Functional Fibronectin Adsorption on Aptamer-Doped Chitosan Modulates Cell Morphology by Integrin-Mediated Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimens
2.1.1. Chitosan Preparation
2.1.2. Anti-Fibronectin Aptamer
2.1.3. sFBN-CH and CH
2.2. Protein Adsorption Studies
2.2.1. Bradford Assay
2.2.2. Western Blot
2.3. Cell Assays
2.3.1. Cell Culture
2.3.2. Cytoskeleton Inhibitors Cytotoxicity Analysis
2.3.3. Cell Morphology Analysis
2.4. Statistical Analysis
3. Results
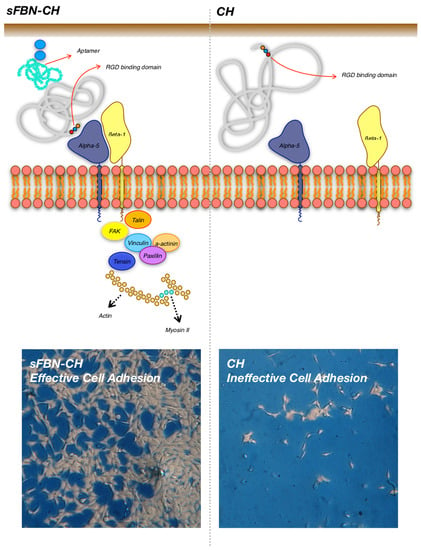
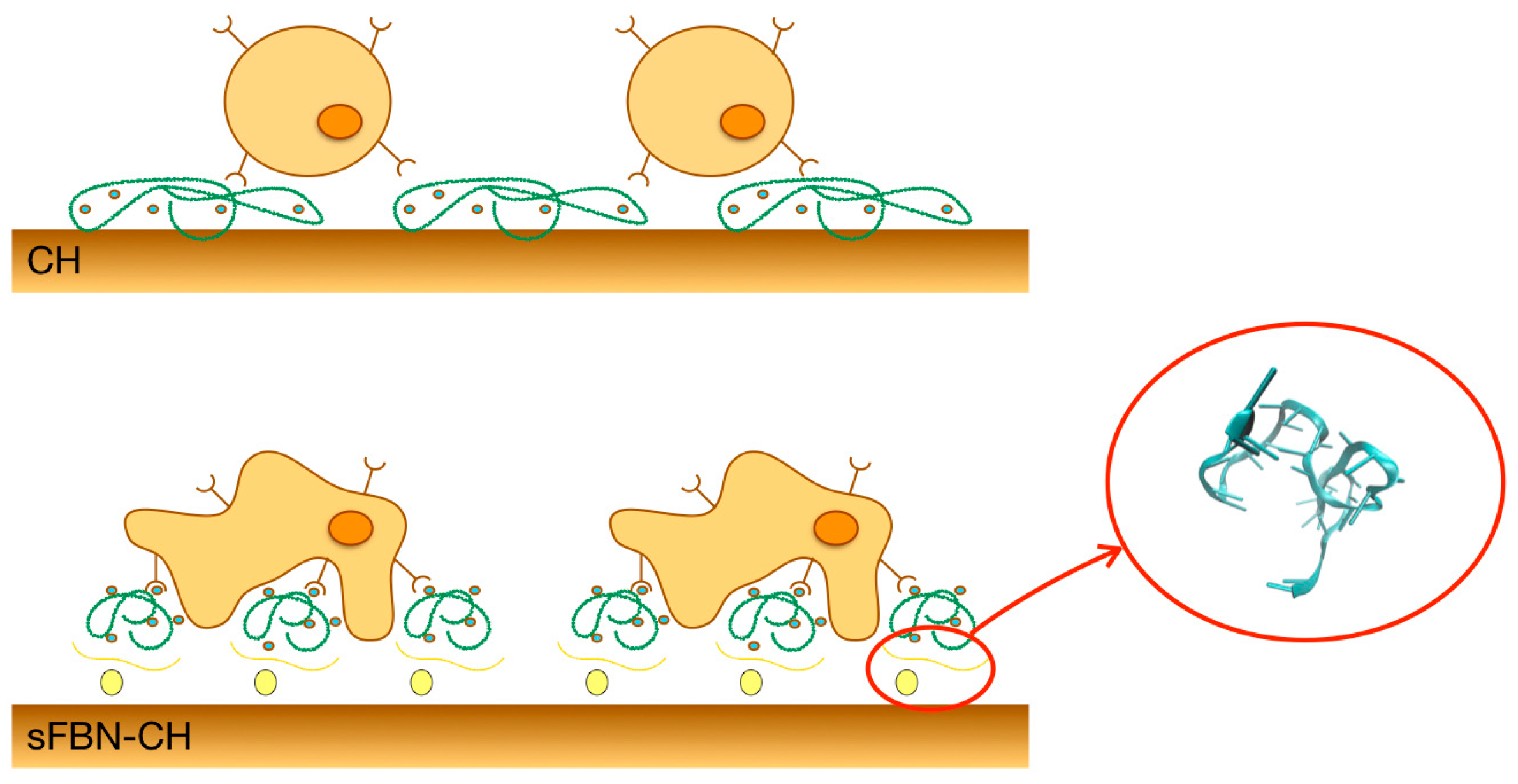
3.1. Anti-FBN Aptamers Interface Modification Induces Firm FBN Adsorption
3.2. Anti-FBN Aptamers Interface Modification Promotes Epithelial Cells Adhesion in A Dose-Dependent Manner
3.3. Integrin-Mediated Pathway Controls Epithelial Cells Adhesion at the Anti-FBN Aptamers Modified Interface
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Parisi, L.; Toffoli, A.; Ghiacci, G.; Macaluso, G. Tailoring the interface of biomaterials to design effective scaffolds. J. Funct. Biomater. 2018, 9, E50. [Google Scholar] [CrossRef] [PubMed]
- Pavinatto, F.J.; Caseli, L.; Oliveira, O.N. Chitosan in nanostructured thin films. Biomacromolecules 2010, 11, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jerome, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Galli, C.; Parisi, L.; Elviri, L.; Bianchera, A.; Smerieri, A.; Lagonegro, P.; Lumetti, S.; Manfredi, E.; Bettini, R.; Macaluso, G.M. Chitosan scaffold modified with D-(+) raffinose and enriched with thiol-modified gelatin for improved osteoblast adhesion. Biomed. Mater. 2016, 11, 015004. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.; Hlady, V. Protein adsorption and materials biocompatibility—A tutorial review and suggested hypotheses. In Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1986; Volume 79, pp. 1–63. [Google Scholar]
- Benbow, N.L.; Webber, J.L.; Karpiniec, S.; Krasowska, M.; Ferri, J.K.; Beattie, D.A. The influence of polyanion molecular weight on polyelectrolyte multilayers at surfaces: Protein adsorption and protein-polysaccharide complexation/stripping on natural polysaccharide films on solid supports. Phys. Chem. Chem. Phys. 2017, 19, 23790–23801. [Google Scholar] [CrossRef] [PubMed]
