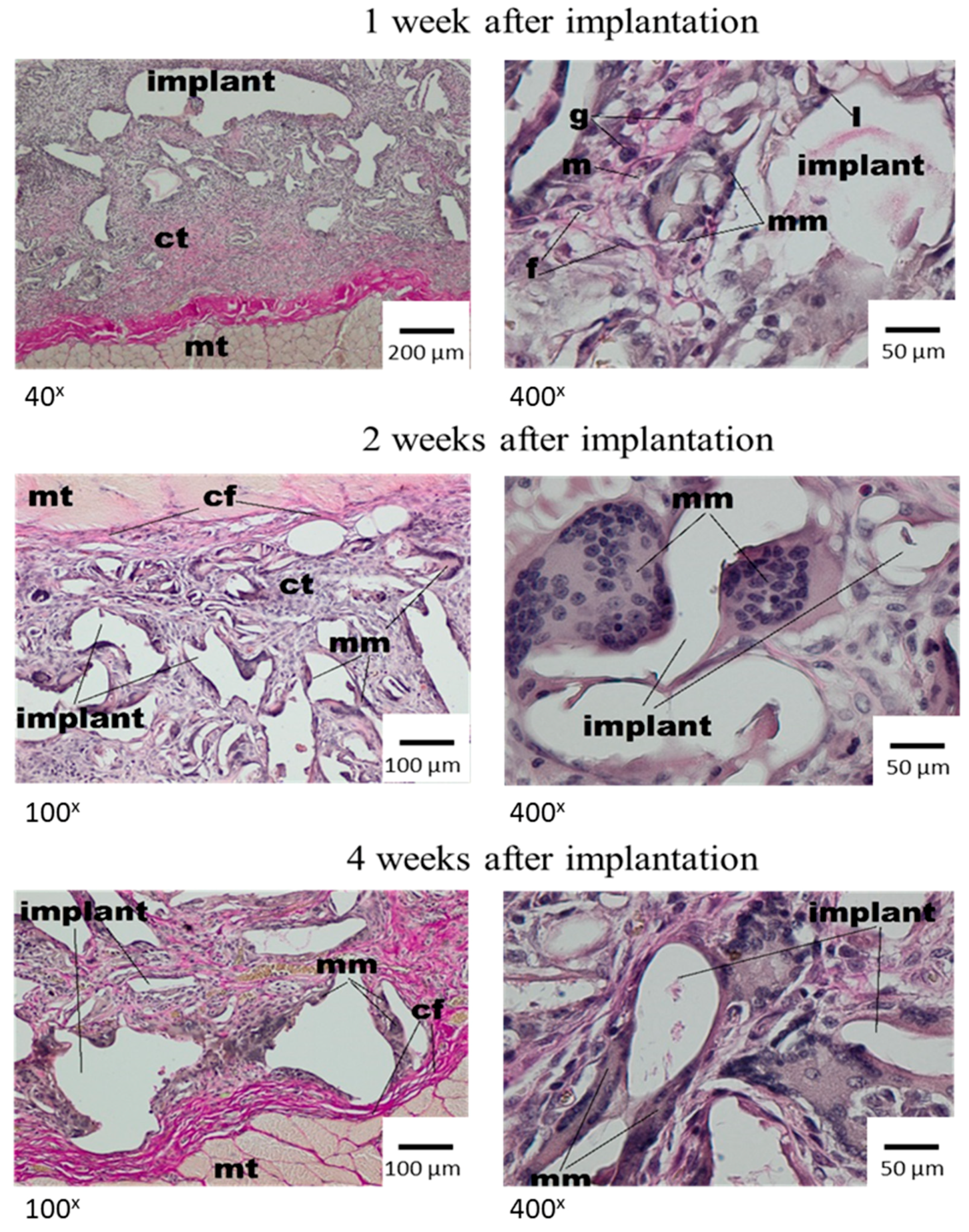
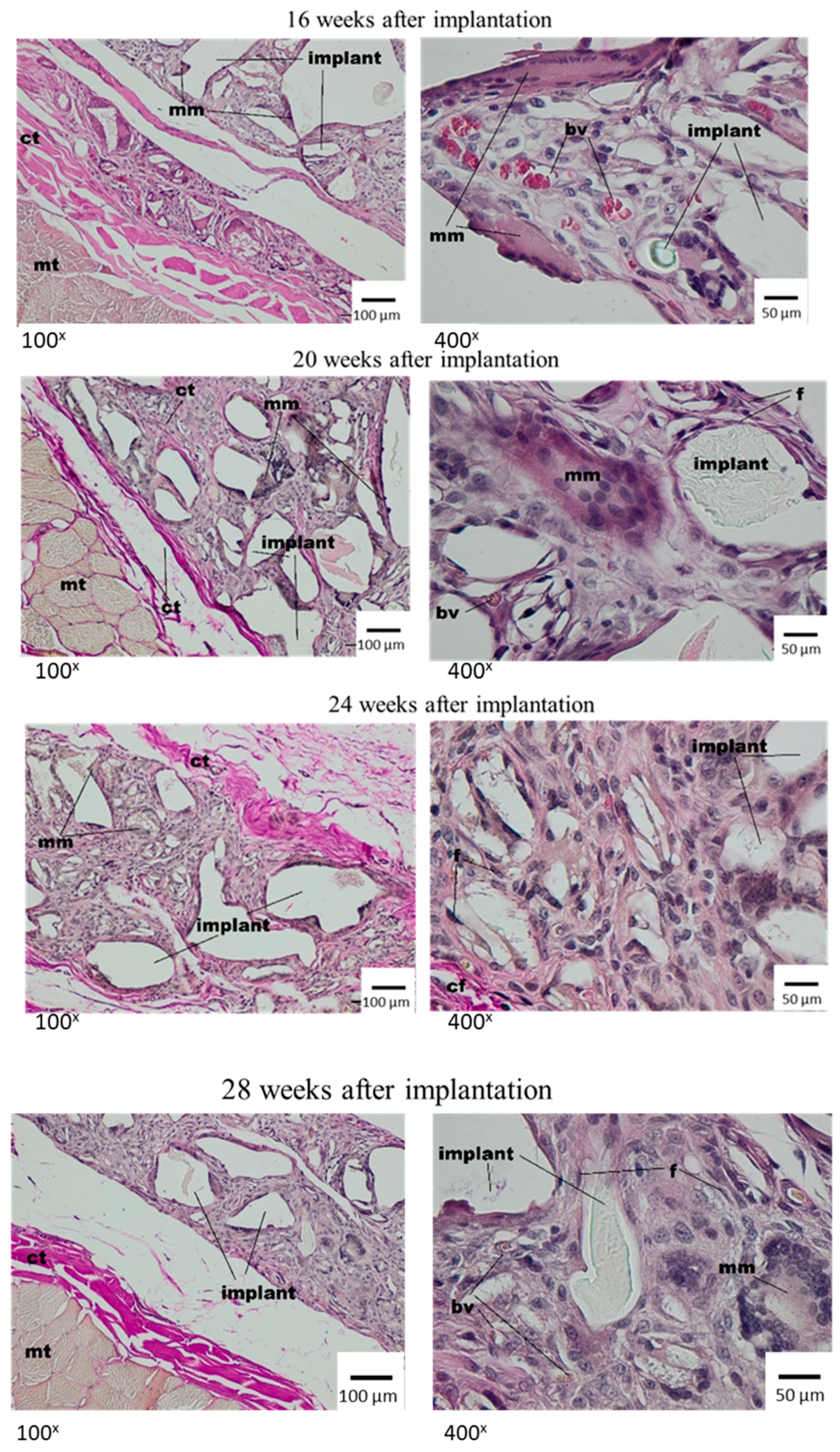
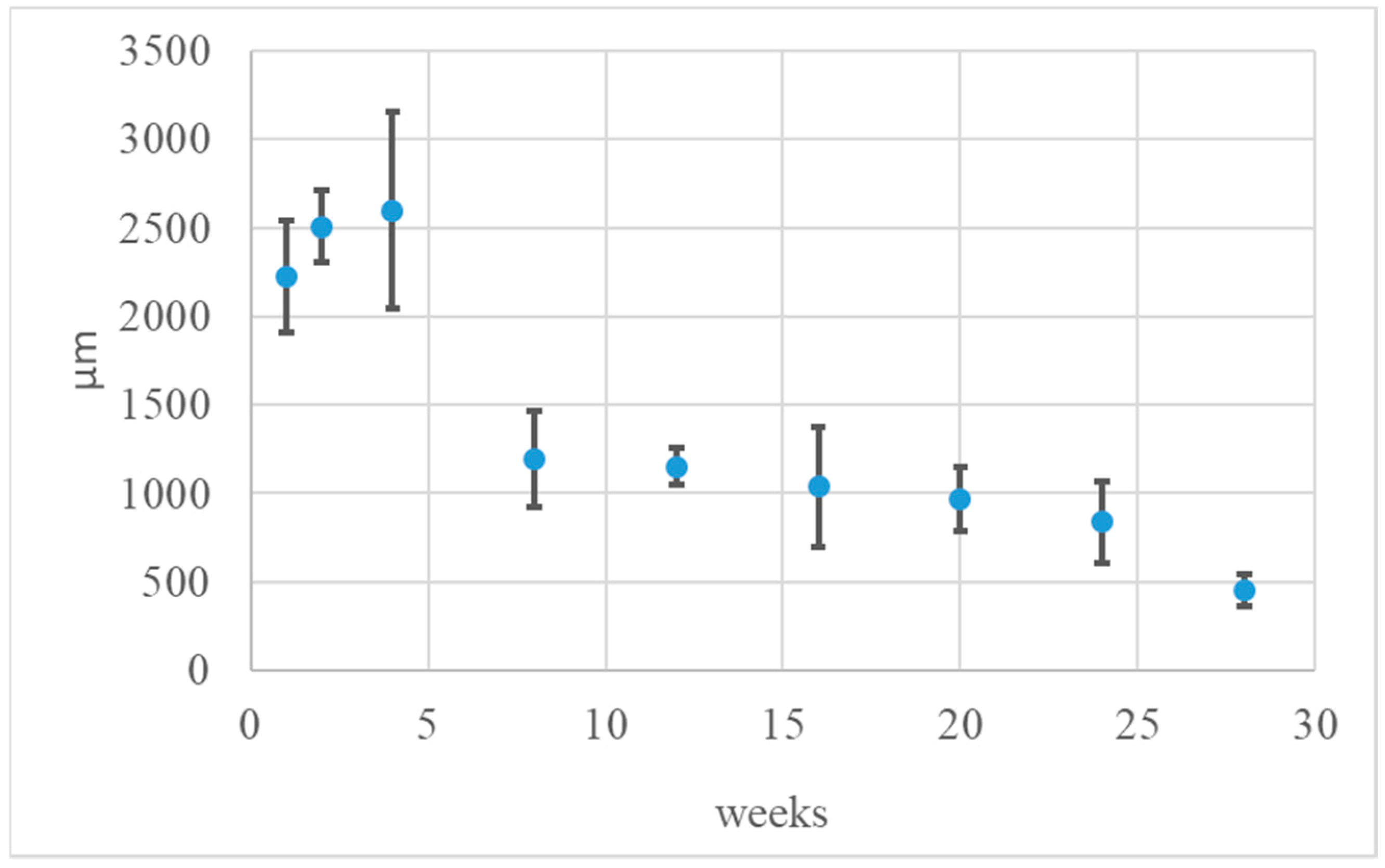
