Composite Materials Design: Biomineralization Proteins and the Guided Assembly and Organization of Biomineral Nanoparticles
Abstract
:1. Introduction
2. Biogenic Mesocrystals and Their Nanoparticle Components
2.1. The Sea Urchin Spicule
2.2. The Mollusk Shell Nacre Layer
3. Hydrogels and Biomineralization
4. Biomineral-Associated Protein Families Are “Smart” Hydrogelators
5. Evidence for Protein-Driven Particle Assembly and Organization
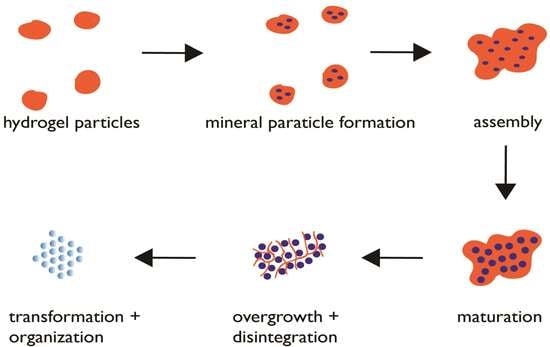
6. Proposed Mechanism of Protein-Directed Nanoparticle Assembly into a Crystal Format
7. The Future: Where Do We Go from Here?
Funding
Acknowledgments
Conflicts of Interest
References
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press: New York, NY, USA, 1989; pp. 1–134. ISBN 0-19-504977-2. [Google Scholar]
- Mann, S. Biomineralization. Principles and Concepts in Bioinorganic Materials Chemistry; Oxford University Press: New York, NY, USA, 2001; pp. 6–9, 24–108. [Google Scholar]
- Sodergren, E.; Weinstock, G.M.; Davidson, E.H.; Cameron, R.A.; Gibbs, R.A.; Angerer, R.C.; Angerer, L.M.; Arnone, M.I.; Burgess, D.R.; Burke, R.D.; et al. The genome of the sea urchin Strongylocentrotus purpuratus. Science 2006, 314, 941–952. [Google Scholar] [CrossRef]
- Zhang, G.; Fang, X.; Guo, X.; Li, L.; Luo, R.; Xu, F.; Yang, P.; Zhang, L.; Wang, X.; Qi, H.; et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 2012, 490, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, D.; Xu, G.; Hu, Y.; Pan, C.; Xie, L.; Zhang, R. Identification of genes directly involved in shell formation and their functions in pearl oyster, Pinctada fucata. PLoS ONE 2011, 6, 1–13. [Google Scholar] [CrossRef]
- Jackson, D.J.; McDougall, C.; Woodcroft, B.; Moase, P.; Rose, R.A.; Kube, M.; Reinhart, R.; Rokhsar, D.S.; Montagnani, C.; Joube, C.; et al. Parallel evolution of nacre building gene sets in mollusks. Mol. Biol. Evol. 2010, 27, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.L.; Mass, T.; Haramaty, L.; Zelzion, E.; Bhattacharya, D.; Falkowski, P.G. Proteomic analysis of skeletal organic matrix from the stony coral Stylophora pistillata. Proc. Natl. Acad. Sci. USA 2013, 110, 3788–3793. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Iwashima, A.; Kimura, M.; Kogure, T.; Nagasawa, H. The molecular evolution of the Pif family proteins in various species of mollusks. Mar. Biotechnol. 2013, 15, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Su, J.; Zheng, G.; Liang, J.; Zhang, G.; Wang, H.; Xie, L.; Zhang, R. Patterns of expression in the matrix proteins responsible for nucleation and growth of aragonite crystals in flat pearls of Pinctada fucata. PLoS ONE 2013, 8, e66564. [Google Scholar] [CrossRef]
- Marie, B.; Joubert, C.; Tayale, A.; Zanella-Cleon, I.; Belliard, C.; Piquemal, D.; Cochennec-Laureau, N.; Marin, F.; Gueguen, Y.; Montagnani, C. Different secretory repertoires control the biomineralization processes of prism and nacre deposition of the pearl oyster shell. Proc. Natl. Acad. Sci. USA 2012, 109, 20986–20991. [Google Scholar] [CrossRef] [Green Version]
- Mann, L.; Poustka, A.J.; Mann, M. The sea urchin (Strongylocentrotus purpuratus) test and spine proteomes. Proteome Sci. 2008, 6, 1–10. [Google Scholar] [CrossRef]
- Mann, K.; Wilt, F.H.; Poustka, A.J. Proteomic analysis of the sea urchin (Strongylocentrotus purpuratus) spicule matrix. Proteome Sci. 2010, 8, 1–12. [Google Scholar] [CrossRef]
