Effective Medium Method for Chloride Diffusion Coefficient of Mature Fly Ash Cement Paste
Abstract
:1. Introduction
2. Numerical Estimate for Degrees of Hydration of Cement and Fly Ash
2.1. Degree of Hydration of Cement
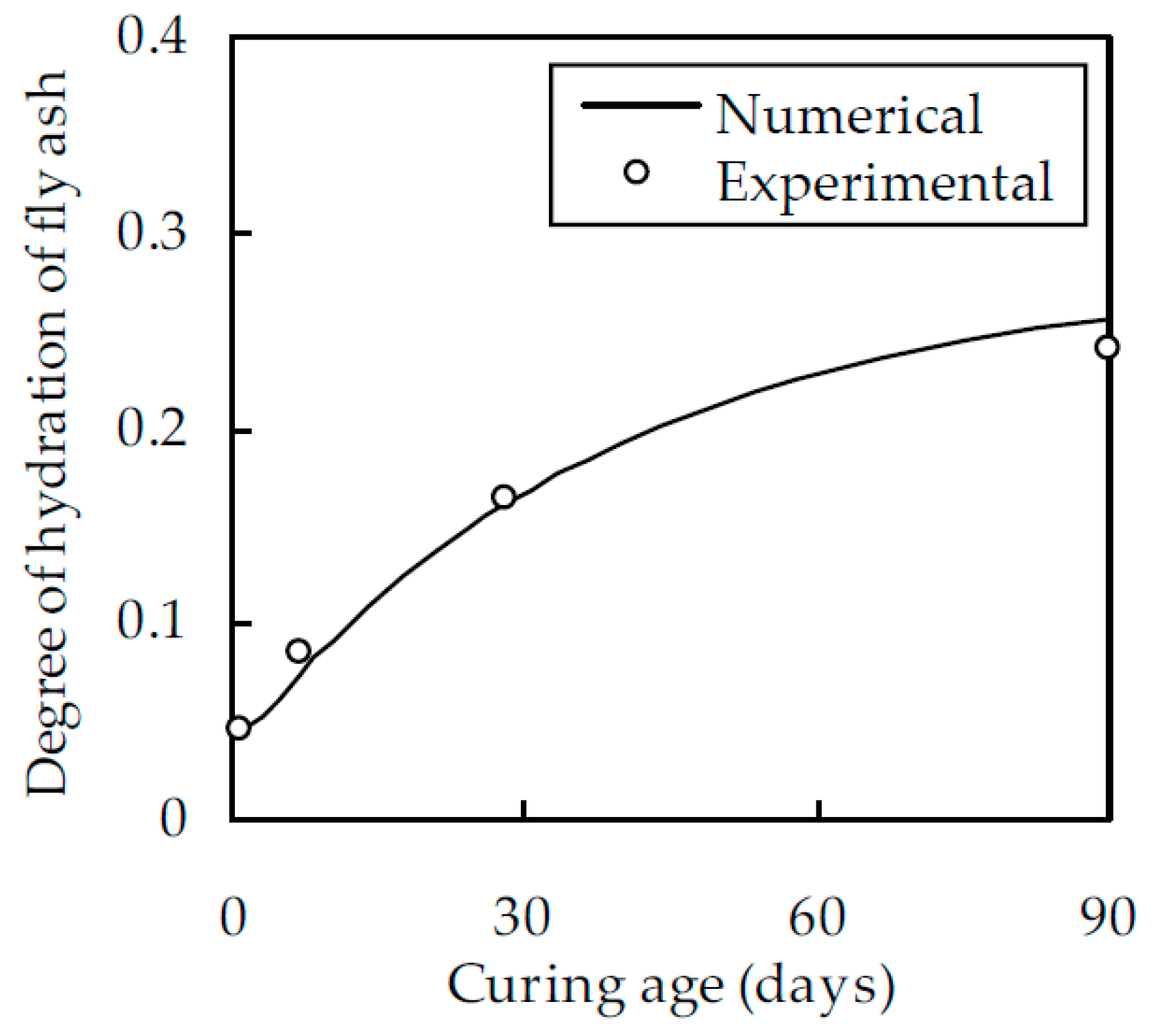
2.2. Degree of Hydration of Fly Ash
2.3. Experimental Verification
3. EMM for Chloride Diffusion Coefficient of Fly Ash Cement Paste
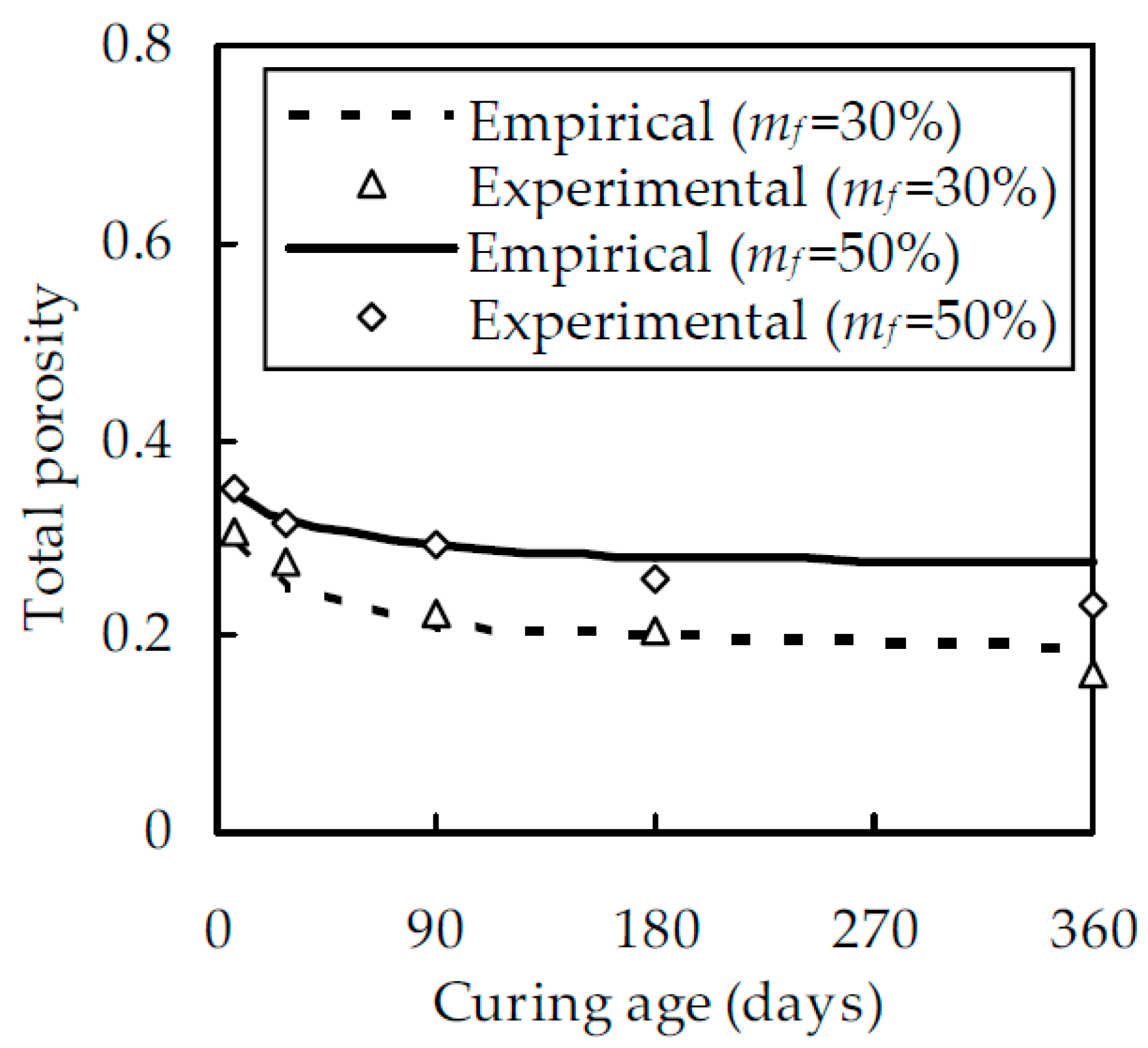