3.3. Local Evaluation of Subcutaneous Tissue Reaction after Medisorb R Implantation
Histological Tests
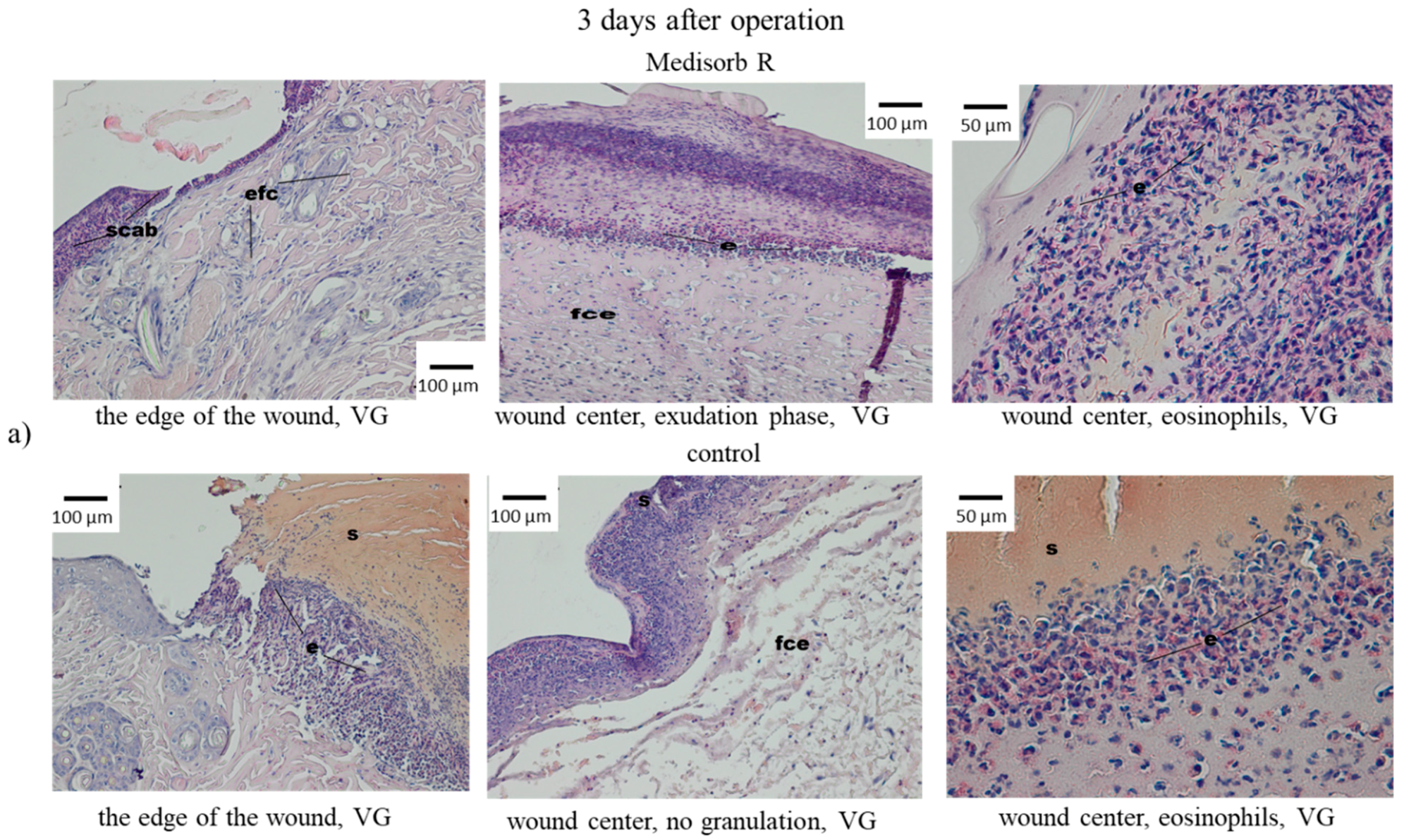
