A Double Barrier Technique with Hydrotalcites for Pb Immobilisation from Electric Arc Furnace Dust
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterisation of Mortar Component Materials
2.2. Leaching and pH Dependence of EAFD
2.3. Immobilisation Mortar Dosage
2.4. Mixing Procedure
2.5. Compressive Strength and Leaching Behaviour of Immobilisation Mortars
3. Results and Discussion
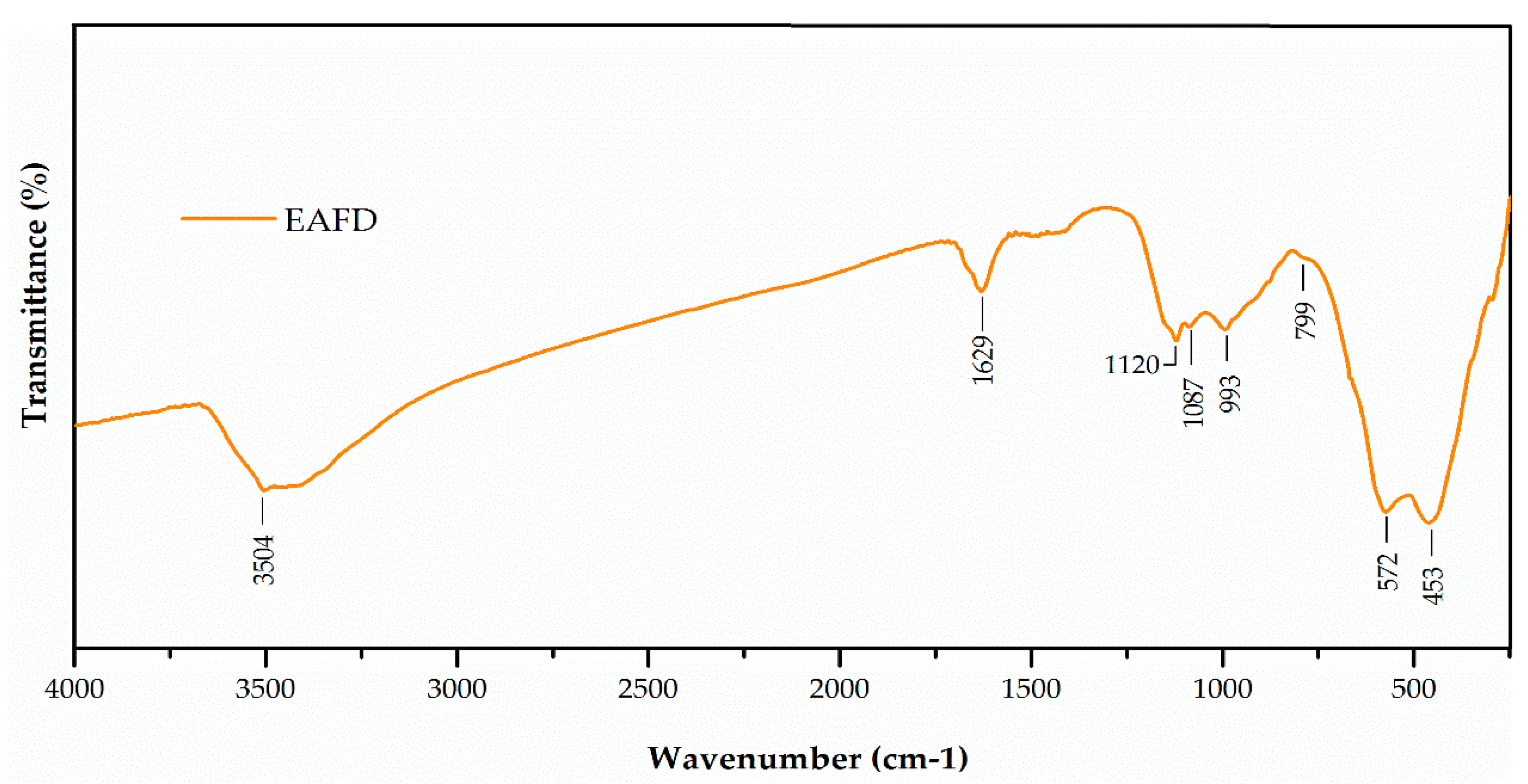
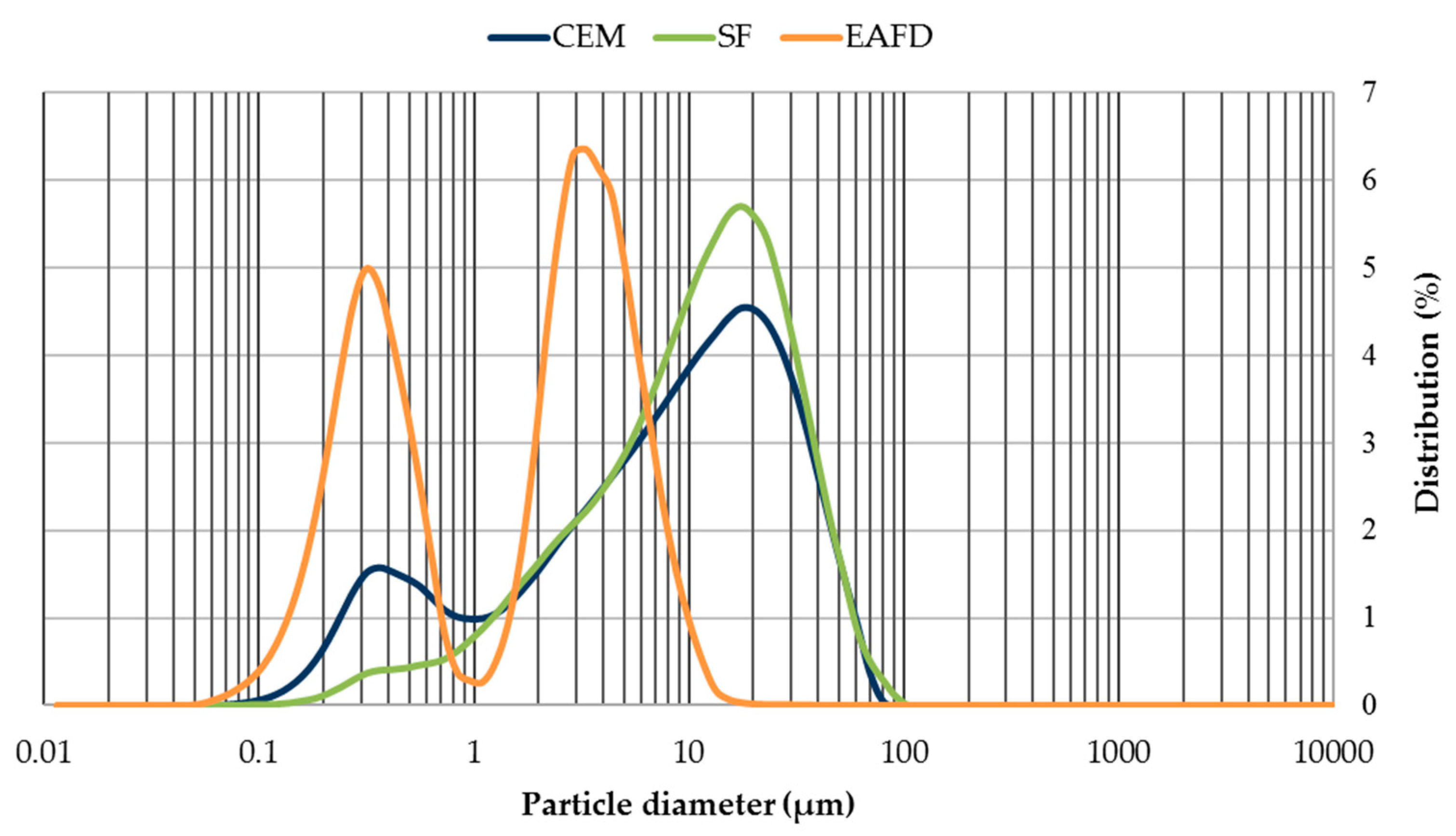
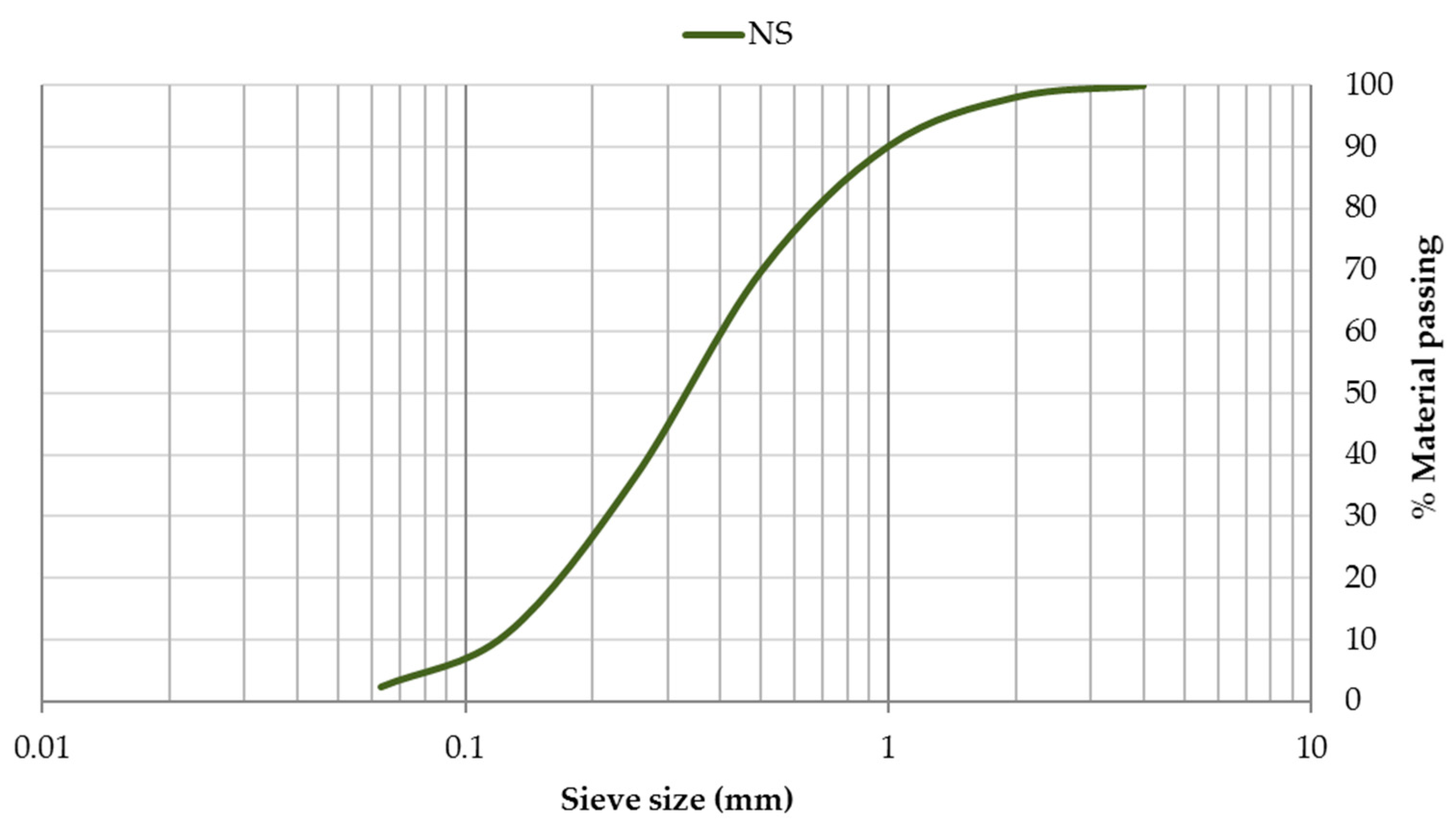
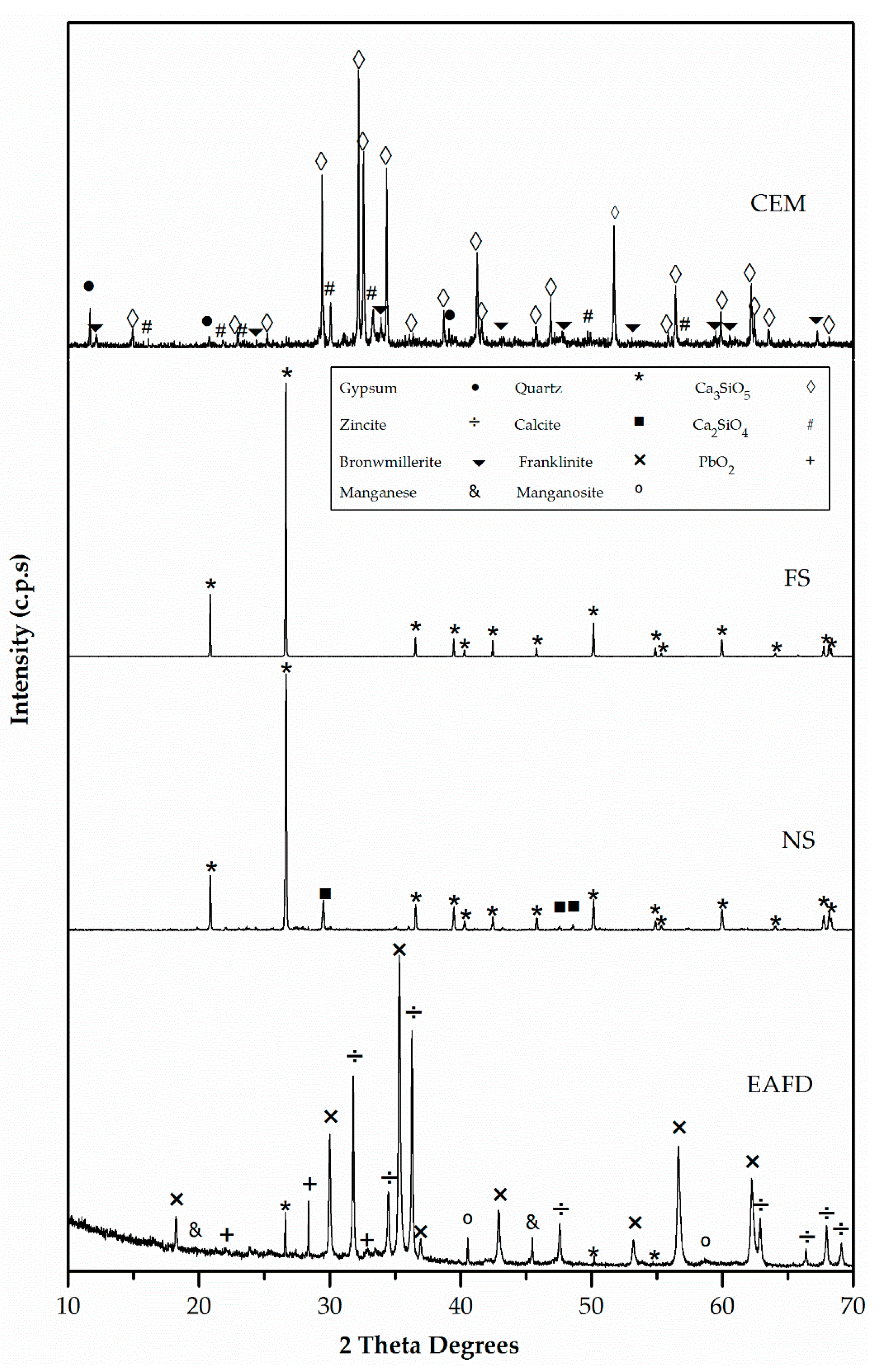
3.1. Characterisation of Mortar Component Materials
3.2. Leaching and pH Dependence of EAFD
3.3. Compressive Strength at 28 Days
3.4. Leaching Behaviour of Immobilisation and DB Mortars
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Issa, H.; Korac, M.; Gavrilovski, M.; Kamberovic, Z. Possibility of carbon steel EAFD solidification/stabilization in concrete. Metal. Int. 2013, 18, 182–188. [Google Scholar]
- Sofilic, T.; Rastovcan-Mioc, A.; Cerjan-Stefanovic, S.; Novosel-Radovic, V.; Jenko, M. Characterization of steel mill electric-arc furnace dust. J. Hazard. Mater. 2004, 109, 59–70. [Google Scholar] [CrossRef] [PubMed]
- European Waste Catalog. 2002. Available online: https://www.boe.es/boe/dias/1999/01/08/pdfs/A00570-00580.pdf (accessed on 5 January 2019).
- Maslehuddin, M.; Awan, F.R.; Shameem, M.; Ibrahim, M.; Ali, M.R. Effect of electric arc furnace dust on the properties of OPC and blended cement concretes. Constr. Build. Mater. 2011, 25, 308–312. [Google Scholar] [CrossRef]
- Oustadakis, P.; Tsakiridis, P.E.; Katsiapi, A.; Agatzini-Leonardou, S. Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD): Part I: Characterization and leaching by diluted sulphuric acid. J. Hazard. Mater. 2010, 179, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.M.; Kim, B.S.; Lee, J.C.; Kim, M.S.; Nam, C.W. Kinetics of the volatilization removal of lead in electric arc furnace dust. Mater. Trans. 2005, 46, 323–328. [Google Scholar] [CrossRef]
- Lenz, D.M.; Martins, F.B. Lead and zinc selective precipitation from leach electric arc furnace dust solutions. Matéria (Rio J.) 2007, 12, 503–509. [Google Scholar] [CrossRef] [Green Version]
