Ceramic-Reinforced γ-TiAl-Based Composites: Synthesis, Structure, and Properties
Abstract
:1. Introduction
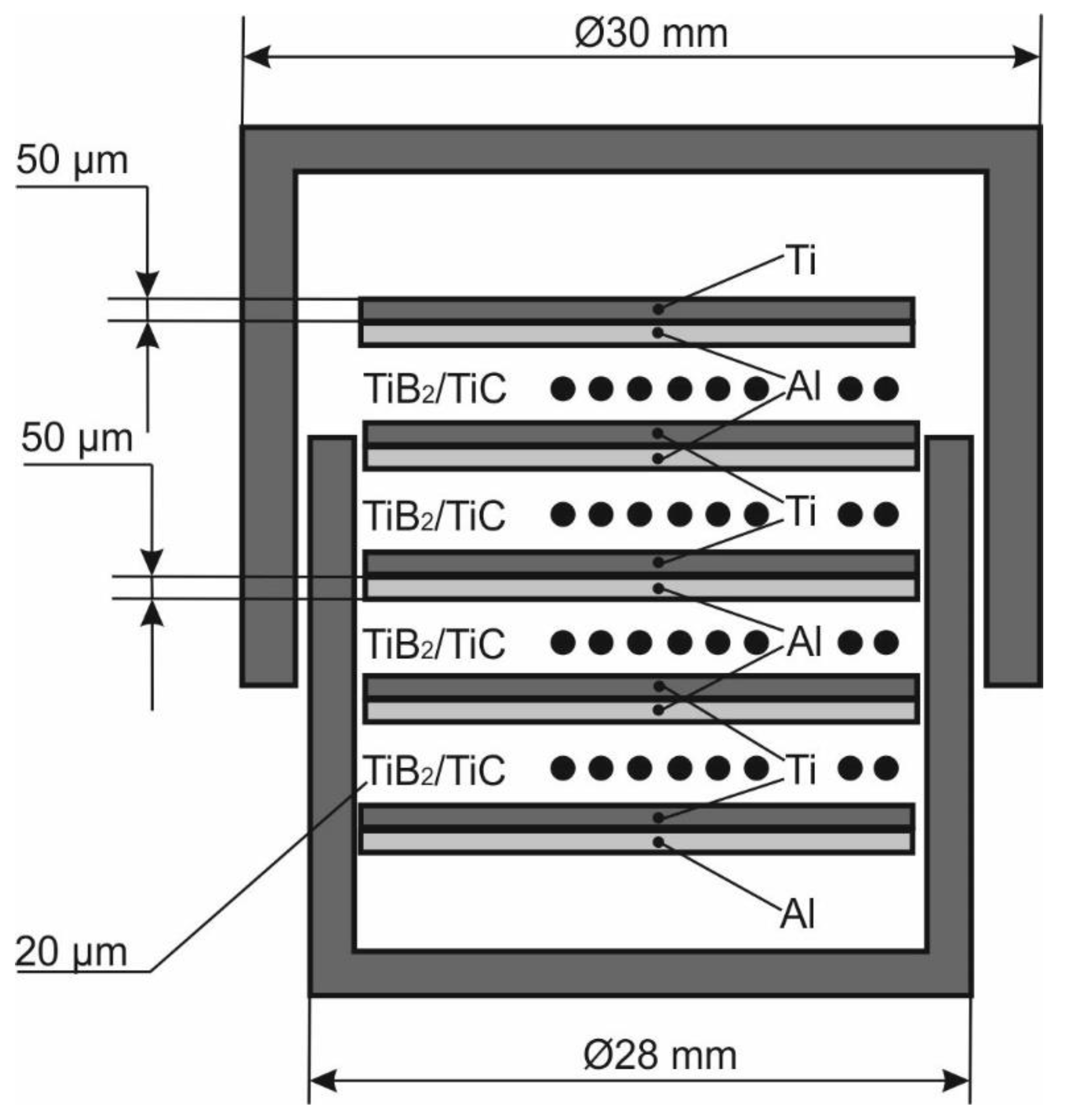
2. Materials and Methods
3. Results and Discussion
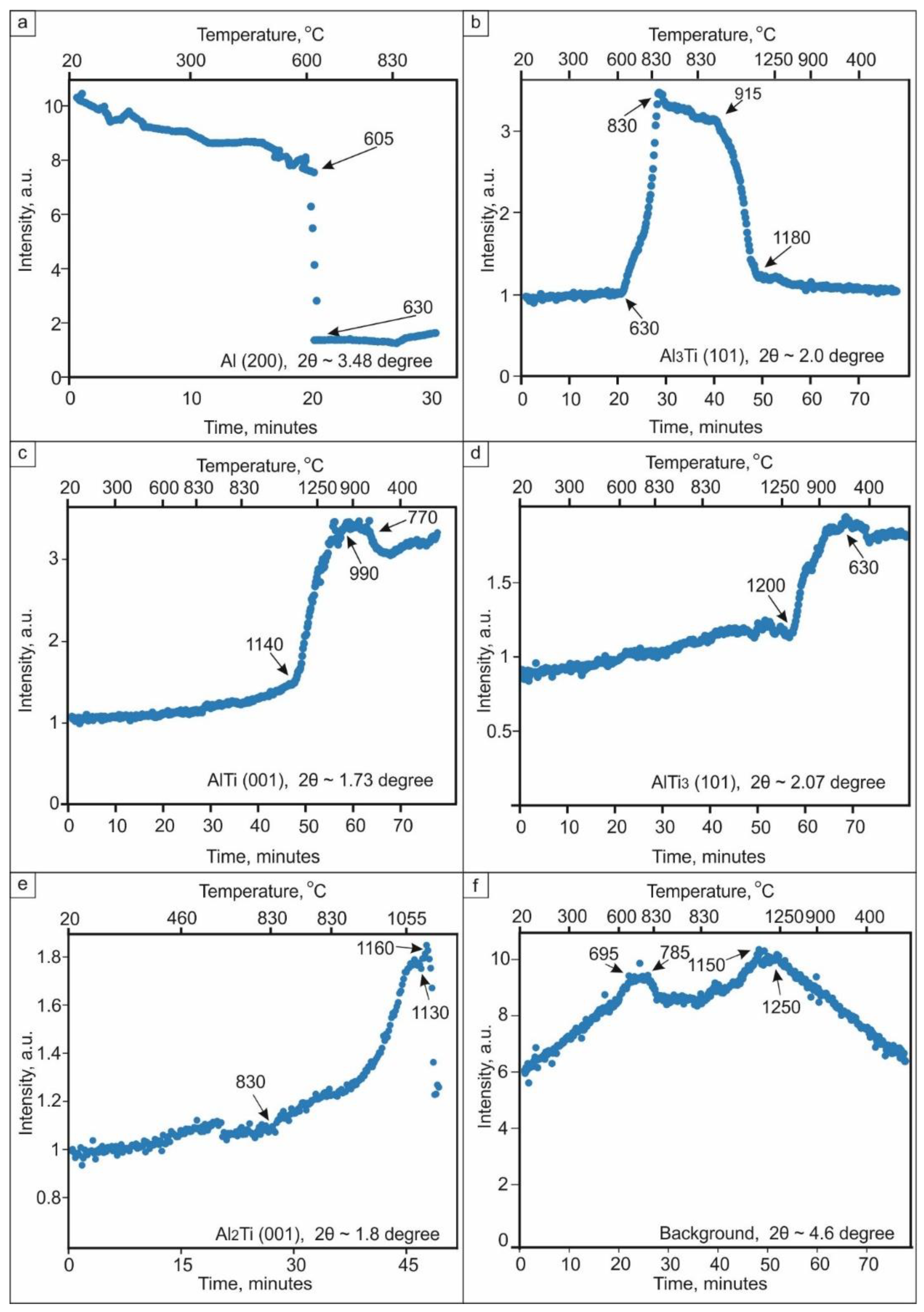
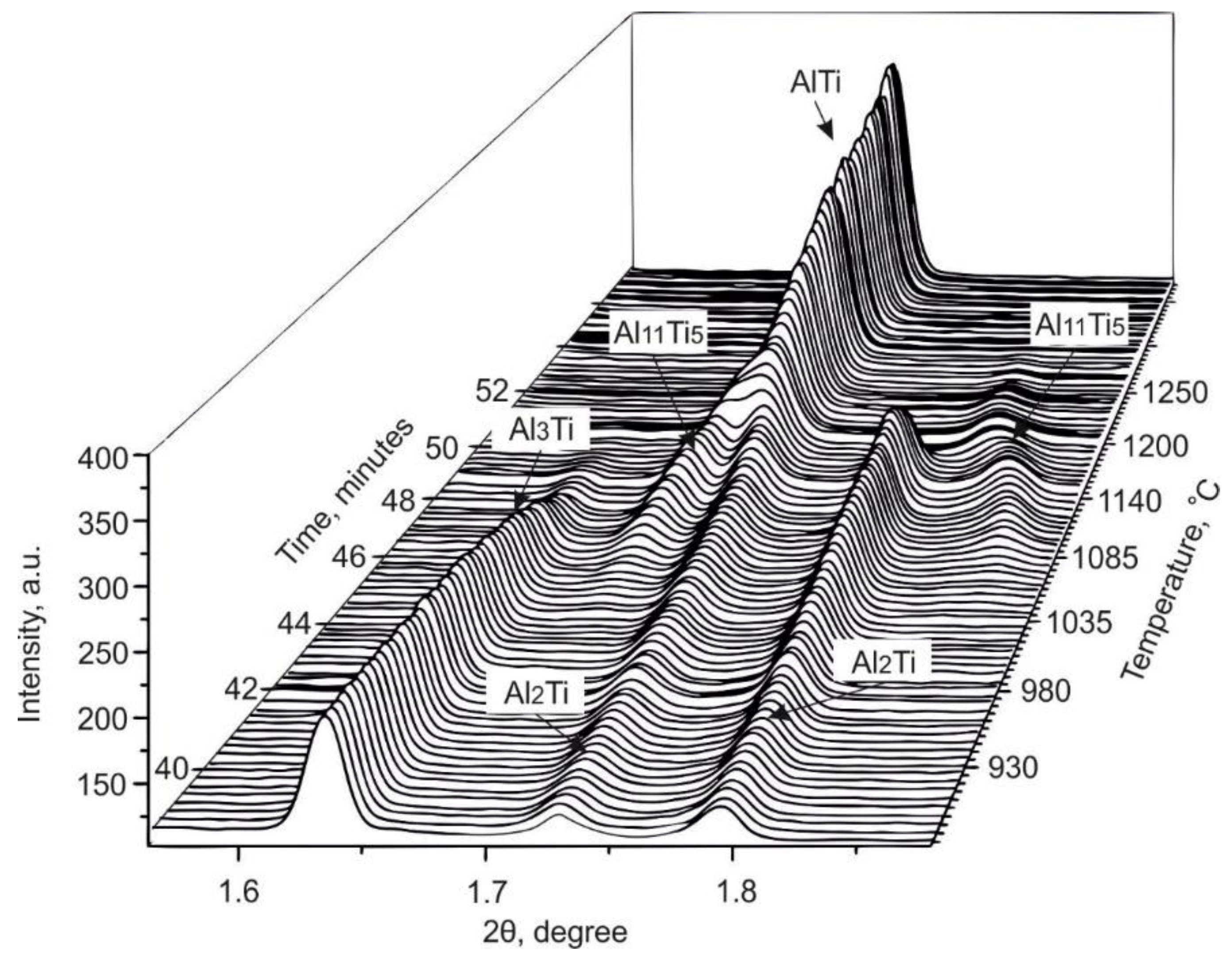
3.1. Observation of the Structural Transformations in the Ti–Al System Using in Situ Synchrotron Diffraction Analysis