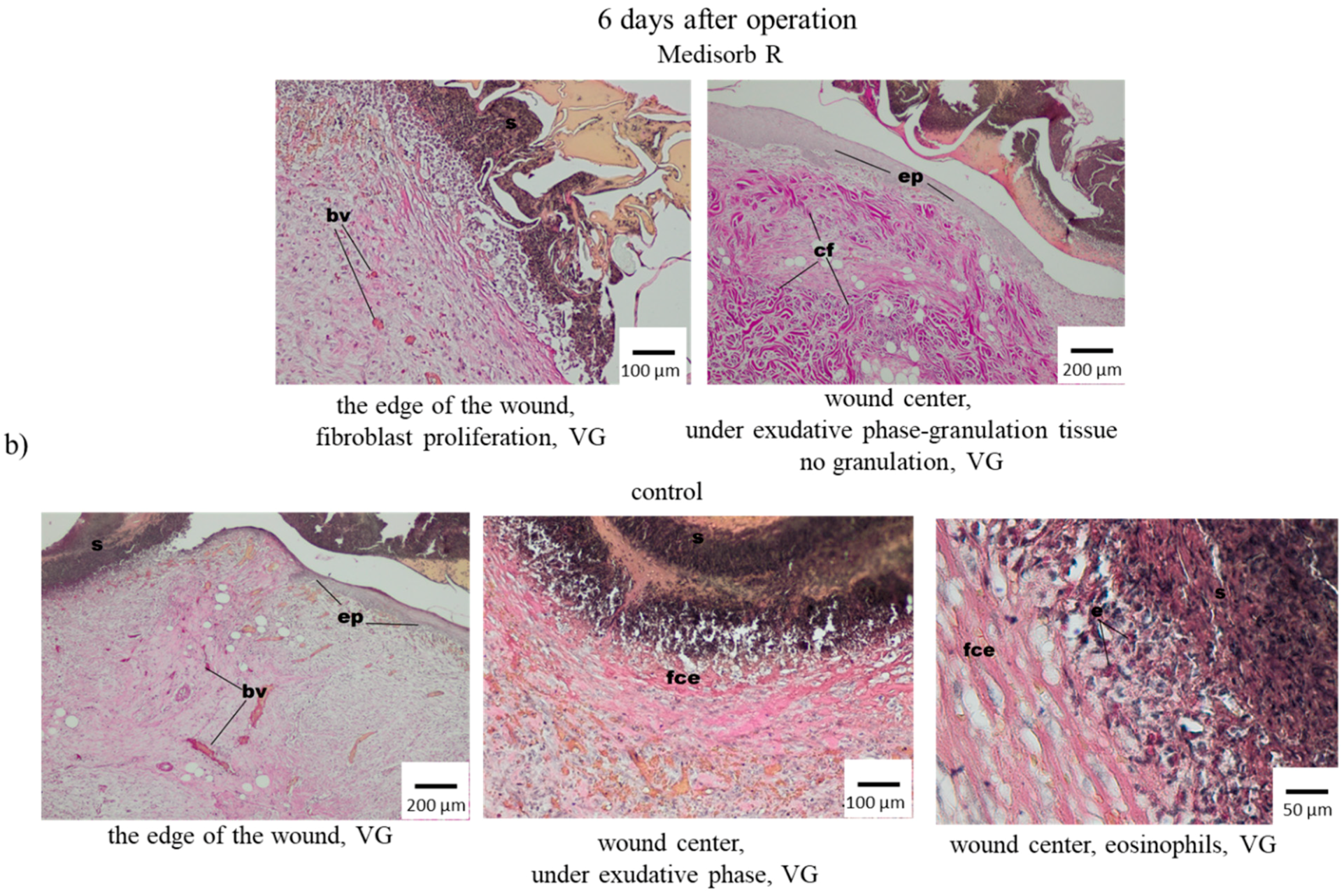
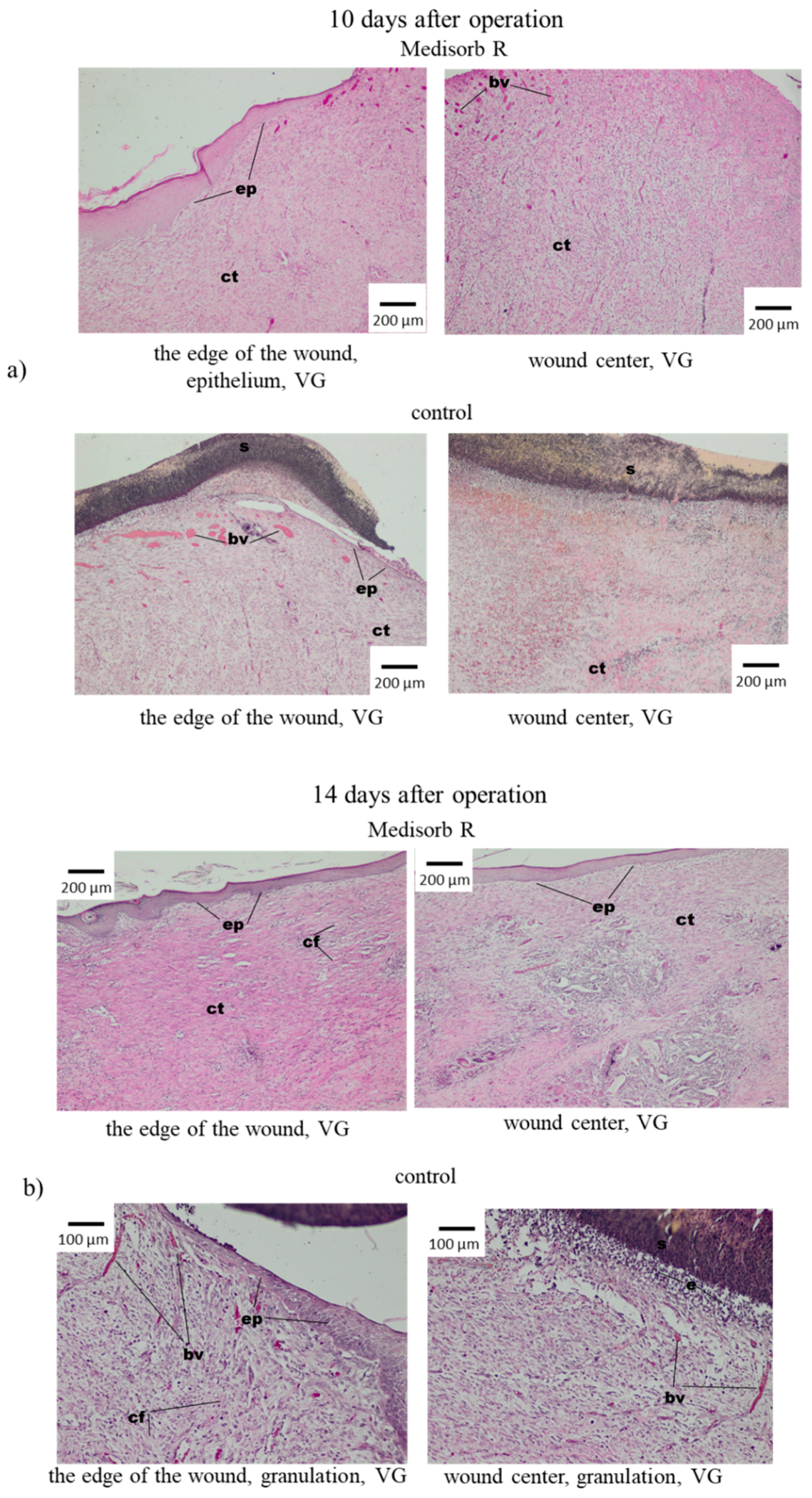
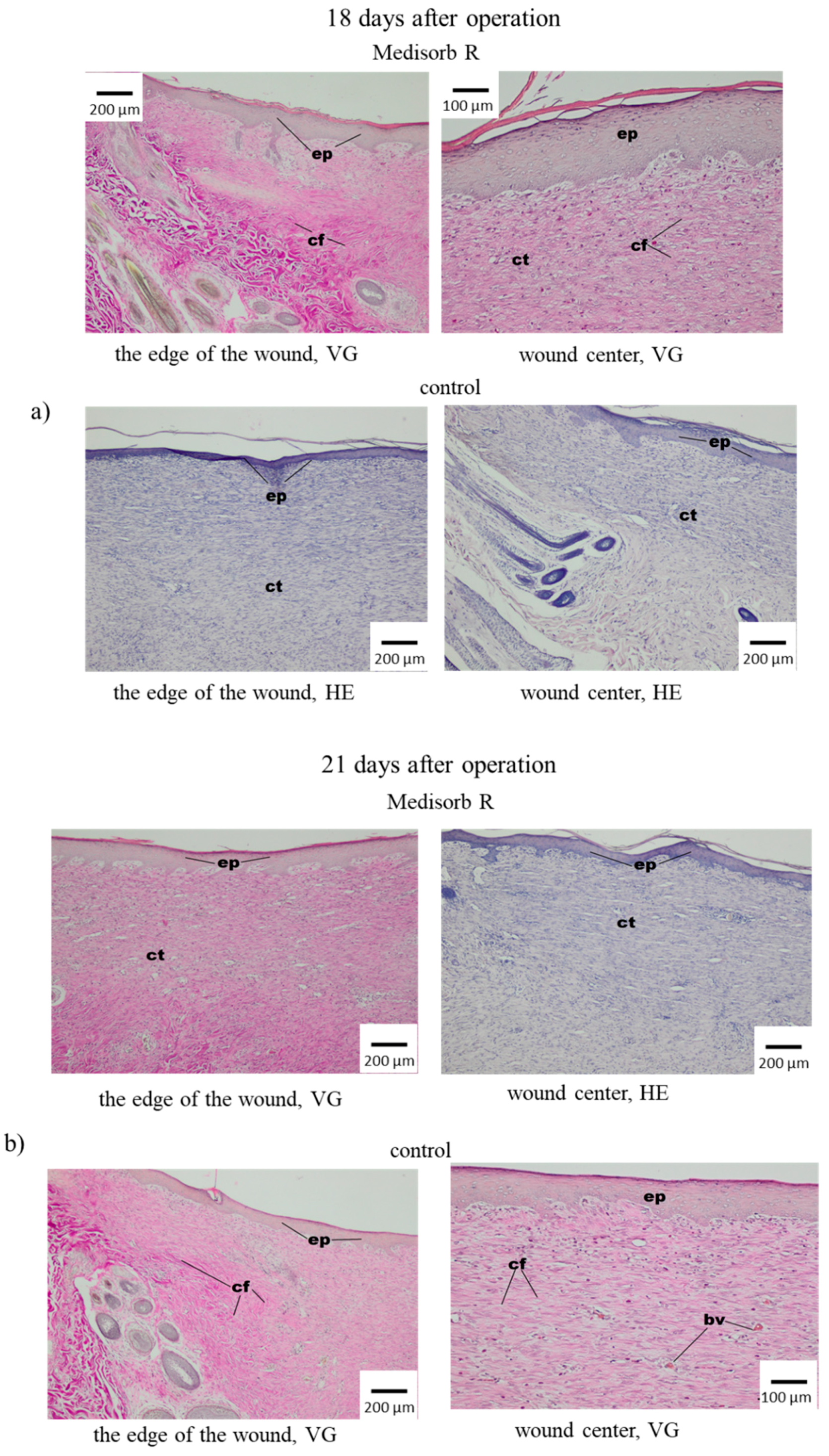
3.4. Wound Treatment of Full-Thickness Skin Loss with Medisorb R Dressing in Rabbits
3.5. Antibacterial Activity of the Medisorb Ag Dressing
4. Conclusions
- In all scheduled tests (up to week 28), Medisorb R dressing covered with thin bag was visible in macroscopic image in unchanged subcutaneous tissue.