- Parisi, L.; Galli, C.; Bianchera, A.; Lagonegro, P.; Elviri, L.; Smerieri, A.; Lumetti, S.; Manfredi, E.; Bettini, R.; Macaluso, G.M. Anti-fibronectin aptamers improve the colonization of chitosan films modified with D-(+) Raffinose by murine osteoblastic cells. J. Mater. Sci. Mater. Med. 2017, 28, 136. [Google Scholar] [CrossRef] [PubMed]
- Mascini, M.; Palchetti, I.; Tombelli, S. Nucleic acid and peptide aptamers: Fundamentals and bioanalytical aspects. Angew. Chem. Int. Ed. 2012, 51, 1316–1332. [Google Scholar] [CrossRef] [PubMed]
- Nuttelman, C.R.; Mortisen, D.J.; Henry, S.M.; Anseth, K.S. Attachment of fibronectin to poly(vinyl alcohol) hydrogels promotes NIH3T3 cell adhesion, proliferation, and migration. J. Biomed. Mater. Res. 2001, 57, 217–223. [Google Scholar] [CrossRef]
- Alberts, B. Integrins. In Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2002; pp. 1227–1242. [Google Scholar]
- Krammer, A.; Craig, D.; Thomas, W.E.; Schulten, K.; Vogel, V. A structural model for force regulated integrin binding to fibronectin`s RGD-synergy site. Matrix Biol. 2002, 21, 139–147. [Google Scholar] [CrossRef]
- Saccani, M.; Parisi, L.; Bergonzi, C.; Bianchera, A.; Galli, C.; Macaluso, G.M.; Bettini, R.L.E. Surface modification of chitosan films with a fibronectin fragment-DNA aptamer complex to enhance osteoblastic cell activity: A mass spectrometry approach probing evidence on protein behavior. Rapid Commun. Mass Spectrom. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Bettini, R.; Romani, A.A.; Morganti, M.M.; Borghetti, A.F. Physicochemical and cell adhesion properties of chitosan films prepared from sugar and phosphate-containing solutions. Eur. J. Pharm. Biopharm. 2008, 68, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Parisi, L.; Piergianni, M.; Smerieri, A.; Passeri, G.; Guizzardi, S.; Costa, F.; Lumetti, S.; Manfredi, E.; Macaluso, G.M. Improved scaffold biocompatibility through anti-Fibronectin aptamer functionalization. Acta Biomater. 2016, 42, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Elviri, L.; Asadzadeh, M.; Cucinelli, R.; Bianchera, A.; Bettini, R. Macroporous chitosan hydrogels: Effects of sulfur on the loading and release behaviour of amino acid-based compounds. Carbohydr. Polym. 