- Conner, J.R.; Hoeffner, S.L. The History of Stabilization/Solidification Technology. Crit. Rev. Environ. Sci. Technol. 1998, 28, 325–396. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Tyrer, M.; Hills, C.D.; Yang, X.M.; Carey, P. Immobilisation of heavy metal in cement-based solidification/stabilisation: A review. Waste Manag. 2009, 29, 390–403. [Google Scholar] [CrossRef]
- Chaabane, L.; Moussaceb, K.; Ait-Mokhtar, A. Factors affecting the leaching of heavy metals (Ni+2, Pb+2, Cr+3) contained in sludge waste stabilization/solidification by hydraulic benders, Part I: Water/cement and waste/cement ratio in S/S mortars. Environ. Prog. Sustain. Energy 2017, 36, 93–103. [Google Scholar] [CrossRef]
- Katsioti, M.; Katsiotis, N.; Rouni, G.; Bakirtzis, D.; Loizidou, M. The effect of bentonite/cement mortar for the stabilization/solidification of sewage sludge containing heavy metals. Cem. Concr. Comp. 2008, 30, 1013–1019. [Google Scholar] [CrossRef]
- Hills, C.D.; Sollars, C.J.; Perry, R. A calorimetric and microstructural study of solidifiekd toxic wastes—Part 1: A classification of OPC waste interference effects. Waste Manag. 1994, 14, 589–599. [Google Scholar] [CrossRef]
- Salihoglu, G.; Pinarli, V. Steel foundry electric arc furnace dust management: Stabilization by using lime and Portland cement. J. Hazard. Mater. 2008, 153, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Cubukcuoglu, B.; Ouki, S.K. Solidification/stabilisation of electric arc furnace waste using low grade MgO. Chemosphere 2012, 86, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Ledesma, E.F.; Lozano-Lunar, A.; Ayuso, J.; Galvín, A.P.; Fernández, J.M.; Jiménez, J.R. The role of pH on leaching of heavy metals and chlorides from electric arc furnace dust in cement-based mortars. Constr. Build. Mater. 2018, 183, 365–375. [Google Scholar] [CrossRef]
- Halim, C.E.; Scott, J.A.; Amal, R.; Short, S.A.; Beydoun, D.; Low, G.; Cattle, J. Evaluating the applicability of regulatory leaching tests for assessing the hazards of Pb-contaminated soils. J. Hazard. Mater. 2005, 120, 101–111. [Google Scholar] [CrossRef]
- Navarro, A.; Cardellach, E.; Corbella, M. Immobilization of Cu, Pb and Zn in mine-contaminated soils using reactive materials. J. Hazard. Mater. 2011, 186, 1576–1585. [Google Scholar] [CrossRef]