- Evans, J.S. Polymorphs, Proteins, and Nucleation Theory: A Critical Analysis. Minerals 2017, 7, 62. [Google Scholar] [CrossRef]
- Evans, J.S. “Liquid-like” biomineralization protein assemblies: A key to the regulation of non-classical nucleation. Cryst. Eng. Commun. 2013, 15, 8388–8394. [Google Scholar] [CrossRef]
- Evans, J.S. “Tuning in” to mollusk shell nacre- and prismatic-associated protein terminal sequences. Implications for biomineralization and the construction of high-performance inorganic-organic composites. Chem. Rev. 2008, 108, 4455–4462. [Google Scholar] [CrossRef] [PubMed]
- Aizenberg, J.; Lambert, G.; Addadi, L.; Weiner, S. Stabilization of amorphous calcium carbonate by specialized macromolecules in biological and synthetic precipitates. Adv. Mater. 1996, 8, 222–226. [Google Scholar] [CrossRef]
- Addadi, L.; Raz, S.; Weiner, S. Taking advantage of disorder: Amorphous calcium carbonate and its roles in biomineralization. Adv. Mater. 2003, 15, 959–970. [Google Scholar] [CrossRef]
- Stephens, C.J.; Ladden, S.F.; Meldrum, F.C.; Christenson, H.K. Amorphous calcium carbonate is stabilized in confinement. Adv. Mater. 2010, 20, 2108–2115. [Google Scholar] [CrossRef]
- Politi, Y.; Arad, T.; Klein, E.; Weiner, S.; Addadi, L. Sea urchin spine calcite forms via a transient amorphous calcium carbonate phase. Science 2004, 306, 1161–1164. [Google Scholar] [CrossRef]
- Gal, A.; Habraken, W.; Gur, D.; Fratzl, P.; Weiner, S.; Addadi, L. Calcite crystal growth by a solid-state transformation of stabilized amorphous calcium carbonate nanospheres in a hydrogel. Angew. Chem. 2013, 52, 4967–4970. [Google Scholar] [CrossRef]
- Gong, Y.U.T.; Killian, C.E.; Olson, I.C.; Appathurai, N.P.; Amasino, A.L.; Martin, M.C.; Holt, L.J.; Wilt, F.H.; Gilbert, P.U.P.A. Phase transitions in biogenic amorphous calcium carbonate. Proc. Natl. Acad. Sci. USA 2012, 109, 6088–6093. [Google Scholar] [CrossRef] [Green Version]
- Wallace, A.F.; Hedges, L.O.; Fernandez-Martinez, A.; Raiteri, P.; Gale, J.D.; Waychunas, G.A.; Whitelam, S.; Banfield, J.F.; De Yoreo, J.J. Microscopic evidence for liquid-liquid separation in supersaturated calcium carbonate solutions. Science 2013, 341, 885–889. [Google Scholar] [CrossRef]
- Radha, A.V.; Forbes, T.Z.; Killian, C.E.; Gilbert, P.U.P.A.; Navrotsky, A. Transformation and crystallization energetics of synthetic and biogenic amorphous calcium carbonate. Proc. Natl. Acad. Sci. USA 2010, 107, 16438–16443. [Google Scholar] [CrossRef] [Green Version]
- Colfen, H.; Antonietti, M. Mesocrystals: Inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew. Chem. 2005, 44, 5576–5591. [Google Scholar] [CrossRef] [PubMed]
- Wucher, B.; Yue, W.; Kulak, A.N.; Meldrum, F.C. Designer crystals: Single crystals with complex morphologies. Chem. Mater. 2007, 19, 1111–1119. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Schenk, A.S.; Ihli, J.; Kulak, A.N.; Hetherington, N.B.J.; Tang, C.C.; Schmahl, W.W.; Griesshaber, E.; Hyett, G.; Meldrum, F.C. A critical analysis of calcium carbonate mesocrystals. Nat. Commun. 2014, 5, 4341. [Google Scholar] [CrossRef] [PubMed]