3.1. Volume Fractions of Solid Phase and Pore Space
3.2. Chloride Diffusion Coefficient of Fly Ash Cement Paste
4. Experimental Verification and Discussions
4.1. Materials, Methods, and Results
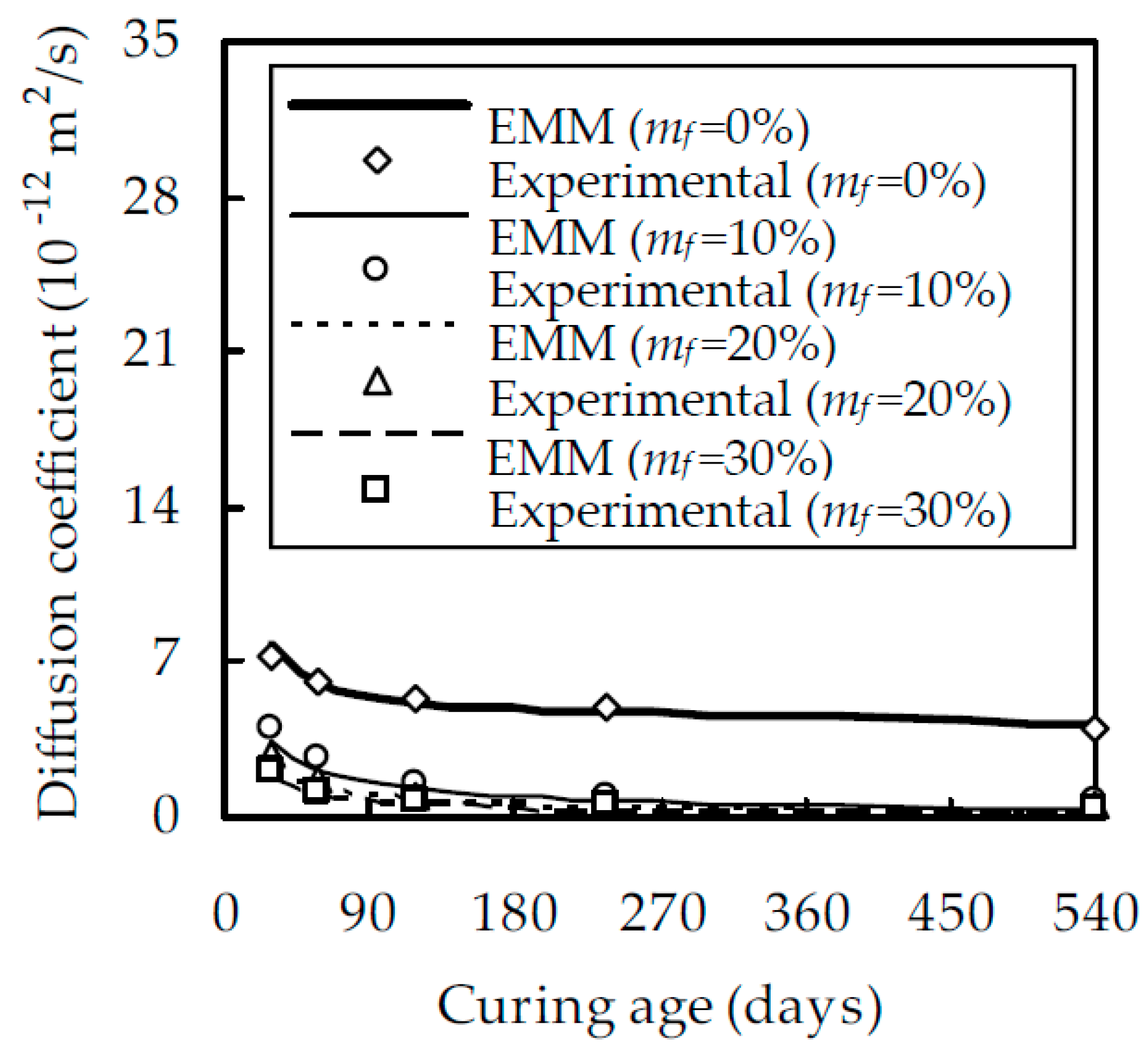
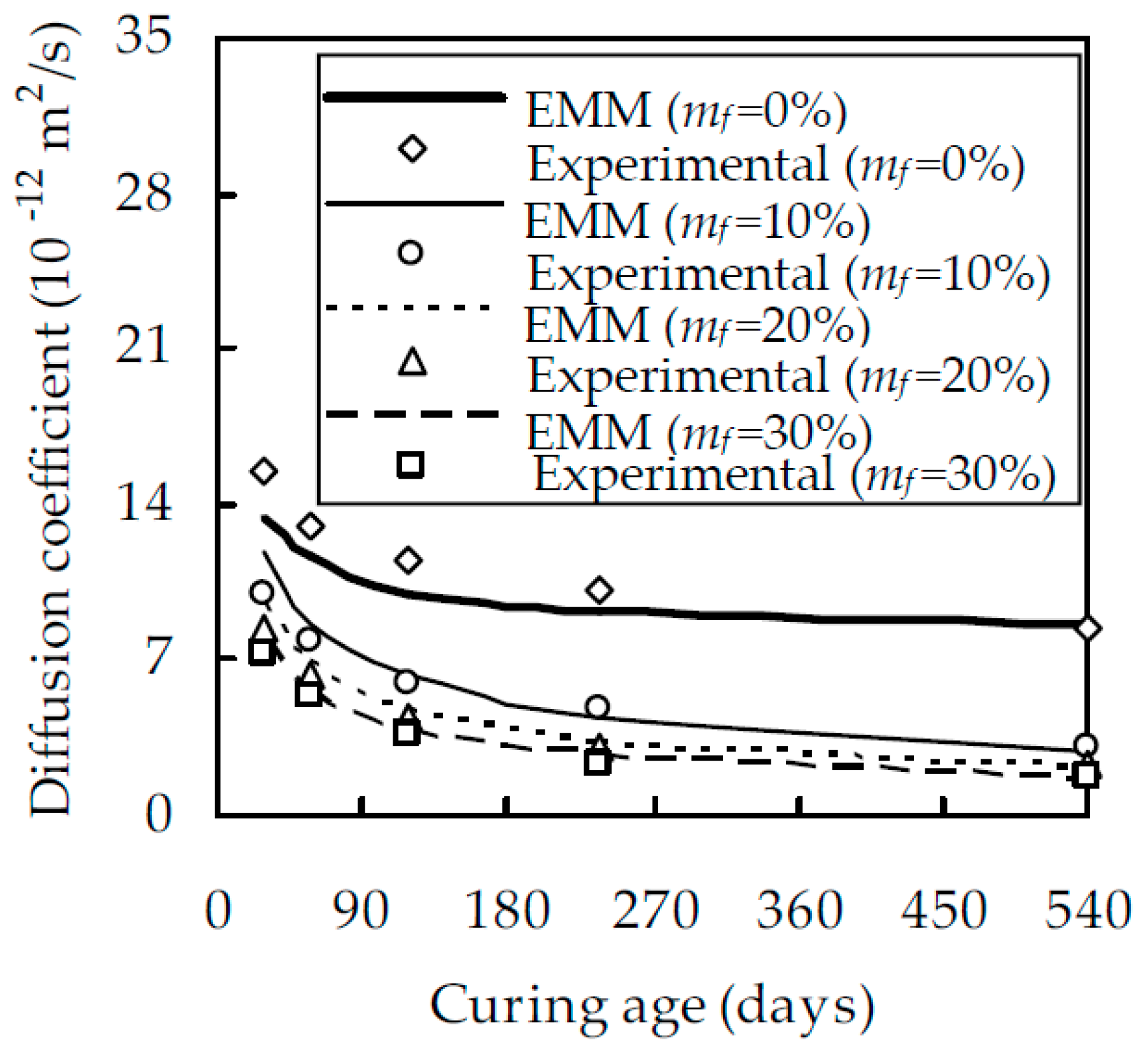
4.2. Experimental Verification
5. Conclusions
- Fly ash cement paste was modeled as a two-phase material, and the volume fraction of each phase constituent was formulated in terms of the water/binder ratio and the numerically estimated degrees of hydration of cement and fly ash.
- By considering the percolation characteristics close to the percolation threshold, the percolation theory was incorporated into the EMM and the chloride diffusion coefficient of fly ash cement paste was derived and verified with experimental results.
- The experimental results show that, when w/b was equal to 0.5 and t was 28 and 540 days, Dfc at mf = 30% was smaller than that at mf = 0% by 55.7% and 90.0%, respectively. When w/b was equal to 0.5 and mf was 0% and 30%, Dfc at 540 days was smaller than that at 28 days by 43.1% and 86.2%, respectively. When t was 540 days and w/b decreased from 0.6 to 0.4 Dfc decreased by 32.4% and 81.7% for mf = 0% and 30%, respectively.