- Ledesma, E.F.; Jimenez, J.R.; Ayuso, J.; Fernandez, J.M.; de Brito, J. Experimental study of the mechanical stabilization of electric arc furnace dust using fluid cement mortars. J. Hazard. Mater. 2017, 326, 26–35. [Google Scholar] [CrossRef]
- De Angelis, G.; Medici, F.; Montereali, M.R.; Pietrelli, L. Reuse of residues arising from lead batteries recycle: a feasibility study. Waste Manag. 2002, 22, 925–930. [Google Scholar] [CrossRef]
- Lozano-Lunar, A.; Raposeiro da Silva, P.; de Brito, J.; Fernández, J.M.; Jiménez, J.R. Safe use of electric arc furnace dust as secondary raw material in self-compacting mortars production. J. Clean. Prod. 2019, 211, 1375–1388. [Google Scholar] [CrossRef]
- European Council Decision for the Acceptance of Waste at Landfills. 2003. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:011:0027:0049:EN:PDF (accessed on 13 March 2018).
- Ter Heijne, A.; Liu, F.; van der Weijden, R.; Weijma, J.; Buisman, C.J.N.; Hamelers, H.V.M. Copper Recovery Combined with Electricity Production in a Microbial Fuel Cell. Environ. Sci. Technol. 2010, 44, 4376–4381. [Google Scholar] [CrossRef]
- Hashim, M.A.; Mukhopadhyay, S.; Sahu, J.N.; Sengupta, B. Remediation technologies for heavy metal contaminated groundwater. J. Environ. Manag. 2011, 92, 2355–2388. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.A.; Trocoli, R.; Pavlovic, I.; Barriga, C.; La Mantia, F. Capturing Cd(II) and Pb(II) from contaminated water sources by electro-deposition on hydrotalcite-like compounds. Phys. Chem. Chem. Phys. 2016, 18, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, I.; Perez, M.R.; Barriga, C.; Ulibarri, M.A. Adsorption of Cu2+, Cd2+ and Pb2+ ions by layered double hydroxides intercalated with the chelating agents diethylenetriaminepentaacetate and meso-2,3-dimercaptosuccinate. Appl. Clay Sci. 2009, 43, 125–129. [Google Scholar] [CrossRef]
- Gonzalez, M.A.; Pavlovic, I.; Rojas-Delgado, R.; Barriga, C. Removal of Cu2+, Pb2+ and Cd2+ by layered double hydroxide-humate hybrid. Sorbate and sorbent comparative studies. Chem. Eng. J. 2014, 254, 605–611. [Google Scholar] [CrossRef]