- Seto, J.; Ma, Y.; Davis, S.A.; Meldrum, F.; Schilde, U.; Gourrier, A.; Jaeger, C.; Coelfen, H. Structure-property relationships of a biological mesocrystal in the adult sea urchin spine. Proc. Natl. Acad. Sci. USA 2012, 109, 3699–3704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergstrom, L.; Sturm, E.V.; Salazar-Alvarez, G.; Coelfen, H. Mesocrystals in biominerals and colloidal arrays. Acc. Chem. Res. 2015, 48, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.M.; Willinger, M.G.; Pina, C.M.; Checa, A.G. Transformation of ACC into aragonite and the origin of the nanogranular structure of nacre. Sci. Rep. 2017, 7, 12728. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.T.; Yao, Q.Z.; Ni, J.; Jin, G. Formation of aragonite mesocrystals and implication for biominerlization. Am. Mineral. 2009, 94, 293–302. [Google Scholar] [CrossRef]
- De Yoreo, J.J.; Gilbert, P.U.P.A.; Sommerdijk, N.A.J.M.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349, 498–508. [Google Scholar] [CrossRef]
- Levy, Y.; Onuchic, J.N. Mechanisms of protein assembly: Lessons from minimalist models. Acc. Chem. Res. 2006, 39, 135–142. [Google Scholar] [CrossRef]
- Papapostolou, D.; Smith, A.M.; Atkins, E.D.T.; Oliver, S.J.; Ryadnov, M.G.; Serpell, L.C.; Woolfson, D.N. Engineering nanoscale order into a designed protein fiber. Proc. Natl. Acad. Sci. USA 2007, 104, 10853–10858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsh, J.A.; Teichmann, S.A. Structure, dynamics, assembly and evolution of protein complexes. Ann. Rev. Biochem. 2015, 84, 551–575. [Google Scholar] [CrossRef]
- Beniash, E.; Addadi, L.; Weiner, S. Cellular control over spicule formation in sea urchin embryos: A structural approach. J. Struct. Biol. 1999, 125, 50–62. [Google Scholar] [CrossRef]
- Wilt, F.H. Biomineralization of the spicules of sea urchin embryo. Zoolog. Sci. 2002, 19, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Wilt, F.H.; Ettensohn, C.E. Morphogenesis and biomineralization of the sea urchin larval endoskeleton. In Handbook of Biomineralization: Biological Aspects and Structure Formation; Baeuerlein, E., Ed.; Wiley-VCH: Weinheim, Germany, 2008; pp. 183–210. [Google Scholar]
- Zhang, G.; Li, X. Uncovering aragonite nanoparticle self-assembly in nacre—A natural armor. Cryst. Growth Des. 2012, 12, 4306–4310. [Google Scholar] [CrossRef]
- Li, X.; Chang, W.C.; Chao, Y.J.; Wang, R.; Chang, M. Nanoscale structural and mechanical characterization of a natural nanocomposite material. The shell of the red abalone. Nanoletters 2004, 4, 613–617. [Google Scholar] [CrossRef]
- Sun, J.; Bhushan, B. Hierarchical structure and mechanical properties of nacre: A review. RSC Adv. 2012, 2, 7617–7632. [Google Scholar] [CrossRef]
- Li, X.; Huang, Z. Unveiling the formation mechanism of pseudo-single-crystal aragonite platelets in nacre. Phys. Rev. Lett. 2009, 102, 075502–075506. [Google Scholar] [CrossRef]
- Zheng, G.; Xu, J. From colloidal nanoparticles to a single crystal: New insights into the formation of nacre’s aragonite tablets. J. Struct. Biol. 2013, 182, 36–43. [Google Scholar] [CrossRef]
- Metzler, R.A.; Evans, J.S.; Killian, C.E.; Zhou, D.; Churchill, T.H.; Appathurai, P.N.; Coppersmith, S.N.; Gilbert, P.U.P.A. Lamellar self-assembly and aragonite polymorph selection by a single intrinsically disordered protein fragment. J. Am. Chem. Soc. 2010, 132, 6329–6334. [Google Scholar] [CrossRef]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk shell formation: A source of new concepts for understanding biomineralization processes. Chem. Eur. J. 2006, 12, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Mouries, L.; Ribeiro, A.M.J.; Barathelemy, P.J.; Milet, C.; Lopez, E. Soluble silk-like organic matrix in the nacreous layer of the bivalve Pinctada maxima. Eur. J. Biochem. 2002, 269, 4994–5003. [Google Scholar] [CrossRef] [Green Version]