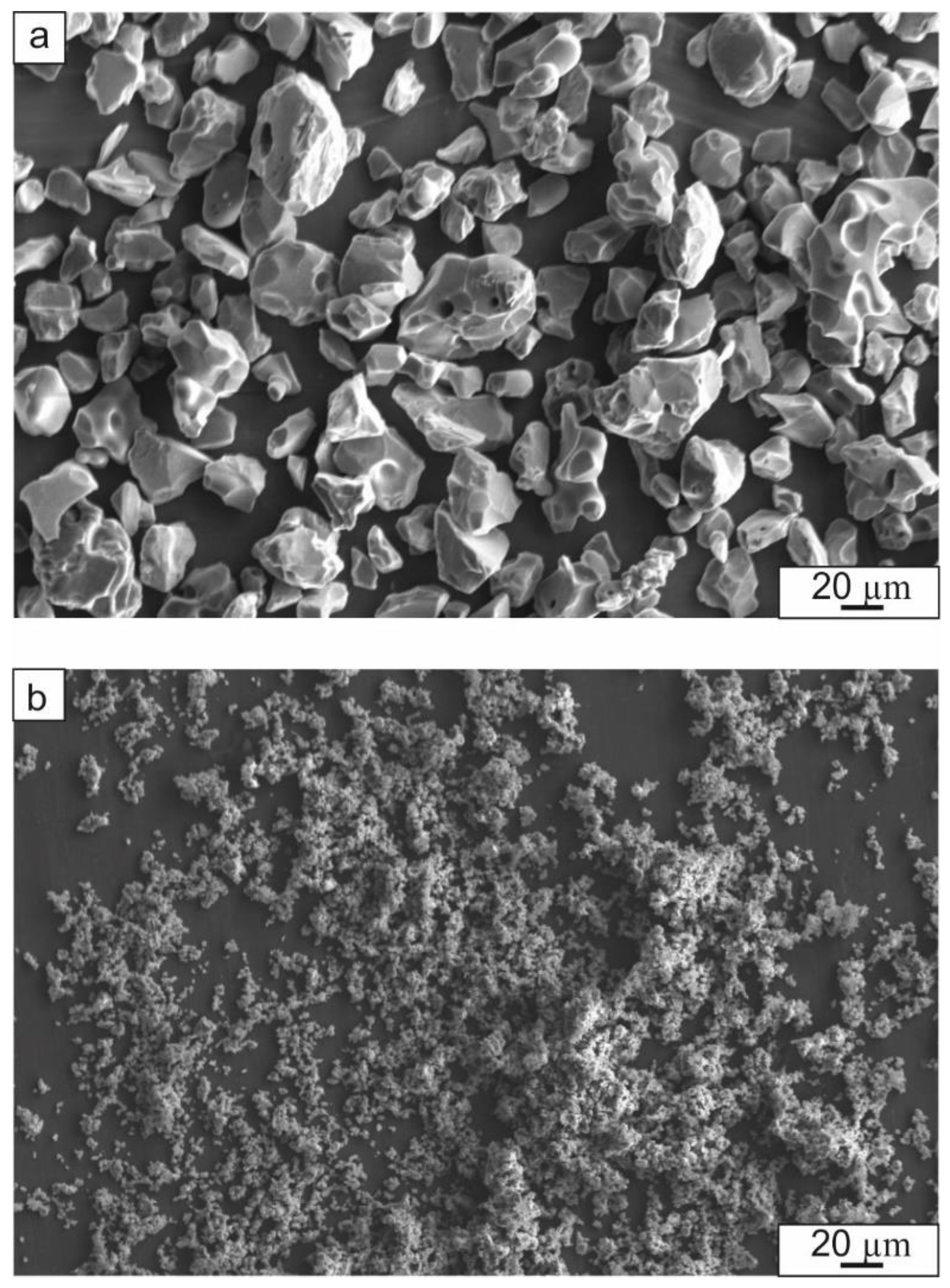
3.2. Characterization of Laminate Composites Obtained by the SPS of Elemental Foils and Ceramic Particles
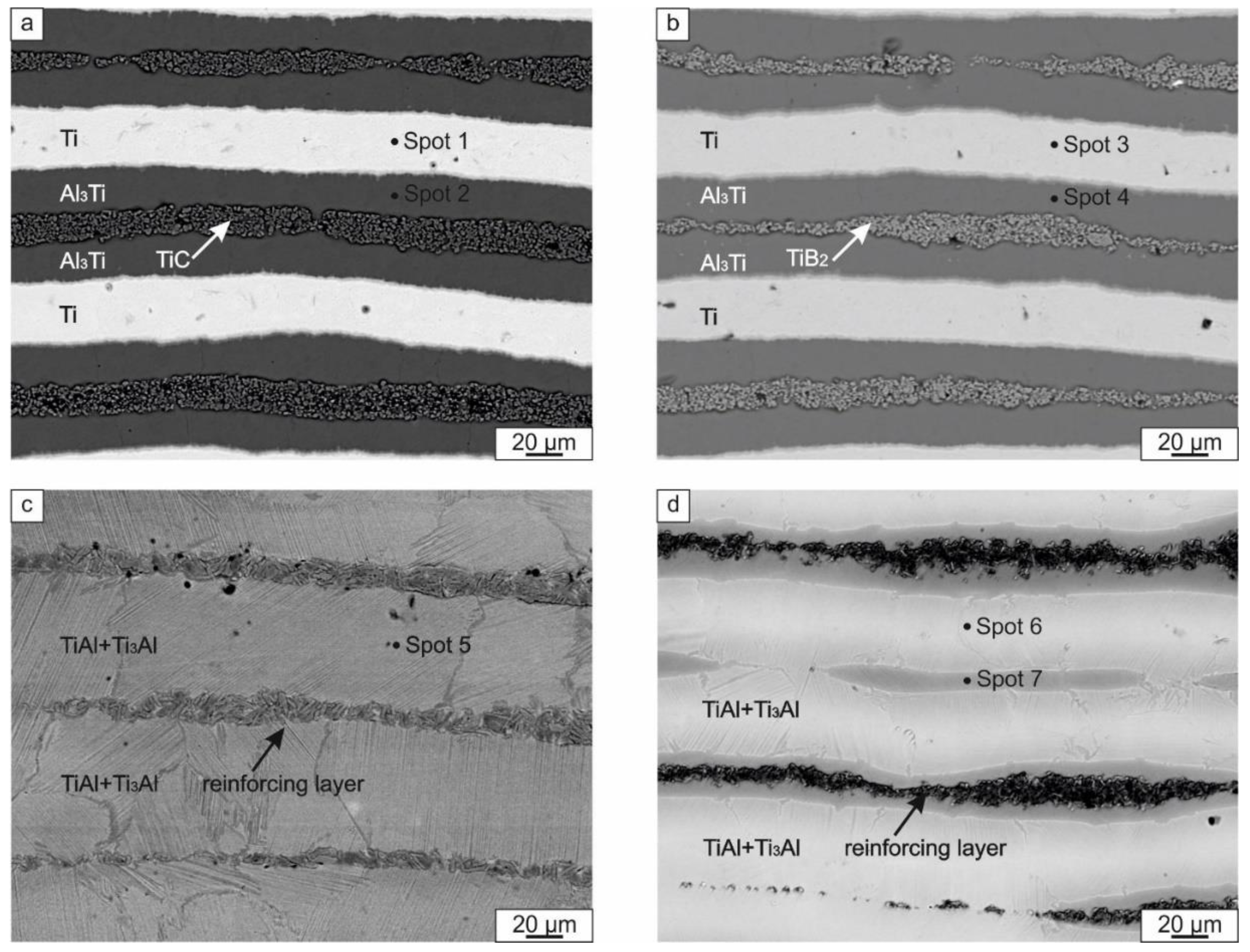
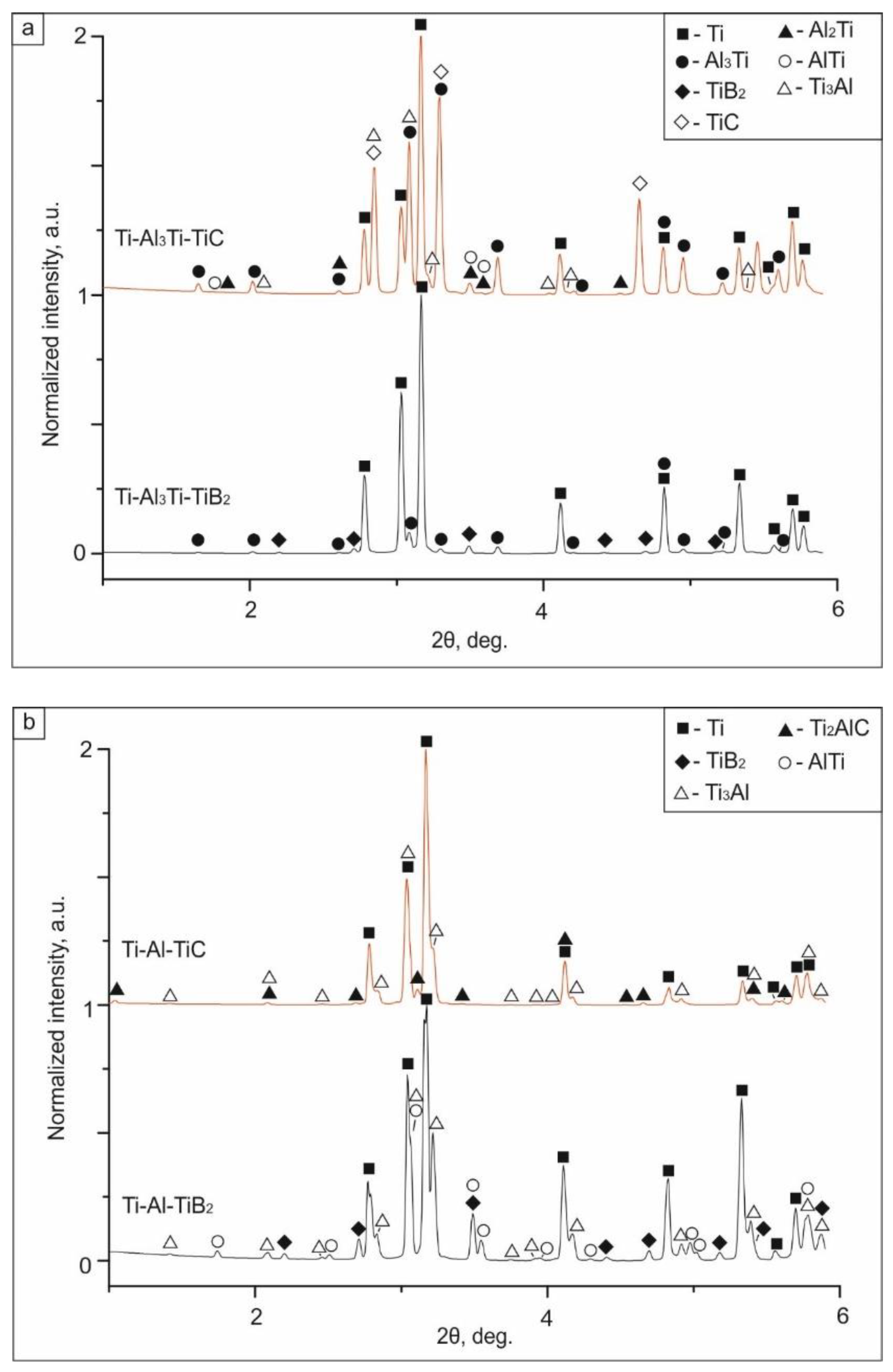
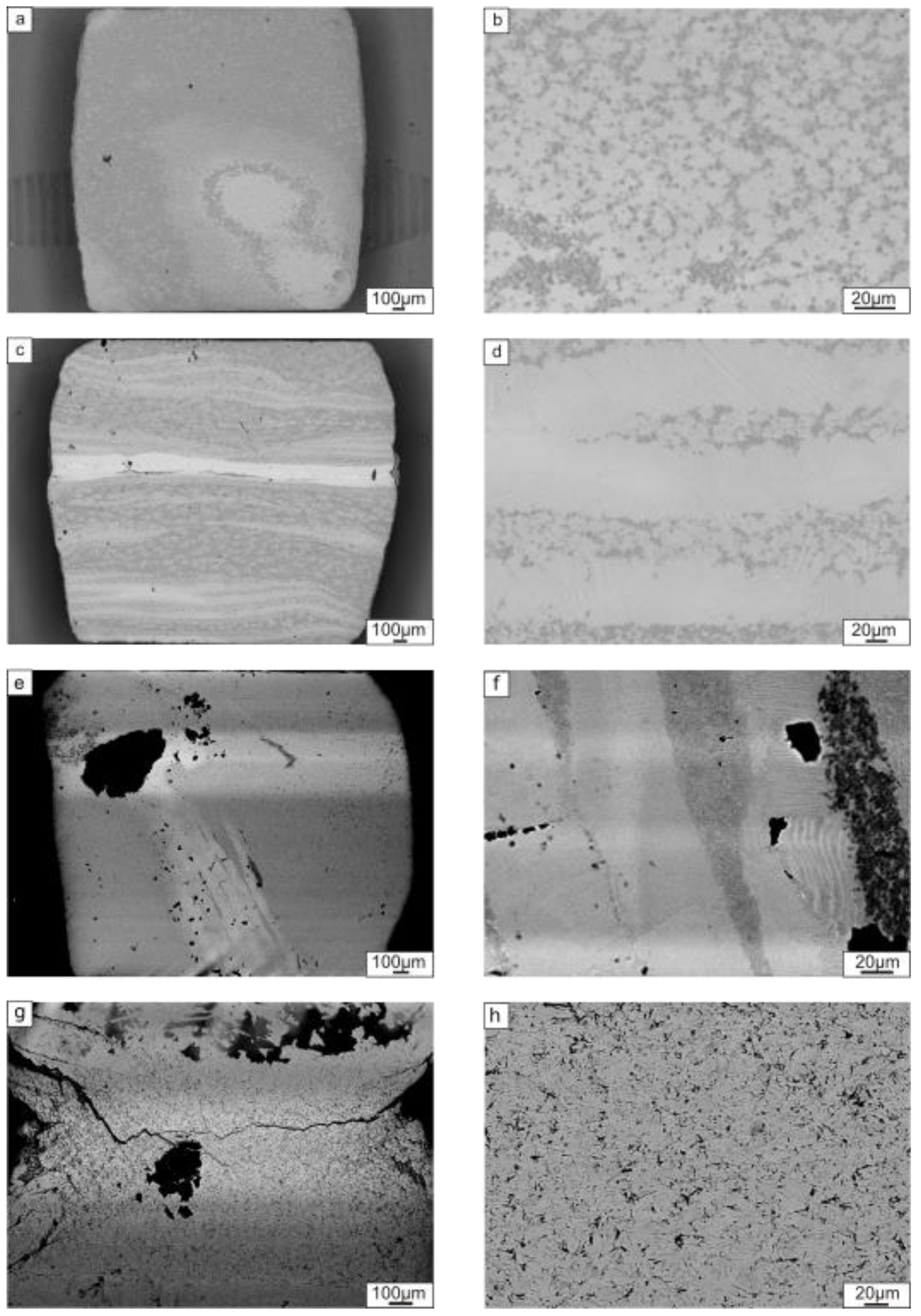
3.2.1. Characterization of Composite Structure
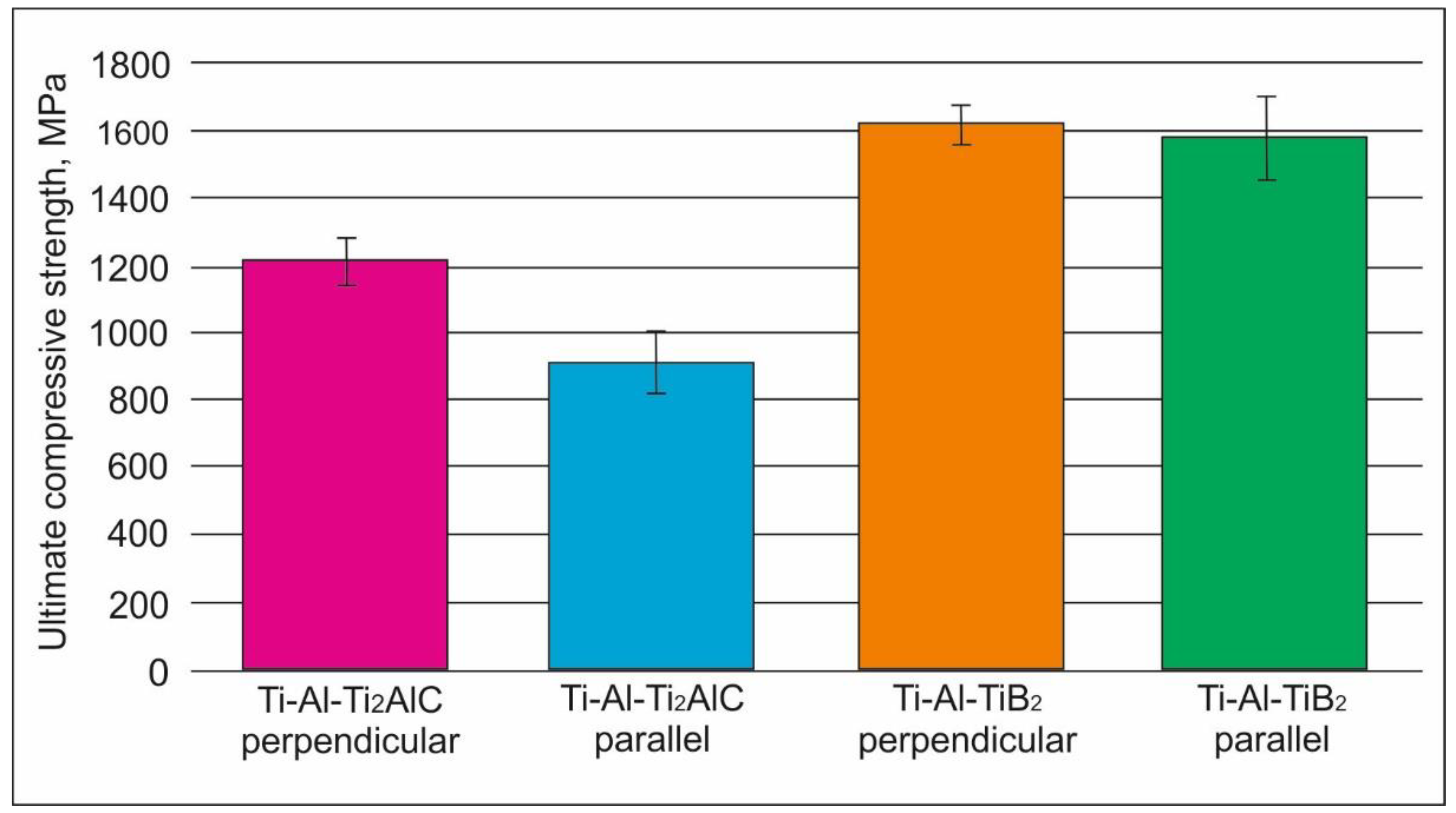
3.2.2. Mechanical Properties of the Materials
