Transient Liquid Phase Bonding of Ti-6Al-4V and Mg-AZ31 Alloys Using Zn Coatings
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Zn Coatings Using the Screen Printing Technique
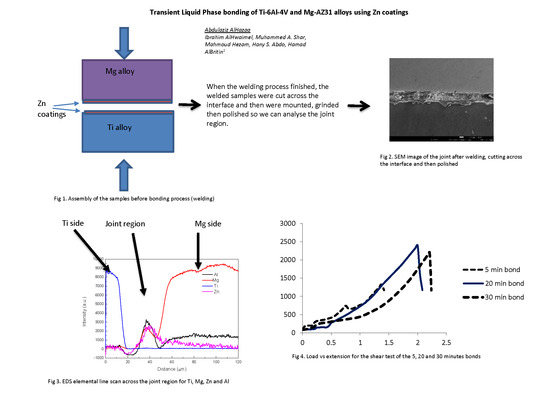
2.2. Transient Liquid Phase Bonding
3. Results and Discussions
3.1. Evolution of the Interfacial Layer
3.2. Morphology and Composition of the Joint Region
4. Analysis of the Fractured Surfaces
5. Shear Strength and Micro-Hardness Measurements
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Joost, W.J.; Krajewski, P.E. Towards magnesium alloys for high-volume automotive applications. Scr. Mater. 2017, 128, 107–112. [Google Scholar] [CrossRef]
- Kulekci, M.K. Magnesium and its alloys applications in automotive industry. Int. J. Adv. Manuf. Technol. 2008, 39, 851–865. [Google Scholar] [CrossRef]
- Sachdev, A.K.; Kulkarni, K.; Fang, Z.Z.; Yang, R.; Girshov, V. Titanium for Automotive Applications: Challenges and Opportunities in Materials and Processing. JOM 2012, 64, 553–565. [Google Scholar] [CrossRef]
- Murray, J.L. The Mg-Ti (Magnesium-Titanium) System. Bull. Alloy Phase Diagr. 1986, 7, 245–248. [Google Scholar] [CrossRef]
- Cook, G.O., III; Sorensen, C.D. Overview of transient liquid phase and partial transient liquid phase bonding. J. Mater. Sci. 2011, 46, 5305–5323. [Google Scholar] [CrossRef] [Green Version]
- Tuah-Poku, I.; Dollar, M.; Massalski, T.B. A Study of the Transient Liquid Phase Bonding Process Applied to a Ag/Cu/Ag Sandwich Joint. Metall. Trans. A 1988, 19A, 675. [Google Scholar] [CrossRef]
- Illingworth, T.C.; Golosnoy, I.O.; Clyne, T.W. Modelling of transient liquid phase bonding in binary systems—A new parametric study. Mater. Sci. Eng. A 2007, 445–446, 493–500. [Google Scholar] [CrossRef]
- Azqadan, E.; Ekrami, A. Transient liquid phase bonding of dual phase steels using Fe-based, Ni-based, and pure Cu interlayers. J. Manuf. Process. 2017, 30, 106–115. [Google Scholar] [CrossRef]
- Assadi, H.; Shrzadi, A.A.; Wallach, E.R. Transient Liquid Phase Diffusion Bonding under a temperature gradient: Modeling of the interface morphology. Acta Mater. 2011, 49, 31–39. [Google Scholar] [CrossRef]
- Cooke, K. Diffusion Bonding and Characterization of a Dispersion Strengthen Aluminum Alloy. Ph.D. Thesis, University of Calgary, Calgary, AB, Canada, 2011. [Google Scholar]
- Jin, Y.J.; Khan, T.I. Effect of bonding time on microstructure and mechanicalproperties of transient liquid phase bonded magnesium AZ31 alloy. Mater. Des. 2012, 38, 32–37. [Google Scholar] [CrossRef]
- AlHazaa, A.; Khan, T.I.; Haq, I. Transient liquid phase (TLP) bonding of Al7075 to Ti–6Al–4V alloy. Mater. Charact. 2010, 61, 312–317. [Google Scholar] [CrossRef]
- Alhazaa, A.N.; Khan, T.I. Diffusion bonding of Al7075 to Ti–6Al–4V using Cu coatings and Sn–3.6Ag–1Cu interlayers. J. Alloys Compd. 2010, 494, 351–358. [Google Scholar] [CrossRef]
- Elthalabawy, W.; Khan, T.I. Eutectic bonding of austenitic stainless steel 316L to magnesium alloy AZ31 using copper interlayer. Int. J. Adv. Manuf. Technol. 2011, 55, 235–241. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, G.; Wang, Y.; Shen, Q.; Zhang, L. An investigation on diffusion bonding of aluminum and magnesium using a Ni interlayer. Mater. Lett. 2012, 83, 189–191. [Google Scholar] [CrossRef]
- Atieh, A.M.; Khan, T.I. TLP bonding of Mg AZ31 to Ti-6Al-4V using pure Ni electro-deposited coats. J. Mater. Process. Technol. 2014, 214, 3158–3168. [Google Scholar] [CrossRef]
- AlHazaa, A.N. Effect of bonding temperature on the microstructure and strength of the joint between magnesium AZ31 and Ti-6Al-4V alloys using Cu coatings and Sn interlayers. Key Eng. Mater. 2017, 735, 34–41. [Google Scholar] [CrossRef]
- Pripanapong, P.; Umeda, J.; Imai, H.; Takahashi, M.; Kondoh, K. Tensile Strength of Ti/Mg Alloys Dissimilar Bonding Material Fabricated by Spark Plasma Sintering. Int. J. Eng. Innov. Res. 2016, 5, 2277–5668. [Google Scholar]
- Murray, J.L. The Titanium-Zinc system. Bull. Alloy Phase Diagr. 1984, 5, 52–56. [Google Scholar] [CrossRef]
