Novel Bi-Functional 14-mer Peptides with Both Ovarian Carcinoma Cells Targeting and Magnetic Fe3O4 Nanoparticles Affinity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Cell Culture
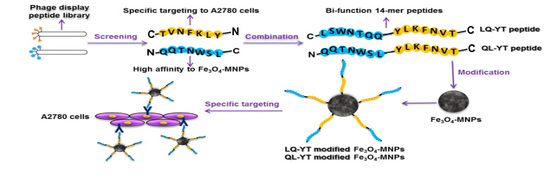
2.2. Design, Prediction, and Characterization of Bi-Functional Peptides
2.3. Characterization of Fe3O4-MNPs
2.4. Modification and Characterization of Fe3O4-MNPs
2.5. Identification of Cellular Affinity
2.6. MTT Assay and Scratch Wound Migration Assay
2.7. Statistical Analysis
3. Results
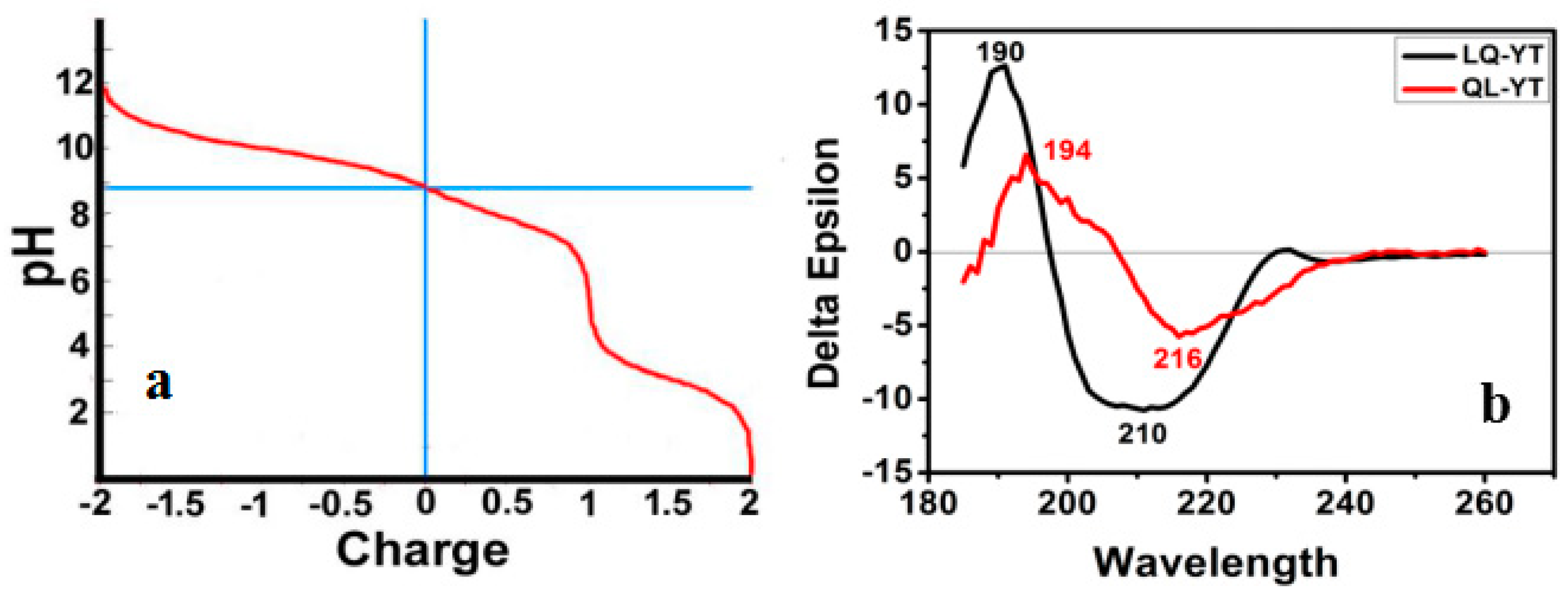
3.1. Composition and Secondary Structure of Synthesized 14-mer Peptides
3.2. Affinity of Synthesized 14-mer Peptides to Fe3O4-MNPs
3.3. Properties and Performances of Modified Fe3O4-MNPs
3.4. Specificity of Modified Fe3O4-MNPs Bound to A2780 Cells
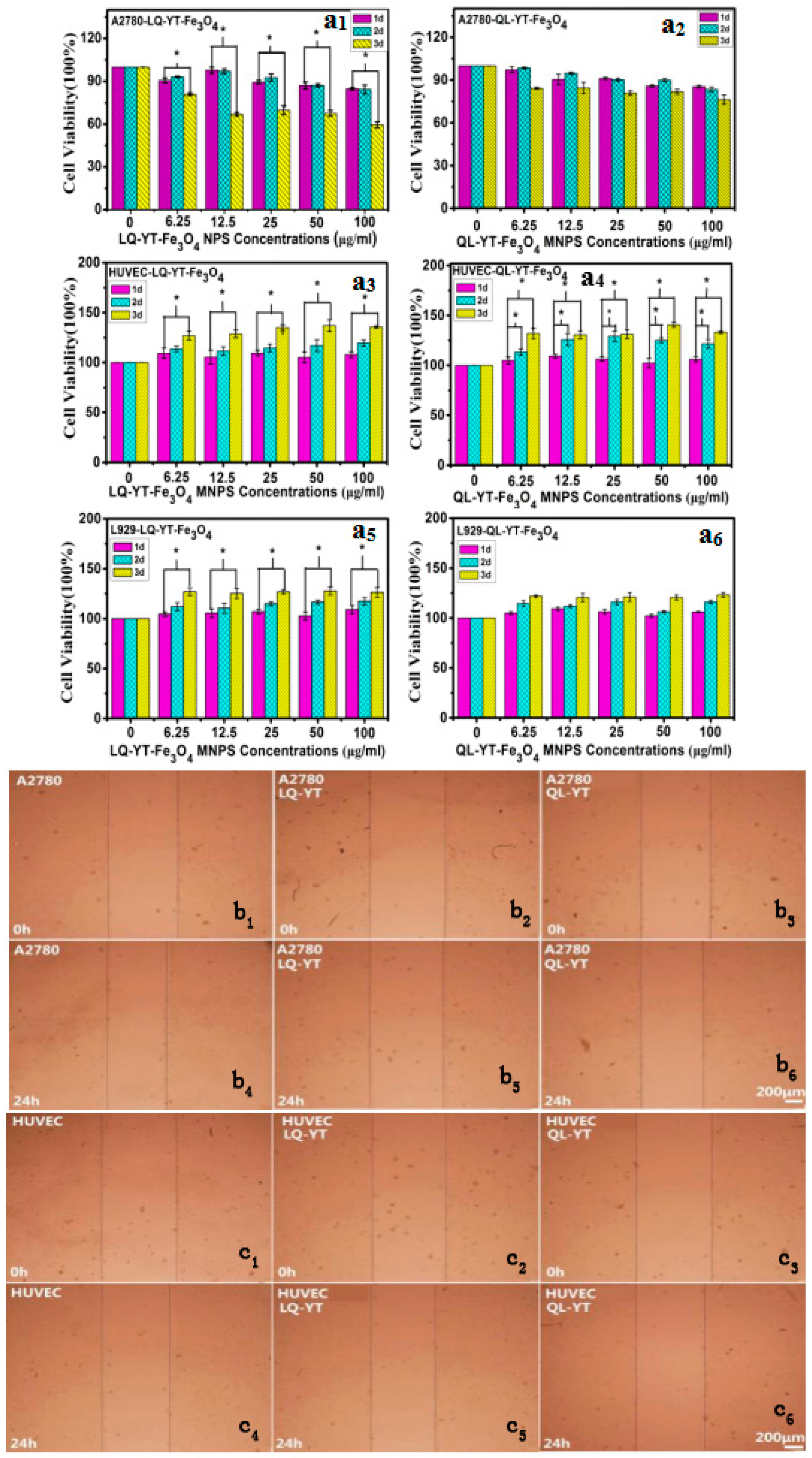
3.5. Cytotoxicity of Polypeptide-Modified Fe3O4-MNPs
3.6. Discussion on Tumor Targeting of Related 14-mer Peptides
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Coburn, S.B.; Bray, F.; Sherman, M.E.; Trabert, B. International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. Int. J. Cancer 2017, 140, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Ray, C.M.; Spira, A.I.; Trepel, J.B.; Carter, C.A.; Cottrill, H.M. A brief review of the management of platinum-resistant-platinum-refractory ovarian cancer. Med. Oncol. 2017, 34. [Google Scholar] [CrossRef] [PubMed]
- Opipari, A.W.; Tan, L.; Boitano, A.E.; Sorenson, D.R.; Aurora, A.; Liu, J.R. Resveratrol-induced Autophagocytosis in Ovarian Cancer Cells. Cancer Res. 2004, 64, 696–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guppy, A.E.; Nathan, P.D.; Rustin, G.J. Epithelial ovarian cancer: A review of current management. Clin. Oncol. 2005, 17, 399–411. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Coleman, R.L.; Markman, M. Ovarian cancer. Lancet 2009, 374, 1371–1382. [Google Scholar] [CrossRef]