- Samchenko, Y.; Ulberg, Z.; Korotych, O. Multipurpose smart hydrogel systems. Adv. Colloid Interfacial Sci. 2011, 147, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–260. [Google Scholar] [CrossRef]
- Lim, H.L.; Hwang, Y.; Kar, M.; Varghese, S. Smart hydrogels as functional biomimetic systems. Biomater. Sci. 2014, 2, 603–618. [Google Scholar] [CrossRef]
- Xia, L.W.; Xie, R.; Ju, X.J.; Wang, W.; Chen, Q.; Chu, L.Y. Nano-structured smart hydrogels with rapid response and high elasticity. Nat. Commun. 2013, 4, 2226–2237. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-hydrogel: A hybrid biomaterial system for localized drug delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061. [Google Scholar] [CrossRef]
- Thoniyot, P.; Tan, M.J.; Loh, X.J. Nanoparticle-hydrogel composites. Concept, design, and applications of these promising, multifunctional materials. Adv. Sci. (Weinh) 2015, 2, 1–12. [Google Scholar] [CrossRef]
- Cantaert, B.; Beniash, E.; Meldrum, F.C. Nanoscale confinement controls the crystallization of calcium phosphate: Relevance to bone formation. Chemistry 2013, 19, 14918–14924. [Google Scholar] [CrossRef]
- Ping, H.; Xie, H.; Wan, Y.; Zhang, Z.; Zhang, J.; Xiang, M.; Xie, J.; Wang, H.; Wang, W.; Fu, Z. Confinement controlled mineralization of calcium carbonate within collagen fibrils. J. Mater. Chem. B 2016, 4, 880–886. [Google Scholar] [CrossRef]
- Risan, J.; Jain, G.; Pendola, M.; Evans, J.S. Intracrystalline incorporation of nacre protein hydrogels modifies the mechanical properties of calcite crystals: A microcompression study. J. Mater. Chem. B 2018, 6, 4191–4196. [Google Scholar] [CrossRef]
- Jain, G.; Pendola, M.; Huang, Y.C.; Gebauer, D.; Koutsoumpeli, E.; Johnson, S.; Evans, J.S. Selective synergism created by interactive nacre framework-associated proteins possessing EGF and vWA motifs. Implications for mollusk shell formation. Biochemistry 2018, 57, 2657–2666. [Google Scholar] [CrossRef] [PubMed]
- Pendola, M.; Evans, J.S. Non-invasive µCT visualization of mineralization directed by sea urchin- and nacre-specific proteins. Cryst. Growth Des. 2018, 18, 1768–1775. [Google Scholar] [CrossRef]
- Pendola, M.; Evans, J.S. Insights into mollusk shell formation: Interlamellar and lamellar specific nacre protein hydrogels differ in ion interaction signatures. J. Phys. Chem. B 2018, 122, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Jain, G.; Pendola, M.; Huang, Y.C.; Colas, J.J.; Gebauer, D.; Johnson, S.; Evans, J.S. Functional prioritization and hydrogel regulation phenomena created by a combinatorial pearl-associated 2-protein biomineralization model system. Biochemistry 2017, 56, 3607–3618. [Google Scholar] [CrossRef] [PubMed]
- Perovic, I.; Davidyants, A.; Evans, J.S. Aragonite-associated mollusk shell protein aggregates to form mesoscale “smart” hydrogels. ACS Omega 2016, 1, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Pendola, M.; Jain, G.; Davidyants, A.; Huang, Y.C.; Gebauer, D.; Evans, J.S. A nacre protein forms mesoscale hydrogels that “hijack” the biomineralization process within a seawater environment. Cryst. Eng. Commun. 2016, 18, 7675–7679. [Google Scholar] [CrossRef] [Green Version]