- Forano, C.; Hibino, T.; Leroux, F.; Taviot-Guého, C. Chapter 13.1 Layered Double Hydroxides. In Developments in Clay Science; Faïza Bergaya, B.K.G.T.a.G.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 1021–1095. [Google Scholar]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Rives, V.; Carriazo, D.; Martín, C. Heterogeneous Catalysis by Polyoxometalate-Intercalated Layered Double Hydroxides. In Pillared Clays and Related Catalysts; Gil, A., Korili, S.A., Trujillano, R., Vicente, M.A., Eds.; Springer: New York, NY, USA, 2010; pp. 319–397. [Google Scholar]
- Tsyganok, A.I.; Suzuki, K.; Hamakawa, S.; Takehira, K.; Hayakawa, T. Mg-Al layered double hydroxide intercalated with [Ni(edta)]2− chelate as a precursor for an efficient catalyst of methane reforming with carbon dioxide. Catal. Lett. 2001, 77, 75–86. [Google Scholar] [CrossRef]
- Perez, M.R.; Pavlovic, I.; Barriga, C.; Cornejo, J.; Hermosin, M.C.; Ulibari, M.A. Uptake of Cu2+, Cd2+ and Pb2+ on Zn-Al layered double hydroxide intercalated with edta. Appl. Clay Sci. 2006, 32, 245–251. [Google Scholar] [CrossRef]
- Kaneyoshi, M.; Jones, W. Formation of Mg-Al layered double hydroxides intercalated with nitrilotriacetate anions. J. Mater. Chem. 1999, 9, 805–811. [Google Scholar] [CrossRef]
- Gutmann, N.H.; Spiccia, L.; Turney, T.W. Complexation of Cu(II) and Ni(II) by nitrilotriacetate intercalated in Zn–Cr layered double hydroxides. J. Mater. Chem. 2000, 10, 1219–1224. [Google Scholar] [CrossRef]
- Rojas, R.; Perez, M.R.; Erro, E.M.; Ortiz, P.I.; Ulibarri, M.A.; Giacomelli, C.E. EDTA modified LDHs as Cu2+ scavengers: Removal kinetics and sorbent stability. J. Colloid Interface Sci. 2009, 331, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Guzman, M.; Celis, R.; Hermosin, M.C.; Koskinen, W.C.; Nater, E.A.; Cornejo, J. Heavy metal adsorption by montmorillonites modified with natural organic cations. Soil Sci. Soc. Am. J. 2006, 70, 215–221. [Google Scholar] [CrossRef]
- Yang, Z.X.; Fischer, H.; Polder, R. Laboratory investigation of the influence of two types of modified hydrotalcites on chloride ingress into cement mortar. Cem. Concr. Comp. 2015, 58, 105–113. [Google Scholar] [CrossRef]
- Hongo, T.; Tsunashima, Y.; Yamasaki, A. Synthesis of Ca-Al layered double hydroxide from concrete sludge and evaluation of its chromate removal ability. Sustain. Mater. Technol. 2017, 12, 23–26. [Google Scholar] [CrossRef]
- AENOR. Asociación Española de Normalización y Certificación, AENOR, Madrid, Spain. Available online: www.aenor.es (accessed on 29 September 2017).