- Long, J.; Yang, Y.; Kang, T.; Zhao, W.; Cheng, H.; Wu, Y.; Du, T.; Liu, B.; Li, Y.; Luo, F.; et al. Ovarian cancer therapy by VSVMP gene mediated by a paclitaxel-enhanced nanoparticle. ACS Appl. Mater. Interfaces 2017, 9, 39152–39164. [Google Scholar] [CrossRef] [PubMed]
- Armijo, L.M.; Brandt, Y.I.; Mathew, D.; Yadav, S.; Maestas, S.; Rivera, A.C.; Cook, N.C.; Withers, N.J.; Smolyakov, G.A.; Adolphi, N.; et al. Iron Oxide Nanocrystals for Magnetic Hyperthermia Applications. Nanomaterials 2012, 2, 134–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobson, J. Magnetic nanoparticles for drug delivery. Drug Dev. Res. 2010, 67, 55–60. [Google Scholar] [CrossRef]
- Serantes, D.; Simeonidis, K.; Angelakeris, M.; Chubykalofesenko, O.; Marciello, M.; Morales, M.D.P.; Baldomir, D.; Martinezboubeta, C. Multiplying Magnetic Hyperthermia Response by Nanoparticle Assembling. J. Phys. Chem. C 2014, 118, 5927–5934. [Google Scholar] [CrossRef] [Green Version]
- Sharifi, I.; Shokrollahi, H.; Amiri, S. Ferrite-based magnetic nanofluids used in hyperthermia applications. J. Magn. Magn. Mater. 2012, 324, 903–915. [Google Scholar] [CrossRef]
- Wagner, S.; Schnorr, J.; Ludwig, A.; Stangl, V.; Ebert, M.; Hamm, B.; Taupitz, M. Contrast-enhanced MR imaging of atherosclerosis using citrate-coated superparamagnetic iron oxide nanoparticles: Calcifying microvesicles as imaging target for plaque characterization. Int. J. Nanomed. 2013, 8, 767–779. [Google Scholar]
- Balivada, S.; Rachakatla, R.S.; Wang, H.; Samarakoon, T.N.; Dani, R.K.; Pyle, M.; Kroh, F.O.; Walker, B.; Leaym, X.; Koper, O.B.; et al. A/C magnetic hyperthermia of melanoma mediated by iron(0)/iron oxide core/shell magnetic nanoparticles: A mouse study. BMC Cancer 2010, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Crowford, S. Nanotechnology Introduction Series: The Energy Sector; HA Hessen Agentur GmbH: Wiesbaden, Germany, 2011. [Google Scholar]