- Cao, X.; Jahazi, M.; Immarigeon, J.P.; Wallace, W. A review of laser welding techniques for magnesium, alloy. J. Mater. Process. Technol. 2006, 171, 188–204. [Google Scholar] [CrossRef]
- Bermudez, B.; Sohn, K. Diffusion Couple Investigation of the Mg-Zn System. In Magnesium Technology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 323–327. [Google Scholar]
- Kammerer, C.; Kulkami, N.; Warmack, R.; Belova, K.P.I.; Murch, G.; Sohn, Y. Impurity Diffusion Coefficients of Al and Zn in Mg determined from solid-to-solid diffusion couples. In Magnesium Technology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 505–509. [Google Scholar]
- Zhao, L.M.; Zhang, Z.D. Effect of Zn alloy interlayer on interface microstructure and strength of diffusion-bonded Mg–Al joints. Scr. Mater. 2008, 58, 283–286. [Google Scholar] [CrossRef]
- Vassilev, G.P.; Liu, X.J.; Ishida, K. Reaction kinetics and phase diagram studies in the Ti–Zn system. J. Alloy. Compd. 2004, 375, 162–170. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.T.; Li, D.W.; Long, Y.T. Recent developments and applications of screen-printed electrodes in environmental assays—A review. Anal. Chim. Acta 2012, 734, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Miriyev, A.; Levy, A.; Kalabukhov, S.; Frage, N. Interface evolution and shear strength of Al/Ti bi-metals processed by a spark plasma sintering (SPS) apparatus. J. Alloy. Compd. 2016, 678, 329–336. [Google Scholar] [CrossRef]
- Xu, L.; Cui, Y.Y.; Hao, Y.L.; Yang, R. Growth of intermetallic layer in multilaminated Ti/Al diffusion couples. Mater. Sci. Eng. A 2016, 435, 638–647. [Google Scholar]
- Materials Science International Team MSIT. Al-Mg-Zn (Aluminium—Magnesium—Zinc). In Light Metal Systems. Part 3. Landolt-Börnstein—Group IV Physical Chemistry (Numerical Data and Functional Relationships in Science and Technology); Effenberg, G., Ilyenko, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; Volume 11A3. [Google Scholar]











| Region | Mg (wt%/at%) | Zn (wt%/at%) | Ti (wt%/at%) | Al (wt%/at%) |
|---|---|---|---|---|
| A1 (eutectic) | 46.9/67.1 | 47.0/25.0 | 0 | 6.1/7.9 |
| A2 (white) | 39.9/60.8 | 53.7/30.4 | 0 | 6.4/8.8 |
| A3 (dark) | 87.1/93.5 | 10.5/4.2 | 0 | 2.4/2.3 |
| B1 (white) | 0.9/1.8 | 1.5/1.1 | 96.6/96.1 | 1.1/1.8 |
| B2 (dark) | 61.4/72.7 | 16.6/7.3 | 7.7/4.6 | 14.4/15.3 |
| Region | Mg (wt%/at%) | Zn (wt%/at%) | Ti (wt%/at%) | Al (wt%/at%) |
|---|---|---|---|---|
| C1 (white) | 65.5/81.3 | 30.1/13.9 | 0 | 4.3/4.9 |
| C2 (dark) | 88.0/92.4 | 6.8/2.6 | 0 | 5.2/4.9 |
| D1 (white) | 2.1/4.2 | 13.6/10.1 | 84.1/85.3 | 0.2/0.4 |
| D2 (dark) | 72.6/81.7 | 4.2/3.1 | 13.7/8.3 | 3.9/4.5 |
| Region | Mg (wt%/at%) | Zn (wt%/at%) | Ti (wt%/at%) | Al (wt%/at%) |
|---|---|---|---|---|
| E1 (white) | 62.8/76.9 | 27.4/12.5 | 0.3/0.2 | 9.5/10.5 |
| E2 (dark) | 85.3/89.0 | 5.2/2.0 | 0 | 9.5/8.9 |
| F1 (white) | 2.8/5.4 | 5.7/4.1 | 91.4/90.2 | 0.2/0.3 |
| F2 (dark) | 78.8/87.7 | 3.9/1.6 | 15.3/8.6 | 2.1/2.1 |
| Sample | 5 min | 10 min | 15 min | 20 min | 25 min | 30 min |
|---|---|---|---|---|---|---|
| Force (N) | 1324 | 1513 | 1954 | 2396 | 2253 | 2197.6 |
| Strength (MPa) | 16.8 | 19.2 | 24.8 | 30.5 | 28.7 | 28.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlHazaa, A.; Alhoweml, I.; Shar, M.A.; Hezam, M.; Abdo, H.S.; AlBrithen, H. Transient Liquid Phase Bonding of Ti-6Al-4V and Mg-AZ31 Alloys Using Zn Coatings. Materials 2019, 12, 769. https://doi.org/10.3390/ma12050769
AlHazaa A, Alhoweml I, Shar MA, Hezam M, Abdo HS, AlBrithen H. Transient Liquid Phase Bonding of Ti-6Al-4V and Mg-AZ31 Alloys Using Zn Coatings. Materials. 2019; 12(5):769. https://doi.org/10.3390/ma12050769
Chicago/Turabian StyleAlHazaa, Abdulaziz, Ibrahim Alhoweml, Muhammad Ali Shar, Mahmoud Hezam, Hany Sayed Abdo, and Hamad AlBrithen. 2019. "Transient Liquid Phase Bonding of Ti-6Al-4V and Mg-AZ31 Alloys Using Zn Coatings" Materials 12, no. 5: 769. https://doi.org/10.3390/ma12050769