- Medisorb R dressing underwent degradation and gradual reduction in subcutaneous tissue.
- The healing process of Medisorb R evoked little inflammation effect, which was connected to material degradation and led to fulfilling the implant with rich-in-cell tissue of granulation feature.
- The process of degradation and Medisorb R dressing healing was not finished by week 28 after implantation, and in the material neighbourhood, rich-in-cell connective tissue with multinuclear macrophages was present,
- Medisorb R dressing was characterised by high biocompatibility.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C 2015, 48, 651–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug. Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Da, L.-C.; Huang, Y.-Z.; Xie, H.-Q. Progress in development of bioderived materials for dermal wound healing. Regen. Biomater. 2017, 4, 325–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glim, J.E.; Everts, V.; Niessen, F.B.; Ulrich, R.M.M.; Beelen, H. Extracellular matrix components of oral mucosa differ from skin and resemble that of foetal skin. Arch. Oral Biol. 2014, 59, 1048–1055. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Chuah, C.H. Synthesis and characterization of nanocellulose reinforced semi-interpenetrating polymer network of chitosan hydrogel. Cellulose 2017, 24, 2215–2228. [Google Scholar]
- Mohd, A.C.M.; Ching, Y.C.; Luqman, C.A.; Poh, S.C.; Chuah, C.H. Review of bionanocomposite coating films and their applications. Polymers 2016, 8, 246. [Google Scholar]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Ching, K.Y.; Chuah, C.H.; Abdullah, L.C. Biomedical and Microbiological Applications of Bio-Based Porous Materials: A Review. Polymers 2017, 9, 160. [Google Scholar] [CrossRef]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skórkowska-Telichowska, K.; Kulma, A.; Żuk, M.; Czuj, T.; Szopa, J. The Effects of Newly Developed Linen Dressings on Decubitus Ulcers. J. Palliat. Med. 2012, 15, 146–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, D.F. There is no such thing as a biocompatible material. Biomaterials 2014, 35, 10009–10014. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef] [PubMed]
- Choo, K.W.; Ching, Y.C.; Chuah, C.H.; Sabariah, J.; Liou, N.S. Preparation and characterization of polyvinyl alcohol-chitosan composite films reinforced with cellulose nanofiber. Materials 2016, 9, 644. [Google Scholar] [CrossRef] [PubMed]
- Ching, Y.C.; Rahman, A.; Ching, K.Y.; Sukiman, N.L.; Cheng, H.C. Preparation and characterization of polyvinyl alcohol-based composite reinforced with nanocellulose and nanosilica. BioResources 2015, 10, 3364–3377. [Google Scholar] [CrossRef]
- Rubentheren, V.; Ward, T.A.; Chee, C.Y.; Nair, P. Physical and chemical reinforcement of chitosan film using nanocrystalline cellulose and tannic acid. Cellulose 2015, 22, 2529–2541. [Google Scholar] [CrossRef]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Yadav, H.; Shah, V.G.; Shah, G.; Dhaka, G. Biomedical biopolymers, their origin and evolution in biomedical sciences: A systematic review. J. Clin. Diagn. Res. 2015, 9, ZE21. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, O.A.; Darwish, S.M. Fabrication of tissue engineering scaffolds using rapid prototyping techniques. World Acad. Sci. Eng. Technol. Int. J. Mech. Aerosp. Ind. Mechatron. Manuf. Eng. 2011, 5, 2317–2325. [Google Scholar]
- Ji, C.; Annabi, N.; Khademhosseini, A.; Dehghani, F. Fabrication of porous chitosan scaffolds for soft tissue engineering using dense gas CO2. Acta Biomater. 2011, 7, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, G.; Pathak, K. Porous carriers for controlled/modulated drug delivery. Indian J. Pharm. Sci. 2009, 71, 599. [Google Scholar] [CrossRef] [PubMed]
- Cam, C.; Zhu, S.; Truong, N.F.; Scumpia, P.O.; Segura, T. Systematic evaluation of natural scaffolds in cutaneous wound healing. J. Mater. Chem. 2015, 3, 7986–7992. [Google Scholar] [CrossRef] [PubMed]
- Laroche, C.; Delattre, C.; Mati-Baouche, N.; Salah, R.; Ursu, A.V.; Moulti-Mati, F.; Michaud, P.; Pierre, G. Bioactivity of Chitosan and its Derivatives. Curr. Org. Chem. 2018, 22, 641–667. [Google Scholar] [CrossRef]