4. Conclusions
- The sequence of phase transformation leading to the formation of a two-phase (α2 + γ) structure from the reaction of Ti and Al was studied using in situ synchrotron X-ray diffraction analysis. The formation process occurred via a series of liquid–solid and solid–solid reactions and was accompanied by the formation of a number of intermediate phases. Some of these intermediate phases (e.g., Al11Ti5) were preserved in the material until the final stage of sintering at 1250 °С. The precipitation of Ti3Al occurred during cooling.
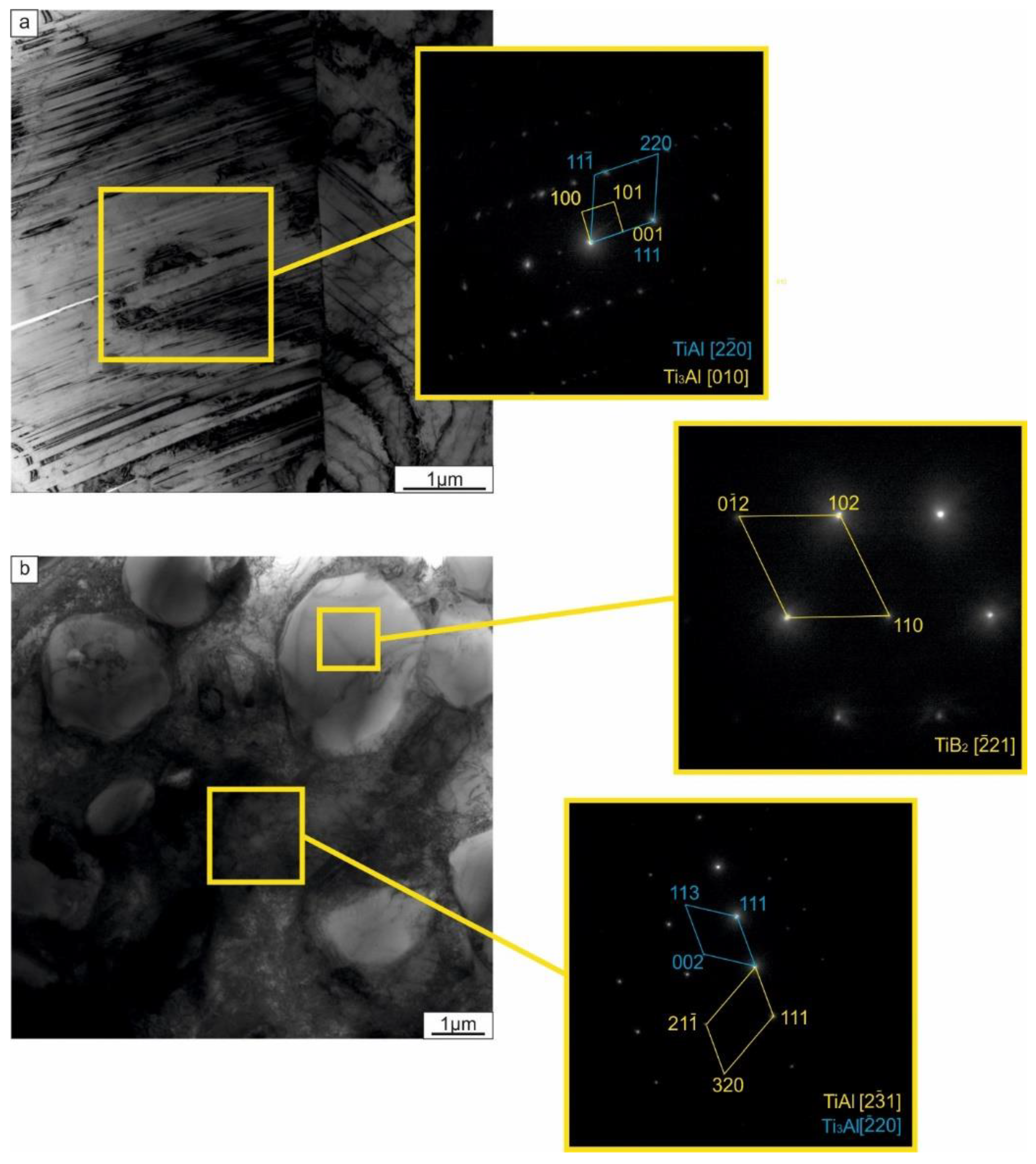
- Heating the ceramic particle containing laminated samples to 1250 °С resulted in reactions between the metallic components themselves and also with the reinforcement particles (in the case of TiC reinforcement). For the composite made with TiC, a reaction between the TiC and the metallic elements produced a Ti2AlC MAX phase. At the same time, the reaction between ceramic particles and the matrix in the sample reinforced by TiB2 was not observed. TiB2 particles did not form a solid layer; TEM investigations revealed that the reinforcement layers consisted of TiB2 within an intermetallic matrix.