- Mahjoubi, F.Z.; Khalidi, A.; Abdennouri, M.; Barka, N. Zn–Al layered double hydroxides intercalated with carbonate, nitrate, chloride and sulphate ions: Synthesis, characterisation and dye removal properties. J. Taibah Univ. Sci. 2017, 11, 90–100. [Google Scholar] [CrossRef]
- Reichle, W.T. Synthesis of anionic clay-minerals (mixed metal-hydroxides, hydrotalcite). Solid State Ion. 1986, 22, 135–141. [Google Scholar] [CrossRef]
- Crisponi, G.; Diaz, A.; Nurchi, V.M.; Pivetta, T.; Estevez, M.J.T. Equilibrium study on Cd(II) and Zn(II) chelates of mercapto carboxylic acids. Polyhedron 2002, 21, 1319–1327. [Google Scholar] [CrossRef]
- AFNOR. Association Française de Normalisation. Available online: www.afnor.org (accessed on 28 September 2017).
- Bayraktar, A.C.; Avsar, E.; Toroz, I.; Alp, K.; Hanedar, A. Stabilization and solidification of electric arc furnace dust originating from steel industry by using low grade MgO. Arch. Environ. Prot. 2015, 41, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Farmer, V.C. The Infrared Spectra of Minerals; Mineralogical Society: London, UK, 1974. [Google Scholar]
- Calvo, J.L.G.; Moreno, M.S.; Alonso, M.C.A.; Lopez, A.H.; Olmo, J.G. Study of the Microstructure Evolution of Low-pH Cements Based on Ordinary Portland Cement (OPC) by Mid- and Near-Infrared Spectroscopy, and Their Influence on Corrosion of Steel Reinforcement. Materials 2013, 6, 2508–2521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiley Interscience: New York, NY, USA, 1986. [Google Scholar]
- Joint Committee on Powder Diffraction Standards; International Center for Diffraction Data: Swarthmore, PA, USA, 1975.
- Suetens, T.; Guo, M.; Van Acker, K.; Blanpain, B. Formation of the ZnFe2O4 phase in an electric arc furnace off-gas treatment system. J. Hazard. Mater. 2015, 287, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.K.; Pickles, C.A. Caustic roasting and leaching of electric arc furnace dust. Can. Metall. Quart. 1999, 38, 175–186. [Google Scholar] [CrossRef]
- Xia, D.K.; Pickles, C.A. Microwave caustic leaching of electric arc furnace dust. Miner. Eng. 2000, 13, 79–94. [Google Scholar] [CrossRef]
- Machado, J.; Brehm, F.A.; Moraes, C.A.M.; dos Santos, C.A.; Vilela, A.C.F.; da Cunha, J.B.M. Chemical, physical, structural and morphological characterization of the electric arc furnace dust. J. Hazard. Mater. 2006, 136, 953–960. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.A.C.; Machado, A.T.; Lima, L.; Cardoso, R.J.C. Stabilization of Electric-Arc Furnace Dust in Concrete. Mater. Res. Ibero Am. J. 2010, 13, 513–519. [Google Scholar] [CrossRef]
- De Paula, L.N.; Giusto, L.A.; Rodrigues, R.C.; Castilho, L.R.; Magalhaes, F.; Rosmaninho, M.G.; Lima, D.Q. Modification and characterization of residue electric arc furnace dust (EAFD) for application in chromium (VI) reduction reactions. Quim. Nova 2013, 36, 1332–1337. [Google Scholar]
- Van der Sloot, H.; Dijkstra, J. Development of Horizontally Standardized Leaching Tests for Construction Materials: A Material Based or Release Based Approach? Identical Leaching Mechanisms for Different Materials. Available online: http://www.ecn.nl/docs/library/report/2004/c04060.pdf (accessed on 18 February 2019).