2015, 132, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.H.; Kyriakides, T.R. Matricellular proteins and biomaterials. Matrix Biol. 2014, 37, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.S.; Dai, L.G.; Yen, B.L.; Hsu, S.H. Spheroid formation of mesenchymal stem cells on chitosan and chitosan-hyaluronan membranes. Biomaterials 2011, 32, 6929–6945. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Huang, G.S.; Feng, F. Isolation of the multipotent MSC subpopulation from human gingival fibroblasts by culturing on chitosan membranes. Biomaterials 2012, 33, 2642–2655. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.Y.; Liu, B.H.; Sieber, M.; Hsu, S.H. Substrate-dependent gene regulation of self-assembled human MSC spheroids on chitosan membranes. BMC Genom. 2014, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Custodio, C.A.; Alves, C.M.; Reis, R.L.; Mano, J.F. Immobilization of fibronectin in chitosan substrates improves cell adhesion and proliferation. J. Tissue Eng. Regen. Med. 2010, 4, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Cuy, J.L.; Beckstead, B.L.; Brown, C.D.; Hoffman, A.S.; Giachelli, C.M. Adhesive protein interactions with chitosan: Consequences for valve endothelial cell growth on tissue-engineering materials. J. Biomed. Mater. Res. Part. A 2003, 67A, 538–547. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisi, L.; Toffoli, A.; Bianchi, M.G.; Bergonzi, C.; Bianchera, A.; Bettini, R.; Elviri, L.; Macaluso, G.M. Functional Fibronectin Adsorption on Aptamer-Doped Chitosan Modulates Cell Morphology by Integrin-Mediated Pathway. Materials 2019, 12, 812. https://doi.org/10.3390/ma12050812
Parisi L, Toffoli A, Bianchi MG, Bergonzi C, Bianchera A, Bettini R, Elviri L, Macaluso GM. Functional Fibronectin Adsorption on Aptamer-Doped Chitosan Modulates Cell Morphology by Integrin-Mediated Pathway. Materials. 2019; 12(5):812. https://doi.org/10.3390/ma12050812
Chicago/Turabian StyleParisi, Ludovica, Andrea Toffoli, Massimiliano G. Bianchi, Carlo Bergonzi, Annalisa Bianchera, Ruggero Bettini, Lisa Elviri, and Guido M. Macaluso. 2019. "Functional Fibronectin Adsorption on Aptamer-Doped Chitosan Modulates Cell Morphology by Integrin-Mediated Pathway" Materials 12, no. 5: 812. https://doi.org/10.3390/ma12050812