Author Contributions
Funding
Conflicts of Interest
References
- Buenfeld, N.R.; Glass, G.K.; Hassanein, A.M.; Zhang, J.Z. Chloride transport in concrete subjected to electric field. J. Mater. Civ. Eng. 1998, 10, 220–228. [Google Scholar] [CrossRef]
- Cady, P.D.; Weyers, R.E. Deterioration rates of bridge concrete decks. J. Transp. Eng. 1984, 110, 34–44. [Google Scholar] [CrossRef]
- Liam, K.C.; Roy, S.K.; Northwood, D.O. Chloride ingress measurements and corrosion potential mapping study of a 24-year-old reinforced-concrete jetty structure in a tropical marine environment. Mag. Concr. Res. 1992, 44, 205–215. [Google Scholar] [CrossRef]
- MacDonald, K.A.; Northwood, D.O. Experimental measurements of chloride ion diffusion rates using a two-compartment diffusion cell: Effects of material and test variables. Cem. Concr. Res. 1995, 25, 1407–1416. [Google Scholar] [CrossRef]
- De Weerdt, K.; Colombo, A.; Coppola, L.; Justnes, H.; Geiker, M.R. Impact of associated cation on chloride binding of Portland cement paste. Cem. Concr. Res. 2015, 68, 196–202. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Shehata, M.H.; Shashiprakash, S.G.; Hopkins, D.S.; Cail, K. Use of ternary cementitious systems containing silica fume and fly ash in concrete. Cem. Concr. Res. 1999, 29, 1207–1214. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jaturapitakkul, C.; Sinsiri, T. Effect of fly ash fineness on compressive strength and pore size of blended cement paste. Cem. Concr. Compos. 2005, 27, 425–428. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E. Plain and ultrafine fly ashes mortars for environmentally friendly construction materials. Sustainability 2018, 10, 874. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E. Rheological and physical performances of mortars manufactured with plain and ultrafine fly ashes. ACI Spec. Publ. 2018, 326, 32. [Google Scholar]
- Zheng, J.J.; Zhou, X.Z. Three-phase composite sphere model for the prediction of chloride diffusion coefficient of concrete. J. Mater. Civ. Eng. 2008, 20, 205–211. [Google Scholar] [CrossRef]
- Page, C.L.; Short, N.R.; Tarra, A.E. Diffusion of chloride ions in hardened cement pastes. Cem. Concr. Res. 1981, 11, 395–406. [Google Scholar] [CrossRef]
- Castellote, M.; Alonso, C.; Andrade, C.; Chadbourn, G.A.; Page, C.L. Oxygen and chloride diffusion in cement pastes as a validation of chloride diffusion coefficients obtained by steady-state migration tests. Cem. Concr. Res. 2001, 31, 621–625. [Google Scholar] [CrossRef]
- Tang, L.P.; Nilsson, L.O. Rapid determination of chloride diffusivity in concrete by applying an electrical field. ACI Mater. J. 1992, 89, 49–53. [Google Scholar]
- Lu, X.Y. Application of the Nernst-Einstein equation to concrete. Cem. Concr. Res. 1997, 27, 293–302. [Google Scholar] [CrossRef]
- Ampadu, K.O.; Torii, K.; Kawamura, M. Beneficial effect of fly ash on chloride diffusivity of hardened cement paste. Cem. Concr. Res. 1999, 29, 585–590. [Google Scholar] [CrossRef]
- Ngala, V.T.; Page, C.T. Effects of carbonation on pore structure and diffusional properties of hydrated cement pastes. Cem. Concr. Res. 1997, 27, 995–1007. [Google Scholar] [CrossRef]
- Chalabi, H.; Khelidj, A.; Bezzar, A. Chloride transport in partially saturated cementitious material: Influence of hydric state and binding chloride. Mag. Concr. Res. 2017, 69, 1103–1114. [Google Scholar] [CrossRef]