- Lasheras-Zubiate, M.; Navarro-Blasco, I.; Álvarez, J.I.; Fernández, J.M. Interaction of carboxymethylchitosan and heavy metals in cement media. J. Hazard. Mater. 2011, 194, 223–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belebchouche, C.; Moussaceb, K.; Tahakourt, A.; Aït-Mokhtar, A. Parameters controlling the release of hazardous waste (Ni2+, Pb2+ and Cr3+) solidified/stabilized by cement-CEM I. Mater. Struct. 2015, 48, 2323–2338. [Google Scholar] [CrossRef]
- Cao, L.; Guo, J.; Tian, J.; Xu, Y.; Hu, M.; Wang, M.; Fan, J. Preparation of Ca/Al-layered double hydroxide and the influence of their structure on early strength of cement. Constr. Build. Mater. 2018, 184, 203–214. [Google Scholar] [CrossRef]

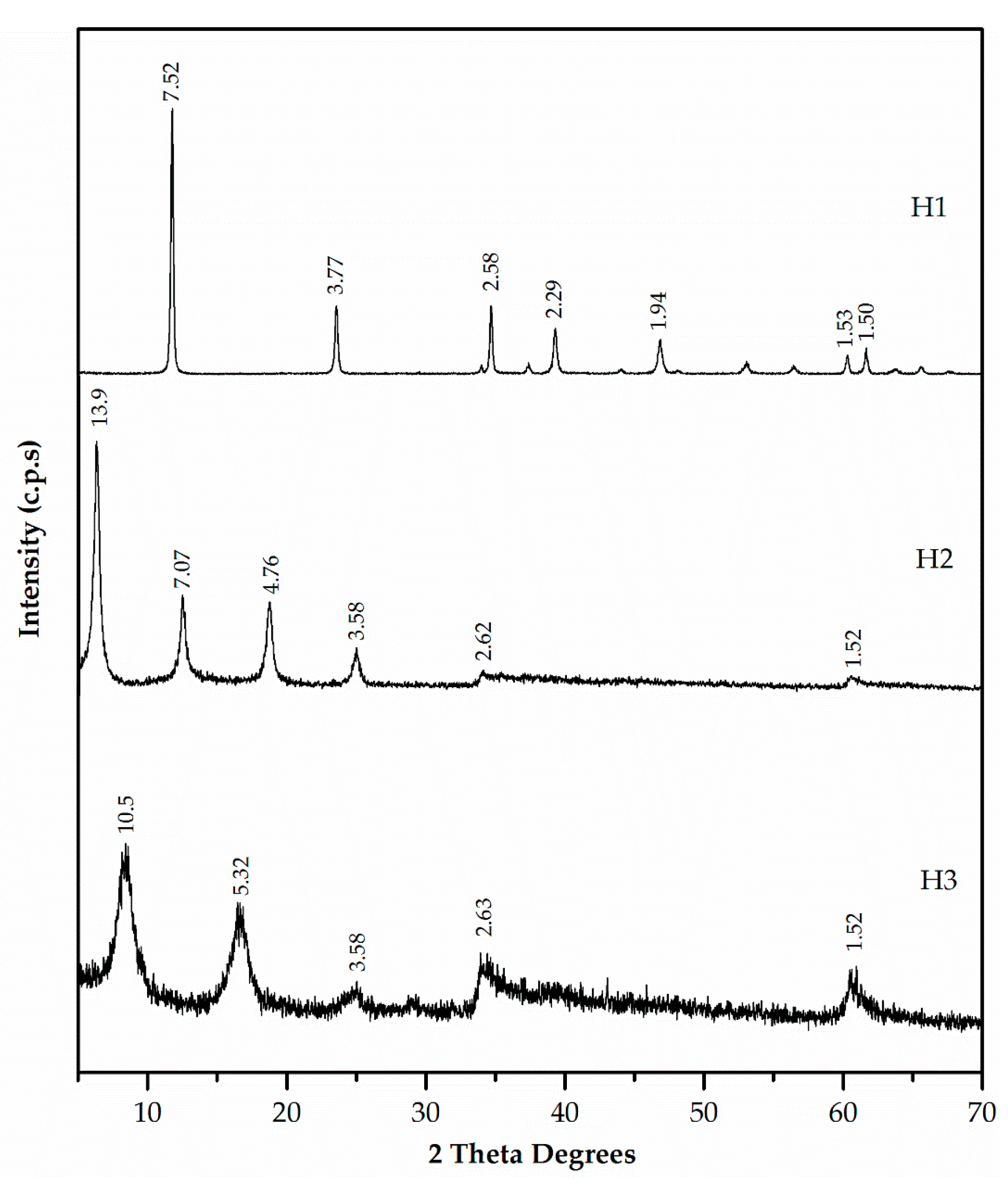
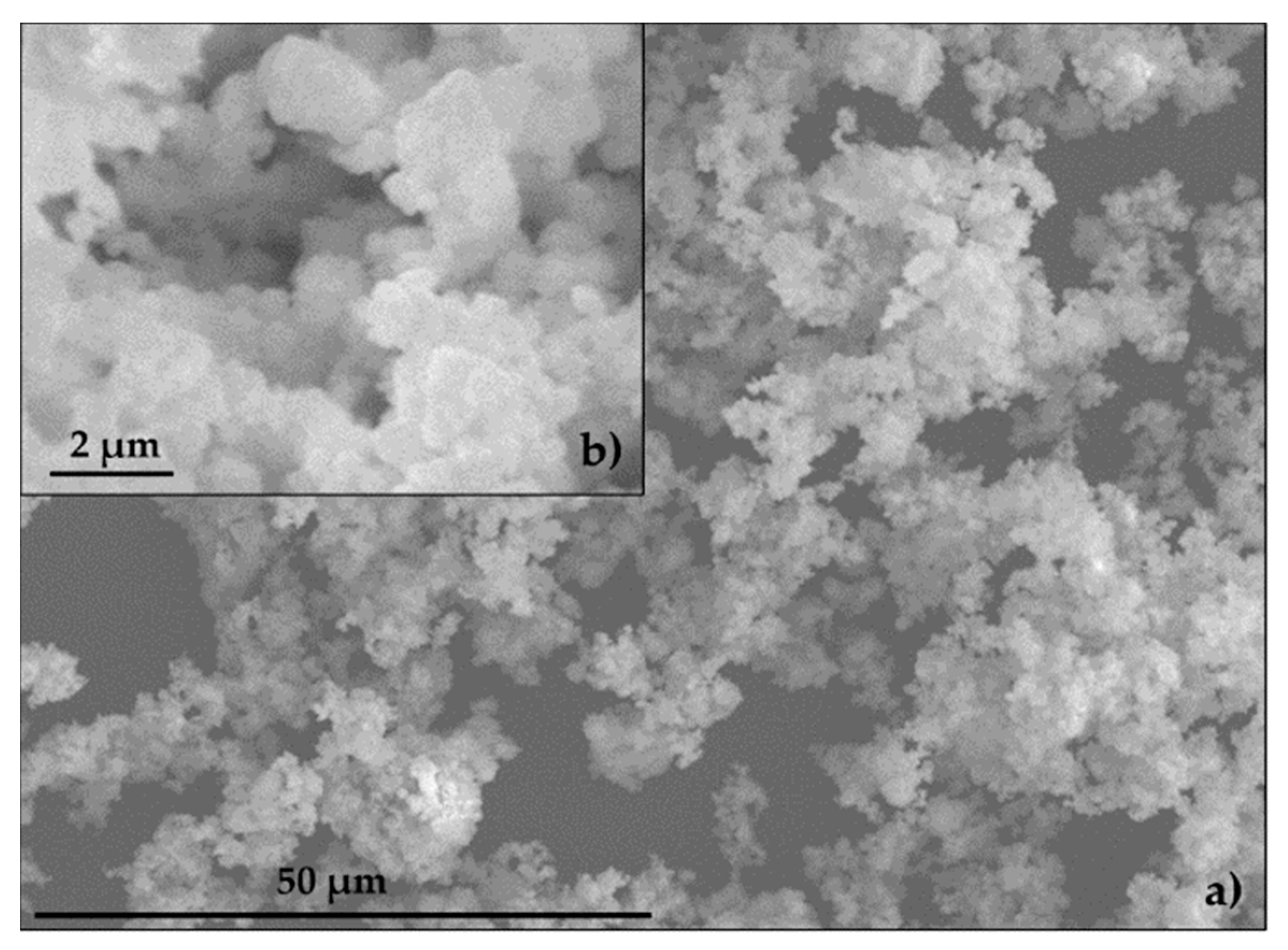

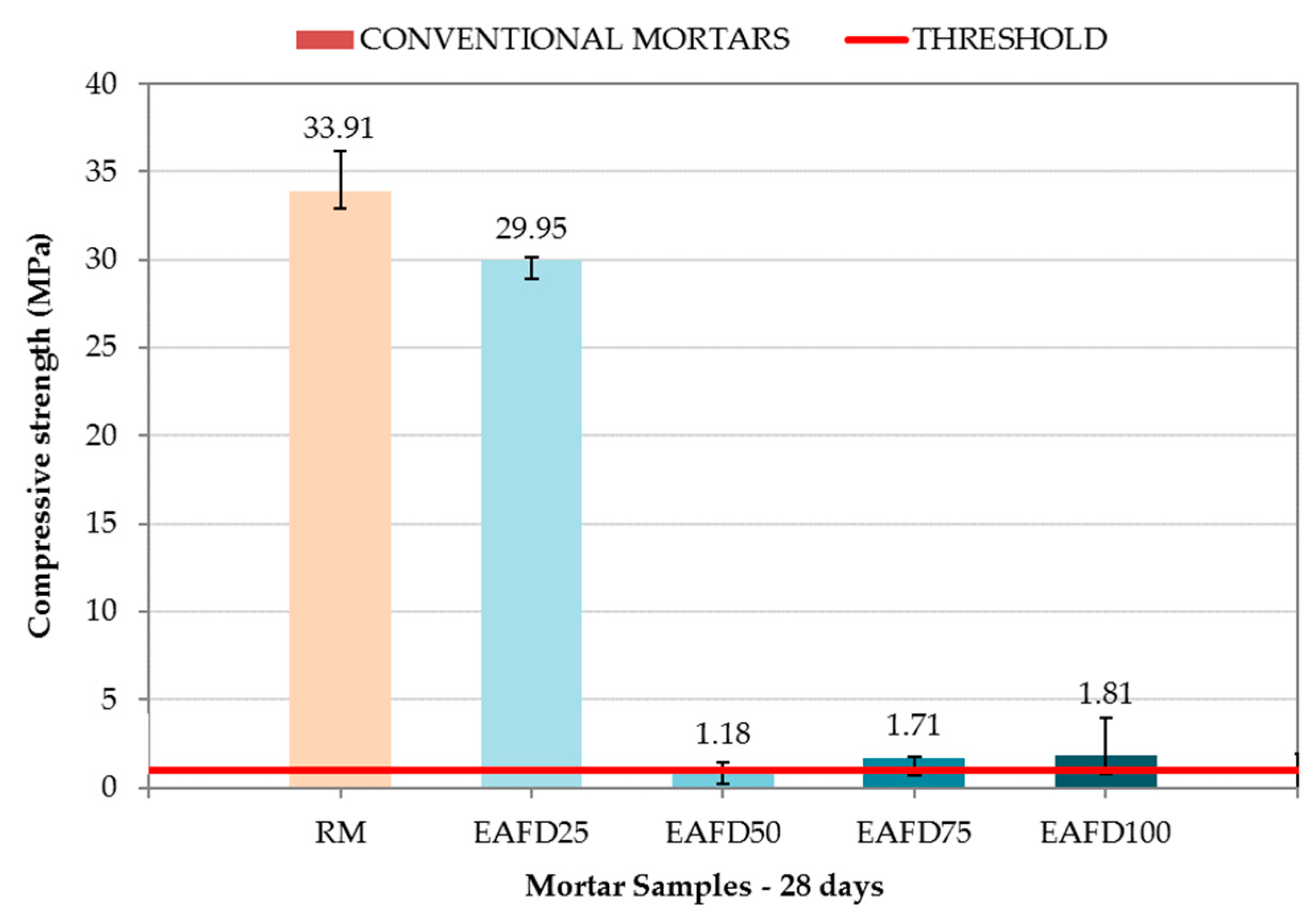
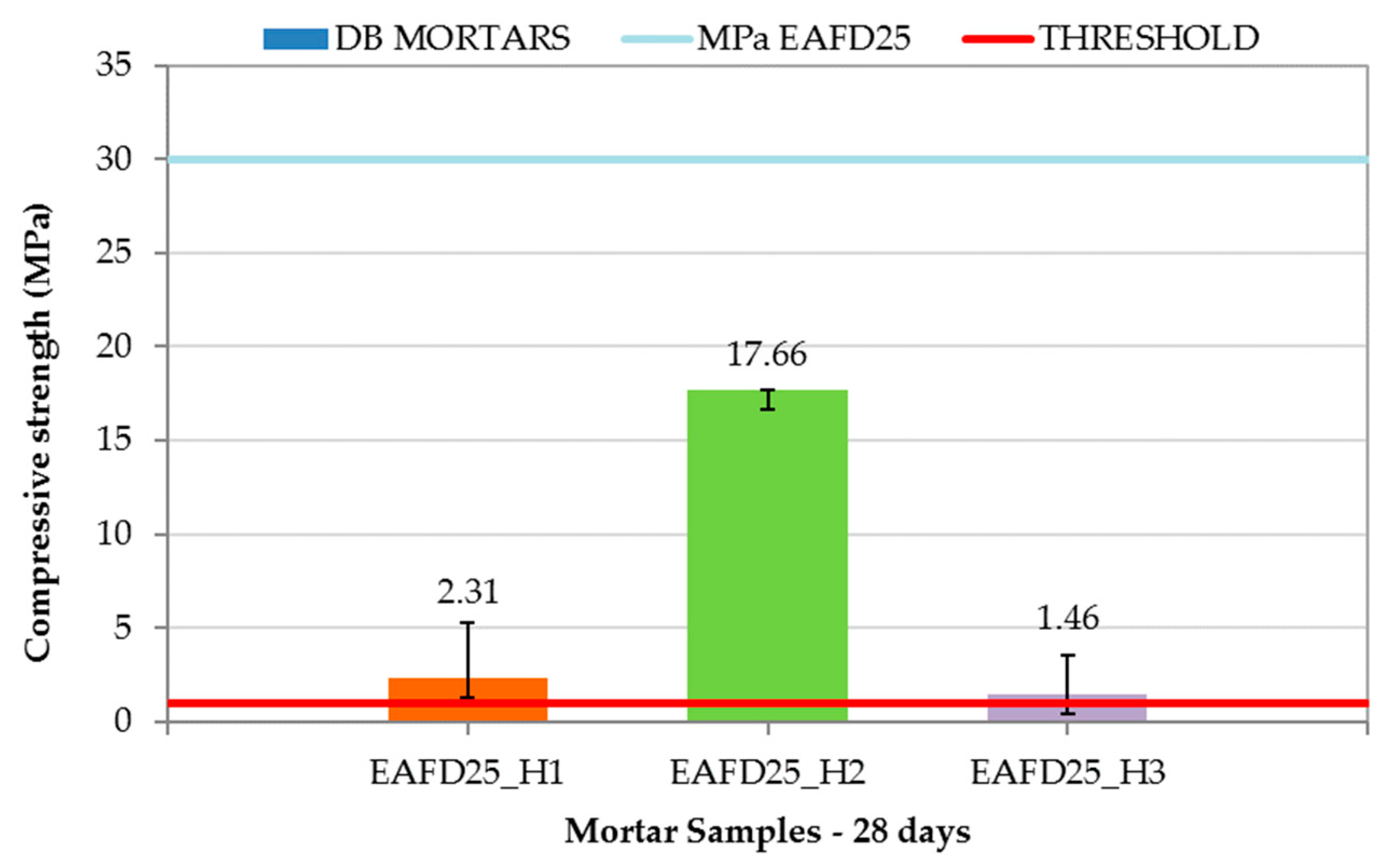

| Mortars’ Composition | CEM (g) | NS (g) | SF (g) | EAFD (g) | H (g) | %W | C (mm) | |
|---|---|---|---|---|---|---|---|---|
| First Stage | ||||||||
| Reference Mortar | RM | 1600 | 800 | 1600 | 0 | 0 | 26.25 | 183.00 |