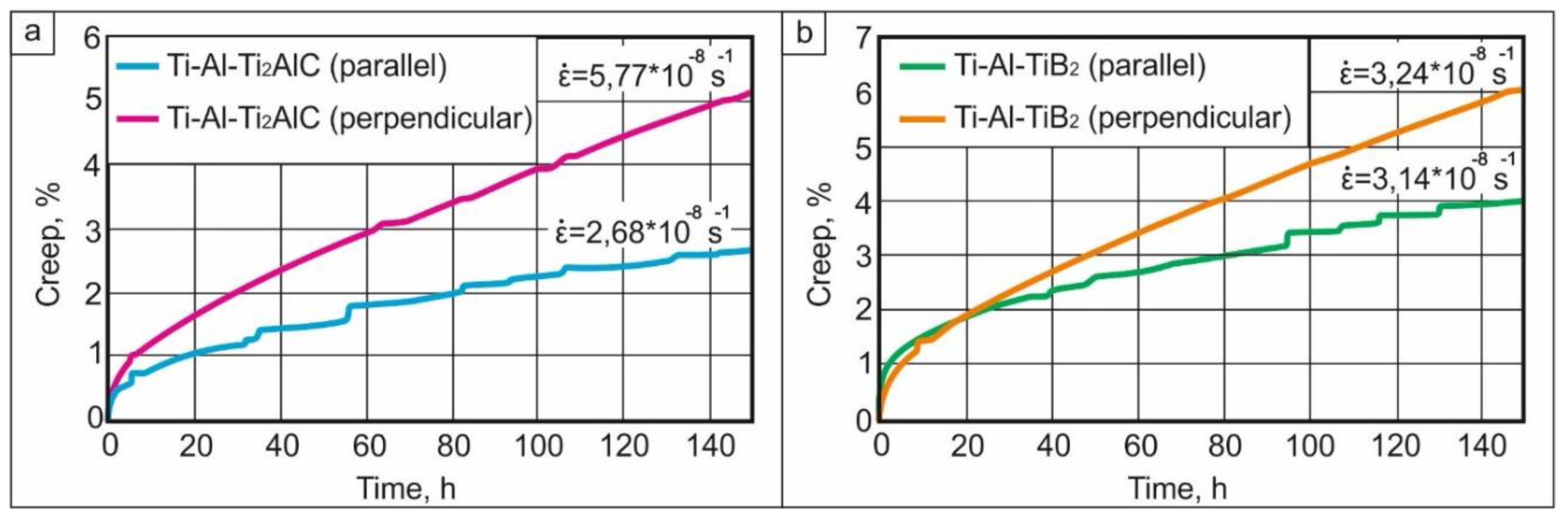
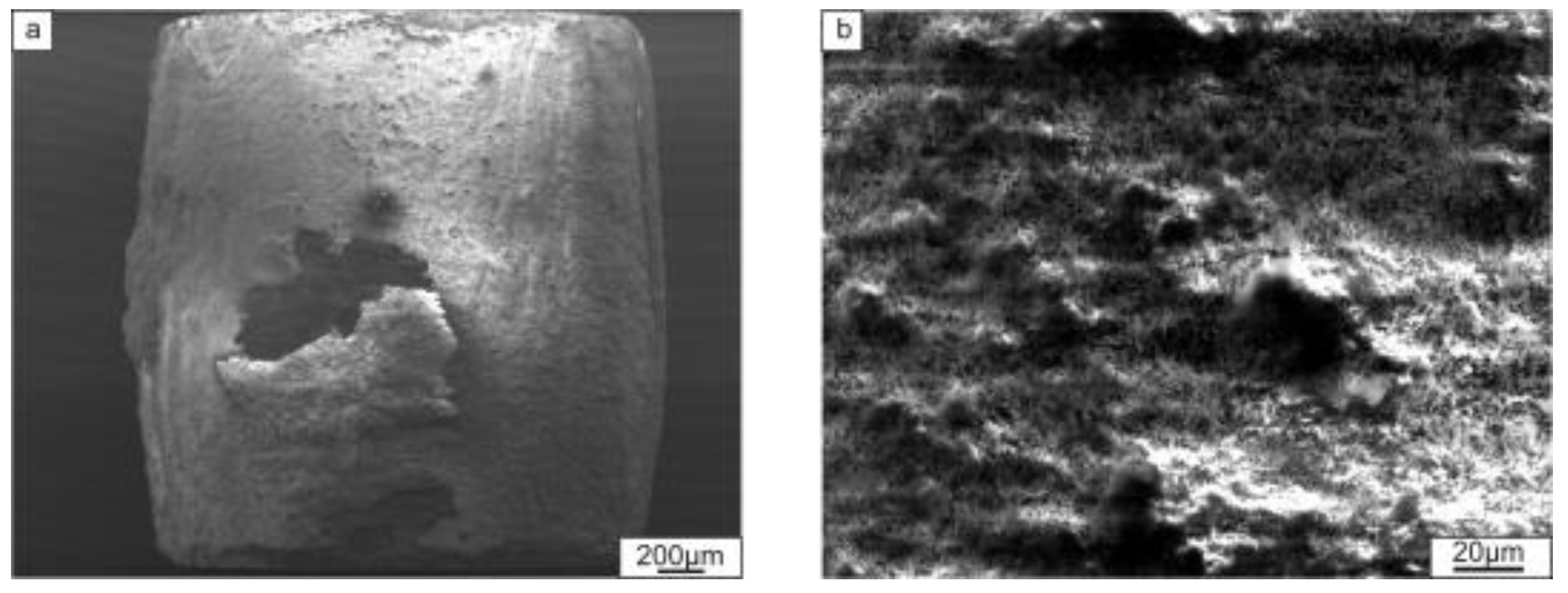
- Compression tests showed that the strength of the material reinforced by TiB2 was higher than that of the Ti2AlC (TiC-based composite) material. The relation between orientation of the layers with respect to the loading direction and mechanical properties was observed only in the case of Ti2AlC reinforcement. The room temperature strength was higher when loading was perpendicular to the layers. However, this loading orientation had worse creep resistance at 750 °C/250 MPa than when loading was performed parallel to the layers.