- Ramirez-Ortiz, A.E.; Castellanos, F.; Cano-Barrita, P.F.D. Ultrasonic detection of chloride ions and chloride binding in Portland cement pastes. Int. J. Concr. Struct. Mater. 2018, 12, UNSP 20. [Google Scholar] [CrossRef]
- Akhavan, A.; Rajabipour, F. Evaluating ion diffusivity of cracked cement paste using electrical impedance spectroscopy. Mater. Struct. 2013, 46, 697–708. [Google Scholar] [CrossRef]
- Millar, S.; Kruschwitz, S.; Wilsch, G. Determination of total chloride content in cement pastes with laser-induced breakdown spectroscopy (LISB). Cem. Concr. Res. 2019, 117, 16–22. [Google Scholar] [CrossRef]
- Pivonka, P.; Hellmich, C.; Smith, D. Microscopic effects on chloride diffusivity of cement pastes—A scale-transition analysis. Cem. Concr. Res. 2004, 34, 2251–2260. [Google Scholar] [CrossRef]
- Zheng, J.J.; Zhou, X.Z. Analytical solution for the chloride diffusivity of hardened cement paste. J. Mater. Civ. Eng. 2008, 20, 384–391. [Google Scholar] [CrossRef]
- Garboczi, E.J.; Bentz, D.P. Computer simulation of the diffusivity of cement-based materials. J. Mater. Sci. 1992, 27, 2083–2092. [Google Scholar] [CrossRef]
- Masi, M.; Colella, D.; Radaelli, G.; Bertolini, L. Simulation of chloride penetration in cement-based materials. Cem. Concr. Res. 1997, 27, 1591–1601. [Google Scholar] [CrossRef]
- Liu, L.; Sun, W.; Ye, G.; Chen, H.S.; Qian, Z.W. Estimation of the ionic diffusivity of virtual cement paste by random walk algorithm. Constr. Build. Mater. 2012, 28, 405–413. [Google Scholar] [CrossRef]
- Ma, H.Y.; Hou, D.S.; Li, Z.J. Two-scale modeling of transport properties of cement paste: Formation factor, electrical conductivity and chloride diffusivity. Comput. Mater. Sci. 2015, 110, 270–280. [Google Scholar] [CrossRef]
- Du, X.L.; Jin, L.; Zhang, R.B. Chloride diffusivity in saturated cement paste subjected to external mechanical loading. Ocean Eng. 2015, 95, 1–10. [Google Scholar] [CrossRef]
- Yang, Y.K.; Patel, R.A.; Churakov, S.V.; Prasianakis, N.I.; Kosakowshi, G.; Wang, M.R. Multiscale modeling of ion diffusion in cement paste: Electrical double layer effect. Cem. Concr. Compos. 2019, 96, 55–65. [Google Scholar] [CrossRef]
- Carrara, P.; De Lorenzis, L.; Wu, T. Chloride diffusivity of hardened cement paste from multiscale modeling. Spec. Publ. 2015, 305, 6. [Google Scholar]
- Damrongwiriyanupap, N.; Scheiner, S.; Pichler, B.; Hellmich, C. Self-consistent channel approach for upscaling chloride diffusivity in cement pastes. Transp. Porous Med. 2017, 118, 495–518. [Google Scholar] [CrossRef]
- Parrot, L.J.; Killoh, D.C. Prediction of cement hydration. Br. Ceram. Proc. 1984, 35, 41–53. [Google Scholar]
- Wang, X.Y.; Lee, H.S. Modeling the hydration of concrete incorporating fly ash or slag. Cem. Concr. Res. 2010, 40, 984–996. [Google Scholar] [CrossRef]
- Papadakis, V.G. Effect of fly ash on Portland cement systems: Part I. low-calcium fly ash. Cem. Concr. Res. 1999, 30, 1647–1654. [Google Scholar] [CrossRef]
- Haha, M.B.; De Weerdt, K.; Lothenbach, B. Quantification of the degree of reaction of fly ash. Cem. Concr. Res. 2010, 40, 1620–1629. [Google Scholar] [CrossRef]
- Hansen, T.C. Physical structure of hardened cement paste: A classical approach. Mater. Struct. 1986, 19, 423–436. [Google Scholar] [CrossRef]