- Chang, E.P.; Roncal-Herrero, T.; Morgan, T.; Dunn, K.E.; Rao, A.; Kunitake, J.A.M.R.; Lui, S.; Bilton, M.; Estroff, L.A.; Kroeger, R.; et al. Synergistic biomineralization phenomena created by a nacre protein model system. Biochemistry 2016, 55, 2401–2410. [Google Scholar] [CrossRef]
- Chang, E.P.; Perovic, I.; Rao, A.; Cölfen, H.; Evans, J.S. Insect cell glycosylation and its impact on the functionality of a recombinant intracrystalline nacre protein, AP24. Biochemistry 2016, 55, 1024–1035. [Google Scholar] [CrossRef]
- Chang, E.P.; Evans, J.S. Pif97, a von Willebrand and Peritrophin biomineralization protein organizes mineral nanoparticles and creates intracrystalline nanochambers. Biochemistry 2015, 54, 5348–5355. [Google Scholar] [CrossRef]
- Chang, E.P.; Williamson, G.; Evans, J.S. Focused ion beam tomography reveals the presence of micro, meso, and microporous intracrystalline regions introduced into calcite by the gastropod nacre protein AP7. Cryst. Growth Des. 2015, 15, 1577–1582. [Google Scholar] [CrossRef]
- Perovic, I.; Chang, E.P.; Verch, A.; Rao, A.; Cölfen, H.; Kroeger, R.; Evans, J.S. An oligomeric C-RING nacre protein influences pre-nucleation events and organizes mineral nanoparticles. Biochemistry 2014, 53, 7259–7268. [Google Scholar] [CrossRef]
- Chang, E.P.; Russ, J.A.; Verch, A.; Kroeger, R.; Estroff, L.A.; Evans, J.S. Engineering of crystal surfaces and subsurfaces by an intracrystalline biomineralization protein. Biochemistry 2014, 53, 4317–4319. [Google Scholar] [CrossRef]
- Chang, E.P.; Russ, J.A.; Verch, A.; Kroeger, R.; Estroff, L.A.; Evans, J.S. Engineering of crystal surfaces and subsurfaces by framework biomineralization protein phases. Cryst. Eng. Commun. 2014, 16, 7406–7409. [Google Scholar] [CrossRef]
- Perovic, I.; Chang, E.P.; Lui, M.; Rao, A.; Cölfen, H.; Evans, J.S. A framework nacre protein, n16.3, self-assembles to form protein oligomers that participate in the post-nucleation spatial organization of mineral deposits. Biochemistry 2014, 53, 2739–2748. [Google Scholar] [CrossRef] [PubMed]
- Perovic, I.; Mandal, T.; Evans, J.S. A pseudo EF-hand pearl protein self-assembles to form protein complexes that amplify mineralization. Biochemistry 2013, 52, 5696–5703. [Google Scholar] [CrossRef]
- Amos, F.F.; Ndao, M.; Ponce, C.B.; Evans, J.S. A C-RING-like domain participates in protein self-assembly and mineral nucleation. Biochemistry 2011, 50, 8880–8887. [Google Scholar] [CrossRef]
- Amos, F.F.; Ponce, C.B.; Evans, J.S. Formation of framework nacre polypeptide supramolecular assemblies that nucleate polymorphs. Biomacromolecules 2011, 12, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Ndao, M.; Ponce, C.B.; Evans, J.S. Oligomer formation, metalation, and the existence of aggregation-prone and mobile sequences within the intracrystalline protein family, Asprich. Faraday Discuss. 2012, 159, 449–462. [Google Scholar] [CrossRef]
- Pendola, M.; Jain, G.; Huang, Y.-C.; Gebauer, D.; Evans, J.S. Secrets of the sea urchin spicule revealed: Protein cooperativity is responsible for ACC transformation, intracrystalline incorporation, and guided mineral particle assembly in biocomposite material formation. ACS Omega 2018, 3, 11823–11830. [Google Scholar] [CrossRef] [PubMed]