- Bedian, L.; Villalba-Rodríguez, A.M.; Hernández-Vargas, G.; Parra-Saldivar, R.; Iqbal, H.M.N. Bio-based materials with novel characteristics for tissue engineering applications—A review. Int. J. Biol. Macromol. 2017, 98, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Kim, M.-M. Applications of Chitinand Its Derivatives in Biological Medicine. Int. J. Mol. Sci. 2010, 11, 5152–5164. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K. Chitin and Chitosan: Functional Biopolymers from Marine Crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.A.; Shahzad, S.A.; Hussain, F.; Skene, W.G.; Ali Khan, Z.; Yar, M. Synthetic modifications of chitin and chitosan as multipurpose biopolymers: A review. Synth. Commun. 2018, 48, 1893–1908. [Google Scholar] [CrossRef]
- Rameshthangam, P.; Solairaj, D.; Arunachalam, G.; Ramasamy, P. Chitin and Chitinases: Biomedical and Environmental Applications of Chitin and its Derivatives. J. Enzym. 2018, 1, 20–43. [Google Scholar]
- Van Luyen, D.; Rossbach, V. Mixed esters of chitin. J. Appl. Polym. Sci. 1995, 55, 679–685. [Google Scholar] [CrossRef]
- Bhatt, L.R.; Kim, B.M.; Hyun, K.; Kang, K.H.; Lu, C.; Chai, K.Y. Preparation of chitin butyrate by using phosphoryl mixed anhydride system. Carbohydr. Res. 2011, 346, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, L.R.; Kim, B.M.; An, C.Y.; Lu, C.C.; Chung, Y.S.; Soung, M.G.; Park, S.H.; Chai, K.Y. Synthesis of chitin cycloalkyl ester derivatives and their physical properties. Carbohydr. Res. 2010, 345, 2102–2106. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, L.R.; Kim, B.M.; Hyun, K.; Kwak, G.B.; Lee, C.H.; Chai, K.Y. Preparation and characterization of chitin benzoic acid esters. Molecules 2011, 16, 3029–3036. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, H.; Yoshida, J.; Yamamoto, K.; Kadokawa, J. Facile acylation of α-chitin in ionic liquid. Carbohydr. Polym. 2018, 200, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J. Dissolution, derivatization, and functionalization of chitin in ionic liquid. Int. J. Biol. Macromol. 2019, 123, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, D.; Schoukens, G.; Göktepe, Ö.; Göktepe, F. Preparation of di-butyryl-chitin scaffolds by using salt leaching method for tissue engineering and their characteristics. J. Appl. Polym. Sci. 2008, 109, 2882–2887. [Google Scholar] [CrossRef]
- Skołucka-Szary, K.; Ramięga, A.; Piaskowska, W.; Janicki, B.; Grala, M.; Rieske, P.; Stoczyńska-Fidelus, E.; Piaskowski, S. Chitin dipentanoate as the new technologically usable biomaterial. Mater. Sci. Eng. 2015, 55, 50–60. [Google Scholar] [CrossRef]
- Skołucka-Szary, K.; Ramięga, A.; Piaskowska, W.; Janicki, B.; Grala, M.; Rieske, P.; Bartczak, Z.; Piaskowski, S. Synthesis and physicochemical characterization of chitin dihexanoate—A new biocompatible chitin derivative—In comparison to chitin dibutyrate. Mater. Sci. Eng. 2016, 60, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Draczynski, Z. Synthesis and solubility properties of chitin acetate/butyrate copolymers. J. Appl. Polym. Sci. 2011, 122, 175–182. [Google Scholar] [CrossRef]
- Draczynski., Z.; Bogun, M.; Sujka, W.; Kolesinska, B. An industrial-scale synthesis of biodegradable soluble in organic solvents butyric—Acetic chitin copolyesters. Adv. Polym. Technol. 2018, 1–12. [Google Scholar]
- Draczynski, Z.; Kolesinska, B.; Latanska, I.; Sujka, W. Preparation Method of Porous Dressing Materials Based on Butyric-Acetic Chitin Co-Polyesters. Materials 2018, 11, 2359. [Google Scholar] [CrossRef] [PubMed]
- Draczynski, Z.; Szosland, L.; Janowska, G.; Sujka, W.; Rogaczewska, A. Dressing with Chitin Esters and the Method of Making the Dressing from Chitin Esters. PL Patent 229513, 7 June 2013. [Google Scholar]
- Włodarczyk, B.; Sujka, W. New Absorbent Device and Method of its Producing. PL Patent Application P.425438, 30 April 2018. [Google Scholar]
- ISO-EN 10993-6-2009. Biological Evaluation of Medical Devieces—Part 6: Test for Local Effects after Implantation. Available online: https://pkn.pl/en (accessed on 22 March 2019).