| Conventional Immobilisation Mortars | EAFD25 | 1600 | 800 | 1200 | 400 | 0 | 25.97 | 181.25 |
| EAFD50 | 1600 | 800 | 800 | 800 | 0 | 24.32 | 171.50 | |
| EAFD75 | 1600 | 800 | 400 | 1200 | 0 | 25.97 | 179.75 | |
| EAFD100 | 1600 | 800 | 0 | 1600 | 0 | 25.00 | 165.00 | |
| Second Stage | ||||||||
| DB mortars | EAFD25_H1 | 1600 | 800 | 1200 | 400 | 63 | 25.97 | 177.30 |
| EAFD25_H2 | 1600 | 800 | 1200 | 400 | 63 | 25.97 | 177.60 | |
| EAFD25_H3 | 1600 | 800 | 1200 | 400 | 34 | 25.97 | 179.50 | |
| Materials | Fe2O3 | CaO | SiO2 | SO3 | K2O | MgO | Al2O3 |
|---|---|---|---|---|---|---|---|
| CEM 1 | 2.59 | 64.58 | 20.16 | 3.46 | 1.00 | 0.98 | 4.52 |
| SF 2 | - | - | 100.00 | - | - | - | - |
| NS 2 | 6.80 | 4.73 | 72.14 | - | 2.53 | 0.70 | 10.31 |
| Element | C | O | Mg | Al | Si | S | Cl | K | Ca | Ti | Cr | Mn | Fe | Zn | Pb |
| % Weight | 6.53 | 16.18 | 0.91 | 0.37 | 1.21 | 0.79 | 5.08 | 1.30 | 2.20 | - | - | 2.35 | 18.31 | 41.58 | 3.20 |
| Leaching of Pb | EAFD | RM | EAFD25 | EAFD25_H1 | EAFD25_H2 | EAFD25_H3 |
|---|---|---|---|---|---|---|
| Pb | 6.30 NH | 0.00 I | 20.29 H | 13.17 H | 11.33 H | 9.88 NH |