- Mou, Y.; Yang, H.; Xu, Z.L. Morphology, surface layer evolution and structure-dye adsorption relationship of porous Fe3O4 MNPs prepared by solvothermal/gas generation process. ACS Sustain. Chem. Eng. 2017, 5, 2339–2349. [Google Scholar] [CrossRef]
- Gullotti, E.; Yeo, Y. Extracellularly activated nanocarriers: A new paradigm of tumor targeted drug delivery. Mol. Pharm. 2009, 6, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.L.; Gillies, R.J. Molecular imaging and targeted therapies. Biochem. Pharmacol. 2010, 80, 731–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutter, J.P.; Kahn, M.L. Magnetism: Molecules to Materials: Models and Experiments. Magn. Mol. Mater. V 1999, 36, 161–187. [Google Scholar]
- Chang, D.K.; Lin, C.T.; Wu, C.H.; Wu, H.C. A novel peptide enhances therapeutic efficacy of liposomal anti-cancer drugs in mice models of human lung cancer. PLoS ONE 2009, 4, e4171. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ma, H.; Yang, P.; Zhang, X.; Wu, X.; Yin, W.; Wang, H.; Xu, D. Targeted polymer-drug conjugates: Current progress and future perspective. Colloids Surf. B Biointerfaces 2015, 136, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Akita, N.; Maruta FSeymour, L.W.; Kerr, D.J.; Parker, A.L.; Asai, T.; Oku, N.; Nakayama, J.; Miyagawa, S. Identification of oligopeptides binding to peritoneal tumors of gastric cancer. Cancer Sci. 2010, 97, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Donovan, R.J.; Egger, G.; Francas, M. TARPARE: A method for selecting target audiences for public health interventions. Aust. N. Z. J. Public Health 2010, 23, 280–284. [Google Scholar] [CrossRef]
- Bai, F.; Liang, J.; Wang, J.; Shi, Y.; Zhang, K.; Liang, S.; Hong, L.; Zhai, H.; Yuanyuan, L.U.; Han, Y.U.; et al. Inhibitory effects of a specific phage-displayed peptide on high peritoneal metastasis of gastric cancer. J. Mol. Med. 2007, 85, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Han, H.; Wang, Z.; Kuang, L.; Wang, L.; Yu, L.; Wu, M.; Zhou, Z.; Qian, M. Targeted