Author Contributions
Funding
Conflicts of Interest
References
- Mouritz, A.P. (Ed.) Superalloys for gas turbine engines. In Introduction to Aerospace Materials; Woodhead Publishing: Cambridge, UK, 2012; pp. 251–267. [Google Scholar]
- Stoloff, N.S. Physical and mechanical metallurgy of Ni3Al and its alloys. Int. Mater. Rev. 1989, 34, 153–184. [Google Scholar] [CrossRef]
- Jozwik, P.; Polkowski, W.; Bojar, Z. Applications of Ni3Al based intermetallic alloys-current stage and potential perceptivities. Materials 2015, 8, 2537–2568. [Google Scholar] [CrossRef]
- Sauthoff, G. Intermetallics; VCH: Weinheim, Germany, 1995. [Google Scholar]
- Gupta, R.K.; Pant, B.; Sinha, P.P. Theory and practice of γ + α2 Ti aluminide: A review. Trans. Indian Inst. Met. 2014, 67, 143–165. [Google Scholar] [CrossRef]
- Tetsui, T. Effects of high niobium addition on the mechanical properties and high-temperature deformability of gamma TiAl alloy. Intermetallics 2002, 10, 239–245. [Google Scholar] [CrossRef]
- Huang, S.C.; Chesnutt, J.C. Gamma TiAl and its Alioys. In Intermetallic Compounds; Westbrook, J.H., Fleischer, R.L., Eds.; John Wiley & Sons: New York, NY, USA, 1995; Volume 2, pp. 73–90. [Google Scholar]
- Nathal, M.V.; Darolia, R.; Liu, C.T.; Martin, P.L.; Miracle, D.B.; Wagner, R.; Yamaguchi, M. Structural Intermetallics; TMS: Warrendale, PA, USA, 1997; p. 157. [Google Scholar]
- Tetsui, T. Development of a TiAl turbocharger for passenger vehicles. Mater. Sci. Eng. A 2002, 329–331, 582–588. [Google Scholar] [CrossRef]
- Loria, E.A. Gamma titanium aluminides as prospective structural materials. Intermetallics 2000, 8, 1339–1345. [Google Scholar] [CrossRef]
- Westbrook, J.H. Applications of Intermetallic Compounds. MRS Bull. 2013, 21, 26–29. [Google Scholar] [CrossRef]
- Westbrook, J.H.; Fleisher, R.L. Magnetic, Electrical and Optical Properties and Applications of Intermetallic Compounds; Buffins Lane: Chichester, UK, 2000; p. 221. [Google Scholar]
- Appel, F.; Paul, J.D.H.; Oehring, M. Gamma Titanium Aluminide Alloys; Wiley-VCH Verlag GmbH&Co. KGaA: Weinheim, Germany, 2011; p. 752. [Google Scholar]
- Cheng, T.T.; Wills, M.R.; Jones, I.P. Effect of major alloying additions on the microstrusture and mechanical properties of γ-TiA. Intermetallics 1999, 7, 89–99. [Google Scholar] [CrossRef]
- Kevorkijan, V.S.; Škapin, D. Fabrication and characterization of TiAl/Ti3Al-based intermetallic composites (IMCS) reinforced with ceramic particles. Assoc. Metall. Eng. Serbia AMES 2009, 15, 75–89. [Google Scholar]
- Dong, L.; Zhang, W.; Li, J.; Yin, Y. Fabrication of TiAl/B4C composites from an Al-Ti-B4C system reinforced by TiC, TiB2 in-situ. Adv. Mater. Res. 2009, 79–82, 477–480. [Google Scholar] [CrossRef]
- Kakitsuji, A.; Miyamoto, H.; Shingu, H.; Mabuchi, H.; Tsuda, H.; Morii, K. Combustion Synthesis of TiAl-Base Composite under High Pressure. Rev. High Press. Sci. Technol./Koatsuryoku No Kagaku To Gijutsu 1998, 7, 1075–1077. [Google Scholar] [CrossRef]
- Ai, T.T. Microstructures and mechanical properties of in-situ Al2O3/TiAl composites by exothermic dispersion method. Acta Metall. Sin. 2008, 21, 437–443. [Google Scholar] [CrossRef]
- Yue, Y.L.; Gong, Y.S.; Wu, H.T.; Wang, C.B.; Zhang, L.M. Fabrication and Mechanical Properties of TiC/TiAl Composites. J. Wuhan Unive. Technol. Mater. Sci. Ed. 2004, 19, 1–4. [Google Scholar]
- Chen, R.; Fang, H.; Chen, X.; Su, Y.; Ding, H.; Guo, J.; Fu, H. Formation of TiC/Ti2AlC and α2+γ in in-situ TiAl composites with different solidification paths. Intermetallics 2017, 81, 9–15. [Google Scholar] [CrossRef]
- Chen, Y.L.; Yan, M.; Sun, Y.M.; Mei, B.C.; Zhu, J.Q. The phase transformation and microstructure of TiAl/T Ti2AlC composites caused by hot pressing. Ceram. Int. 2009, 35, 1807–1812. [Google Scholar] [CrossRef]
- Cheng, J.; Li, F.; Fu, L.; Qiao, Z.; Yang, J.; Liu, W. Dry-sliding tribological properties of TiAl/T Ti2AlC composites. Tribol. Lett. 2014, 53, 457–467. [Google Scholar] [CrossRef]
- Lee, T.W.; Lee, C.H. Microstructure and mechanical properties of TiB2/TiAl composites produced by reactive sintering using a powder extrusion technique. J. Mater. Sci. Lett. 1999, 18, 801–803. [Google Scholar] [CrossRef]
- Hashimoto, K.; Fujino, Y.; Kuramata, T. Oxidation behavior of SiC/TiAl composite material. Solid State Phenom. 2007, 127, 109–114. [Google Scholar] [CrossRef]
- Lee, D.B.; Park, J.H.; Park, Y.H.; Kim, Y.J. High temperature oxidation of TiAl/SiCp composites manufactured by MA-SPS process. Mater. Trans. JIM 1997, 38, 306–311. [Google Scholar] [CrossRef]
- Liang, W.L.; Hu, R.; Liu, Y.W.; Zhang, T.B.; Li, J.S. Mechanical properties and microstructure of in situ formed Ti2AlN/TiAl(WMS) composites. Rare Metals 2014. [Google Scholar] [CrossRef]
- Liu, P.; Sun, D.; Han, X.; Wang, Q. Investigation on the crystallographic orientation relationships and interface atomic structures in an in-situ Ti2AlN/TiAl composite. Mater. Des. 2017, 130, 239–249. [Google Scholar] [CrossRef]
- Nambu, S.; Michiuchi, M.; Inoue, J.; Koseki, T. Effect of interfacial bonding strength on tensile ductility of multilayered steel composites. Compos. Sci. Technol. 2009, 69, 1936–1941. [Google Scholar] [CrossRef]
- Lazurenko, D.V.; Bataev, I.A.; Mali, V.I.; Lozhkina, E.A.; Esikov, M.A.; Bataev, V.A. Structural Transformations Occurring upon Explosive Welding of Alloy Steel and High-Strength Titanium. Phys. Met. Metallogr. 2018, 119, 469–476. [Google Scholar] [CrossRef]
- Vecchio, K.S. Synthetic multifunctional metallic-intermetallic laminate composites. JOM 2005, 57, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Vecchio, K.S.; Jiang, F. Fracture toughness of Ceramic-Fiber-Reinforced Metallic-Intermetallic-Laminate (CFR-MIL) composites. Mater. Sci. Eng. A 2016, 649, 407–416. [Google Scholar] [CrossRef]
- Han, Y.; Lin, C.; Han, X.; Chang, Y.; Guo, C.; Jiang, F. Fabrication, interfacial characterization and mechanical properties of continuous Al2O3 ceramic fiber reinforced Ti/Al3Ti metal-intermetallic laminated (CCFR-MIL) composite. Mater. Sci. Eng. A 2017, 688, 338–345. [Google Scholar] [CrossRef]