- Maessen-Visch, M.B.; van Montfrans, C. Wound dressings, does it matter and why? Phlebology 2016, 31, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Taneja, N.; Chari, P.; Singh, M.; Singh, G.; Biswal, M.; Sharma, M. Evolution of bacterial flora in burn wounds: Key role of environmental disinfection in control of infection. Int. J. Burns Trauma 2013, 3, 102–107. [Google Scholar] [PubMed]
- Drobnik, J.; Krucinska, I.; Komisarczyk, A.; Sporny, S.; Szczepanowska, A.; Ciosek, J. Effects of electrospun scaffolds of di-O-butyrylchitin and poly-(ɛ-caprolactone) on wound healing. Can. J. Surg. 2017, 60, 162–171. [Google Scholar] [CrossRef] [PubMed]




| Physiological Fluid | Source/Parameter |
|---|---|
| Frozen human plasma | Military Centre of Blood Donation and Blood Treatment in Wroclaw, Poland |
| Fresh human plasma | Regional Centre for Blood Donation and Blood Treatment in Wroclaw, Poland |
| Fresh human serum | Own preparation |
| High-purity water | System Direct-Q® Millipore (Merck KGaA, Darmstadt, Germany)/18.2 MΩ cm |
| Incubation Time (Days) | Control Average Mass Loss ± SD (%) | Frozen Plasma Average Mass Loss ± SD (%) |
| 3 | −4.0 ± 0.2 | −2.5 ± 0.2 |
| 9 | −4.6 ± 0.3 | −2.0 ± 0.6 |
| 14 | −4.6 ± 0.2 | −1.9 ± 0.6 |
| Incubation Time (Days) | Control Average Mass Loss ± SD (%) | Fresh Plasma Average Mass Loss ± SD (%) |
| 3 | −4.0 ± 0.2 | −2.0 ± 0.2 |
| 9 | −4.6 ± 0.3 | −2.7 ± 0.6 |
| 14 | −4.6 ± 0.2 | −3.1 ± 0.5 |
| Incubation Time (Days) | Control Average Mass Loss ± SD (%) | Fresh Serum Average Mass Loss ± SD (%) |
| 3 | −4.1 ± 1.9 | −5.1 ± 0.1 |
| 9 | −5.2 ± 2.0 | −5.1 ± 0.3 |
| 14 | −4.4±1.3 | −4.6±0.1 |
| Reactions | Score |
|---|---|
| Erythema and Eschar | |
| No erythema | 0 |
| Very slight erythema (barely perceptible) | 1 |
| Well-defined erythema | 2 |
| Moderate erythema | 3 |
| Severe erythema (beet redness) to eschar formation preventing grading of erythema | 4 |
| Oedema | |
| No oedema | 0 |
| Very slight oedema (barely perceptible) | 1 |
| Well-defined oedema (edges of area well-defined by defined raising) | 2 |
| Moderate oedema (raised approx. 1 mm) | 3 |
| Severe oedema (raised more than 1 mm and extending beyond exposure area) | 4 |
| Maximum Possible Score for Irritation | 8 |
| Average Score | 0–0.4 | 0.5–1.9 | 2–4.9 | 5–8 |
|---|---|---|---|---|
| Response Category | Negligible | Slight | Moderate | Severe |
| Skin Reaction | Grading Scale |
|---|---|
| No visible change | 0 |
| Discrete or patchy erythema | 1 |
| Moderate and confluent erythema | 2 |
| Intense erythema and/or swelling | 3 |
| Implantation Time (weeks) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | 12 | 16 | 20 | 24 | 28 | |
| Implant width (µm) | 2222.88 | 2505.74 | 2598.27 | 1192.35 | 1152.94 | 1039.54 | 966.15 | 840.42 | 453.39 |
| SD | ±315.49 | ±203.96 | ±553.34 | ±273.45 | ±106.88 | ±338.94 | ±179.89 | ±230.58 | ±88.25 |
| Value of Antibacterial Activity for Effusion Method | ||
|---|---|---|
| Bacteria | Staphylococcus aureus ATCC 6538 | Klebsiella pneumonie ATCC4352 |
| Inoculum concentration | 2.7 × 105 | 1.0 × 105 |
| Value growth F (F = log CT − log C0) | 1.7 | 2.78 |
| Value growth G (G = log TT − log T0) | −1.13 | −1.85 |
| A value of antibacterial activity (A = F − G) | 2.83 | 4.63 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sujka, W.; Draczynski, Z.; Kolesinska, B.; Latanska, I.; Jastrzebski, Z.; Rybak, Z.; Zywicka, B. Influence of Porous Dressings Based on Butyric-Acetic Chitin Co-Polymer on Biological Processes In Vitro and In Vivo. Materials 2019, 12, 970. https://doi.org/10.3390/ma12060970
Sujka W, Draczynski Z, Kolesinska B, Latanska I, Jastrzebski Z, Rybak Z, Zywicka B. Influence of Porous Dressings Based on Butyric-Acetic Chitin Co-Polymer on Biological Processes In Vitro and In Vivo. Materials. 2019; 12(6):970. https://doi.org/10.3390/ma12060970
Chicago/Turabian StyleSujka, Witold, Zbigniew Draczynski, Beata Kolesinska, Ilona Latanska, Zenon Jastrzebski, Zbigniew Rybak, and Boguslawa Zywicka. 2019. "Influence of Porous Dressings Based on Butyric-Acetic Chitin Co-Polymer on Biological Processes In Vitro and In Vivo" Materials 12, no. 6: 970. https://doi.org/10.3390/ma12060970