drug delivery to hepatocarcinoma in vivo by phage-displayed specific binding peptide. Mol. Cancer Res. 2010, 8, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Q.; Wang, H.R.; Li, H.P.; Hao, H.J.; Zheng, Y.L.; Gu, J. Targeting of hepatoma cell and suppression of tumor growth by a novel 12mer peptide fused to superantigen TSST-1. Mol. Med. 2006, 12, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pu, X.; Yin, G.; You, F. In Vivo Screening with Ph.D.-7™ and Identification of Ovarian Cancer A2780 Cell Targeted Heptapeptides. World J. Cancer Res. 2015, 5, 15628. [Google Scholar] [CrossRef]
- You, F.; Yin, G.; Pu, X.; Li, Y.; Hu, Y.; Huang, Z.; Liao, X.; Yao, Y.; Chen, X. Biopanning and characterization of peptides with Fe3O4 nanoparticles-binding capability via phage display random peptide library technique. Colloids Surf B Biointerfaces 2016, 141, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Mamluk, R.; Klagsbrun, M.; Detmar, M.; Bielenberg, D.R. Soluble neuropilin targeted to the skin inhibits vascular permeability. Angiogenesis 2005, 8, 217–227. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yin, G.; Pu, X.; Chen, X.; Liao, X.; Huang, Z. Novel Bi-Functional 14-mer Peptides with Both Ovarian Carcinoma Cells Targeting and Magnetic Fe3O4 Nanoparticles Affinity. Materials 2019, 12, 755. https://doi.org/10.3390/ma12050755
Li Y, Yin G, Pu X, Chen X, Liao X, Huang Z. Novel Bi-Functional 14-mer Peptides with Both Ovarian Carcinoma Cells Targeting and Magnetic Fe3O4 Nanoparticles Affinity. Materials. 2019; 12(5):755. https://doi.org/10.3390/ma12050755
Chicago/Turabian StyleLi, Yueting, Guangfu Yin, Ximing Pu, Xianchun Chen, Xiaoming Liao, and Zhongbing Huang. 2019. "Novel Bi-Functional 14-mer Peptides with Both Ovarian Carcinoma Cells Targeting and Magnetic Fe3O4 Nanoparticles Affinity" Materials 12, no. 5: 755. https://doi.org/10.3390/ma12050755