- Lin, C.; Han, Y.; Guo, C.; Chang, Y.; Han, X.; Lan, L.; Jiang, F. Synthesis and mechanical properties of novel Ti-(SiCf/Al3Ti) ceramic-fiber-reinforced metal-intermetallic-laminated (CFR-MIL) composites. J. Alloys Compd. 2017, 722, 427–437. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Jiang, F.; Zhang, M.; Wang, L.; Yun, F. Elasto-Plastic Mechanical Properties and Failure Mechanism of Innovative Ti-(SiCf/Al3Ti) Laminated Composites for Sphere-Plane Contact at the Early Stage of Penetration Process. Materials 2018, 11, 1152. [Google Scholar] [CrossRef] [PubMed]
- Bataev, I.A.; Pavliukova, D.V.; Zhuravina, T.V.; Makarova, E.B.; Terentiev, D.S. Formation by welding by explosion of layered composite materials from diverse steels. Obrab. Metallov Met. Work. Mater. Sci. 2010, 1, 6–8. [Google Scholar]
- Lesuer, D.R.; Syn, C.K.; Sherby, O.D.; Wadsworth, J.; Lewandowski, J.J.; Hunt, W.H., Jr. Mechanical behaviour of laminated metal composites. Int. Mater. Rev. 1996, 41, 169–197. [Google Scholar] [CrossRef]
- Li, T.; Jiang, F.; Olevsky, E.A.; Vecchio, K.S.; Meyers, M.A. Damage evolution in Ti6Al4V-Al3Ti metal-intermetallic laminate composites. Mater. Sci. Eng. A 2007, 443, 1–15. [Google Scholar] [CrossRef]
- Lazurenko, D.V.; Mali, V.I.; Bataev, I.A.; Thoemmes, A.; Bataev, A.A.; Popelukh, A.I.; Anisimov, A.G.; Belousova, N.S. Metal-Intermetallic Laminate Ti-Al3Ti Composites Produced by Spark Plasma Sintering of Titanium and Aluminum Foils Enclosed in Titanium Shells. Metall. Mater. Trans. A 2015, 46, 4326–4334. [Google Scholar] [CrossRef]
- Lazurenko, D.V.; Bataev, I.A.; Mali, V.I.; Bataev, A.A.; Maliutina, I.N.; Lozhkin, V.S.; Esikov, M.A.; Jorge, A.M.J. Explosively welded multilayer Ti-Al composites: Structure and transformation during heat treatment. Mater. Des. 2016, 102, 122–130. [Google Scholar] [CrossRef]
- Schuster, J.C.; Palm, M. Reassessment of the binary aluminum-titanium phase diagram. J. Phase Equilib. Diffus. 2006, 27, 255–277. [Google Scholar] [CrossRef]
- Vanmeensel, K.; Laptev, A.; Hennicke, J.; Vleugels, J.; Van Der Biest, O. Modelling of the temperature distribution during field assisted sintering. Acta Mater. 2005, 53, 4379–4388. [Google Scholar] [CrossRef]
- Harach, D.J.; Vecchio, K.S. Microstructure evolution in metal-intermetallic laminate (MIL) composites synthesized by reactive foil sintering in air. Metall. Mater. Trans. A 2001, 32, 1493–1505. [Google Scholar] [CrossRef]
- Cornish, L.; Cacciamani, G.; Cupid, D.M.; De Keyzer, J.; Materials Science International Team. Partial Isothermal Section at 1250 °C: Datasheet from MSI Eureka in Springer Materials; MSI, Materials Science International Services GmbH: Stuttgart, Germany, 2009; Available online: http://materials.springer.com/msi/phase-diagram/docs/sm_msi_r_10_014870_02_full_LnkDia2 (accessed on 15 October 2018).
- Xiao, Z.; Zhu, X.; Chu, Z.; Xu, W.; Wang, Z.; Wu, B. Investigation of Ti2AlC formation mechanism through carbon and TiAl diffusional reaction. J. Eur. Ceram. Soc. 2018, 38, 1246–1252. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Venkateswaran, T.; Basu, B. Spark plasma sintering may lead to phase instability and inferior mechanical properties: A case study with TiB2. Scr. Mater. 2013, 69, 159–164. [Google Scholar] [CrossRef]
- Eriksson, M.; Salamon, D.; Nygren, M.; Shen, Z. Spark plasma sintering and deformation of Ti–TiB2 composites. Mater. Sci. Eng. A 2008, 475, 101–104. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Shen, X.-B.; Wang, F.-C.; Lee, S.-K.; Fan, Q.-B.; Cao, M.-S. Low-temperature densification of TiB2 ceramic by the spark plasma sintering process with Ti as a sintering aid. Scr. Mater. 2012, 66, 167–170. [Google Scholar] [CrossRef]
- Hong, X.; Mei, B.; Zhu, J.; Zhou, W. Fabrication of Ti2AlC by hot pressing of Ti, TiC, Al and active carbon powder mixtures. J. Mater. Sci. 2004, 39, 1589–1592. [Google Scholar] [CrossRef]
- Barsoum, M.W.; El-Raghy, T.; Ali, M. Processing and characterization of Ti2AlC, Ti2AlN, and Ti2AlC0.5N0.5. Metall. Mater. Trans. A 2000, 31, 1857–1865. [Google Scholar] [CrossRef]
- Shu, S.; Tong, C.; Qiu, F.; Jiang, Q. Effect of Ceramic Content on the Compression Properties of TiB2-Ti2AlC/TiAl Composites. Metals 2015, 5, 2200–2209. [Google Scholar] [CrossRef]
- Shu, S.; Tong, C.; Qiu, F.; Jiang, Q. Effect of Mn, Fe and Co on the compression strength and ductility of in situ nano-sized TiB2/TiAl composites. SpringerPlus 2015, 4, 784. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Qiu, F.; Xing, B.; Jin, S.; Wang, J.; Jiang, Q. Effect of strain rate on the compression behavior of TiAl and TiAl–2Mn alloys fabricated by combustion synthesis and hot press consolidation. Intermetallics 2013, 43, 24–28. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Viswanathan, G.B.; Gouma, P.I.; Vasudevan, V.K.; Kim, Y.W.; Mills, M.J. Mechanisms and effect of microstructure on creep of TiAl-based alloys. Mater. Sci. Eng. A 2002, 329–331, 621–630. [Google Scholar] [CrossRef]
- Wang, J.N.; Schwartz, A.J.; Nieh, T.G.; Liu, C.T.; Sikka, V.K.; Clemens, D. Creep of a Fine-Grained, Fully-Lamellar, Two-Phase TiAl Alloy at 760 °C; Lawrence Livermore National Lab.: Livermore, CA, USA, 1995. [Google Scholar]
- Harrison, W.; Abdallah, Z.; Whittaker, M. A model for creep and creep damage in the γ-titanium aluminide Ti45Al2Mn2Nb. Materials 2014, 7, 2194–2209. [Google Scholar] [CrossRef] [PubMed]
- Dlouhy, A.; Kuchařová, K. Creep and microstructure of near-gamma TiAl alloys. Intermetallics 2004, 12, 705–711. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazurenko, D.V.; Stark, A.; Esikov, M.A.; Paul, J.; Bataev, I.A.; Kashimbetova, A.A.; Mali, V.I.; Lorenz, U.; Pyczak, F. Ceramic-Reinforced γ-TiAl-Based Composites: Synthesis, Structure, and Properties. Materials 2019, 12, 629. https://doi.org/10.3390/ma12040629
Lazurenko DV, Stark A, Esikov MA, Paul J, Bataev IA, Kashimbetova AA, Mali VI, Lorenz U, Pyczak F. Ceramic-Reinforced γ-TiAl-Based Composites: Synthesis, Structure, and Properties. Materials. 2019; 12(4):629. https://doi.org/10.3390/ma12040629
Chicago/Turabian StyleLazurenko, Daria V., Andreas Stark, Maksim A. Esikov, Jonathan Paul, Ivan A. Bataev, Adelya A. Kashimbetova, Vyacheslav I. Mali, Uwe Lorenz, and Florian Pyczak. 2019. "Ceramic-Reinforced γ-TiAl-Based Composites: Synthesis, Structure, and Properties" Materials 12, no. 4: 629. https://doi.org/10.3390/ma12040629