- Jain, G.; Pendola, M.; Koutsoumpeli, E.; Johnson, S.; Evans, J.S. Glycosylation fosters interactions between model sea urchin spicule matrix proteins. Implications for embryonic spiculogenesis and biomineralization. Biochemistry 2018, 57, 3032–3035. [Google Scholar] [CrossRef] [PubMed]
- Pendola, M.; Davidyants, A.; Jung, Y.S.; Evans, J.S. Sea urchin spicule matrix proteins form mesoscale hydrogels that exhibit selective ion interactions. ACS Omega 2017, 2, 6151–6158. [Google Scholar] [CrossRef]
- Jain, G.; Pendola, M.; Huang, Y.C.; Gebauer, D.; Evans, J.S. A model sea urchin spicule matrix protein, rSpSM50, is a hydrogelator that modifies and organizes the mineralization process. Biochemistry 2017, 56, 2663–2675. [Google Scholar] [CrossRef] [PubMed]
- Jain, G.; Pendola, M.; Rao, A.; Cölfen, H.; Evans, J.S. A model sea urchin spicule matrix protein self-associates to form mineral-modifying protein hydrogels. Biochemistry 2016, 55, 4410–4421. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Völkel, A.; Cölfen, H. Stable prenucleation calcium carbonate clusters. Science 2008, 322, 1819–1822. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Kellermeier, M.; Gale, J.D.; Bergström, L.; Cölfen, H. Pre-nucleation clusters as solute precursors in crystallization. Chem. Soc. Rev. 2014, 43, 2348–2371. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Berg, J.K.; Kellermeier, M.; Gebauer, D. Sweet on biomineralization: Effects of carbohydrates on the early stages of calcium carbonate crystallization. Eur. J. Mineral. 2014, 26, 537–552. [Google Scholar] [CrossRef]
- Gebauer, D.; Gunawidjaja, P.N.; Ko, J.Y.P.; Bacsik, Z.; Aziz, B.; Liu, L.; Hu, Y.; Bergström, L.; Tai, C.W.; Sham, T.K.; et al. Proto-calcite and Proto-vaterite in amorphous calcium carbonates. Angew. Chem. Int. Ed. 2010, 49, 8889–8891. [Google Scholar] [CrossRef]
- Kellermeier, M.; Cölfen, H.; Gebauer, D. Investigating the early stages of mineral precipitation by potentiometric titration and analytical ultracentrifugation. In Methods in Enzymology: Research Methods in Biomineralization Science; De Yoreo, J.J., Ed.; Academic Press: New York, NY, USA, 2013; Volume 532, pp. 45–69. [Google Scholar]
- Evans, J.S. Aragonite-associated biomineralization proteins contain interactive and aggregation-prone motifs. Bioinformatics 2012, 28, 3182–3185. [Google Scholar] [CrossRef]
- Ingersoll, E.P.; Wilt, F.H. Matrix metalloproteinase inhibitors disrupt spicule formation by primary mesenchyme cells in the sea urchin embryo. Dev. Biol. 1998, 196, 95–106. [Google Scholar] [CrossRef]



© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, J.S. Composite Materials Design: Biomineralization Proteins and the Guided Assembly and Organization of Biomineral Nanoparticles. Materials 2019, 12, 581. https://doi.org/10.3390/ma12040581
Evans JS. Composite Materials Design: Biomineralization Proteins and the Guided Assembly and Organization of Biomineral Nanoparticles. Materials. 2019; 12(4):581. https://doi.org/10.3390/ma12040581
Chicago/Turabian StyleEvans, John Spencer. 2019. "Composite Materials Design: Biomineralization Proteins and the Guided Assembly and Organization of Biomineral Nanoparticles" Materials 12, no. 4: 581. https://doi.org/10.3390/ma12040581