- Yu, Z.Q. Microstructure Development and Transport Properties of Portland Cement-Fly Ash Binary Systems: In View of Service Life Predictions. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2015. [Google Scholar]
- Torquato, S. Random Heterogenerous Materials: Microstructure and Macroscopic Properties; Springer: New York, USA, 2001. [Google Scholar]
- Cai, W.Z.; Tu, S.T.; Gong, J.M. A physically based percolation model of the effective electrical conductivity of particle filled composites. J. Compos. Mater. 2006, 40, 2131–2142. [Google Scholar] [CrossRef]
- Oh, B.H.; Jang, S.Y. Prediction of diffusivity of concrete based on simple analytic equations. Cem. Concr. Res. 2004, 34, 463–480. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.D.; Qiu, Q.W.; Ou, G.F.; Xing, F. Understanding the effect of curing age on the chloride resistance of fly ash blended concrete by rapid chloride migration test. Mater. Chem. Phys. 2017, 196, 315–323. [Google Scholar] [CrossRef]
- Zheng, J.J.; Zhou, X.Z.; Wu, Z.M. A simple method for predicting the chloride diffusivity of cement paste. Mater. Struct. 2010, 43, 99–106. [Google Scholar] [CrossRef]
- Garboczi, E.J.; Snyder, K.A.; Douglas, J.F. Geometrical percolation threshold of overlapping ellipsoids. Phys. Rev. E 1995, 52, 819–828. [Google Scholar] [CrossRef]
- Atkinson, A.; Nickerson, A.K. The diffusion of ions through water-saturated cement. J. Mater. Sci. 1984, 19, 3068–3078. [Google Scholar] [CrossRef]
- Nan, C.W. Physics of inhomogeneous inorganic materials. Prog. Mater. Sci. 1993, 37, 1–116. [Google Scholar] [CrossRef]
- Wong, P.A.; Koplik, J.; Tomanic, J.P. Conductivity and permeability of rocks. Phys. Rev. B 1984, 30, 6606–6614. [Google Scholar] [CrossRef]
- Archie, G.E. The electrical resistivity log as an aid in determining some reservoir characteristics. Well Logging Technol. 1942, 146, 54–62. [Google Scholar] [CrossRef]

| Kinetic Parameter | Clinker Phase | |||
|---|---|---|---|---|
| C3S | C2S | C3A | C4AF | |
| K1 | 1.5 | 0.5 | 1.0 | 0.37 |
| N1 | 0.7 | 1.0 | 0.85 | 0.7 |
| K2 | 3.3 | 5.0 | 3.2 | 3.7 |
| K3 | 0.05 | 0.006 | 0.04 | 0.015 |
| N3 | 1.1 | 0.2 | 1.0 | 0.4 |
| Bf (cm/h) | Cf (cm/(cm4·h)) | krf (cm/h) | Def0 (cm2/h) |
|---|---|---|---|
| 2.51 × 10−9 | 1.00 × 1015 | 1.71 × 10−6 | 8.58 × 10−8 |
| Material | CaO (%) | SiO2 (%) | Al2O3 (%) | Fe2O3 (%) | MgO (%) | SO3 (%) | K2O (%) | Na2O (%) | Loss of Ignition (%) |
|---|---|---|---|---|---|---|---|---|---|
| Fly ash | 5.40 | 47.00 | 31.30 | 4.40 | 0.49 | 0.77 | 0.90 | 0.78 | 3.22 |
| Cement | 64.40 | 20.36 | 4.96 | 3.17 | 2.09 | 1.98 | 0.64 | 0.14 | 1.27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Zhou, X.-Z.; Zhang, J.; Zheng, J.-J. Effective Medium Method for Chloride Diffusion Coefficient of Mature Fly Ash Cement Paste. Materials 2019, 12, 811. https://doi.org/10.3390/ma12050811
Zhou H, Zhou X-Z, Zhang J, Zheng J-J. Effective Medium Method for Chloride Diffusion Coefficient of Mature Fly Ash Cement Paste. Materials. 2019; 12(5):811. https://doi.org/10.3390/ma12050811
Chicago/Turabian StyleZhou, Hong, Xin-Zhu Zhou, Jian Zhang, and Jian-Jun Zheng. 2019. "Effective Medium Method for Chloride Diffusion Coefficient of Mature Fly Ash Cement Paste" Materials 12, no. 5: 811. https://doi.org/10.3390/ma12050811