| pH | 8.73 | 12.52 | 12.54 | 11.93 | 12.12 | 11.81 |
| Ta (°C) | 29.5 | 24.35 | 24.35 | 25.7 | 25.9 | 26.2 |
| C (mS cm−1) | 17.29 | 9.62 | 10.10 | 6.86 | 9.36 | 5.90 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano-Lunar, A.; Fernández Ledesma, E.; Romero Esquinas, Á.; Jiménez Romero, J.R.; Fernández Rodríguez, J.M. A Double Barrier Technique with Hydrotalcites for Pb Immobilisation from Electric Arc Furnace Dust. Materials 2019, 12, 633. https://doi.org/10.3390/ma12040633
Lozano-Lunar A, Fernández Ledesma E, Romero Esquinas Á, Jiménez Romero JR, Fernández Rodríguez JM. A Double Barrier Technique with Hydrotalcites for Pb Immobilisation from Electric Arc Furnace Dust. Materials. 2019; 12(4):633. https://doi.org/10.3390/ma12040633
Chicago/Turabian StyleLozano-Lunar, Angélica, Enrique Fernández Ledesma, Álvaro Romero Esquinas, José Ramón Jiménez Romero, and José María Fernández Rodríguez. 2019. "A Double Barrier Technique with Hydrotalcites for Pb Immobilisation from Electric Arc Furnace Dust" Materials 12, no. 4: 633. https://doi.org/10.3390/ma12040633






