Influence of Vanadium on the Microstructure of IN718 Alloy by Laser Cladding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
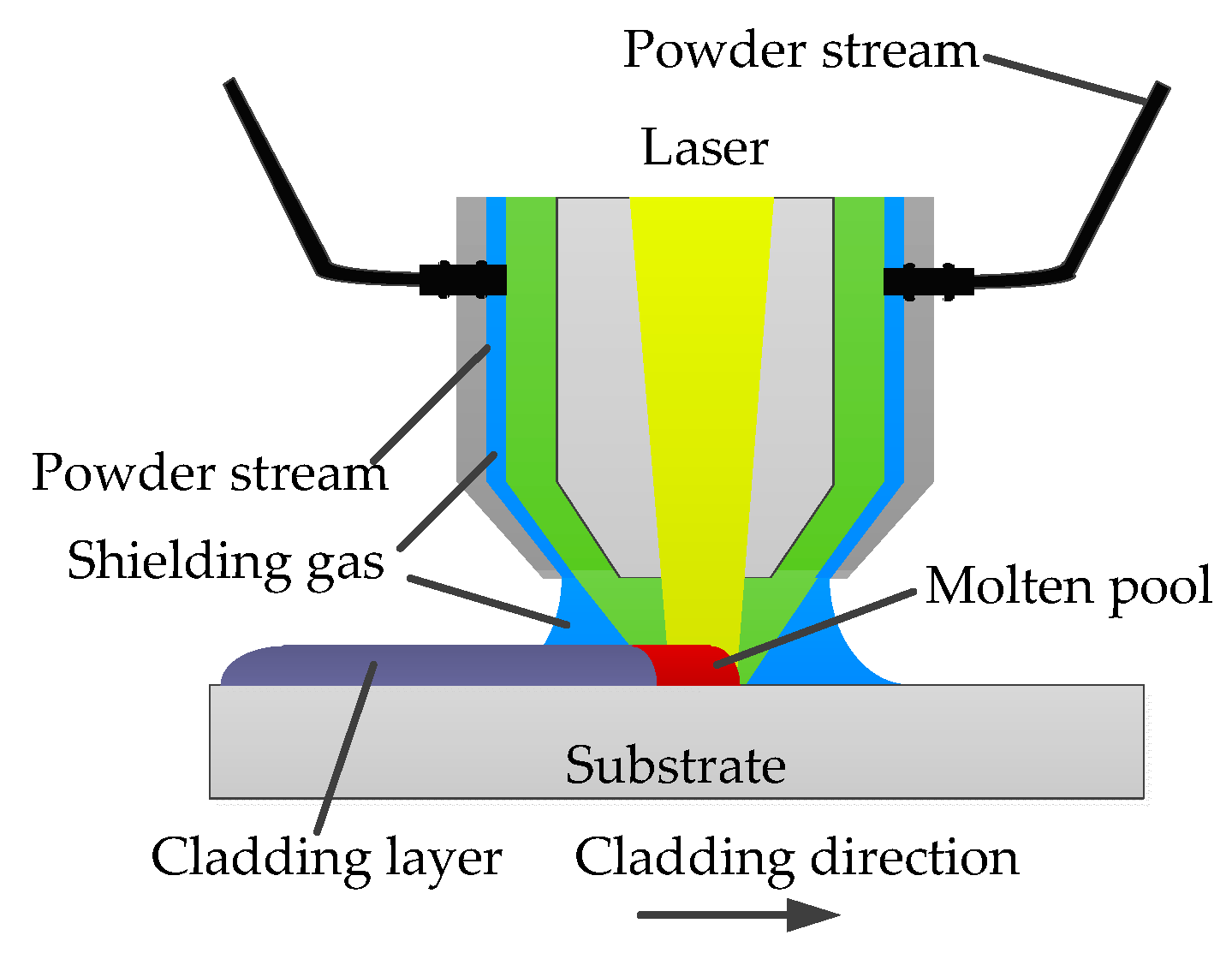
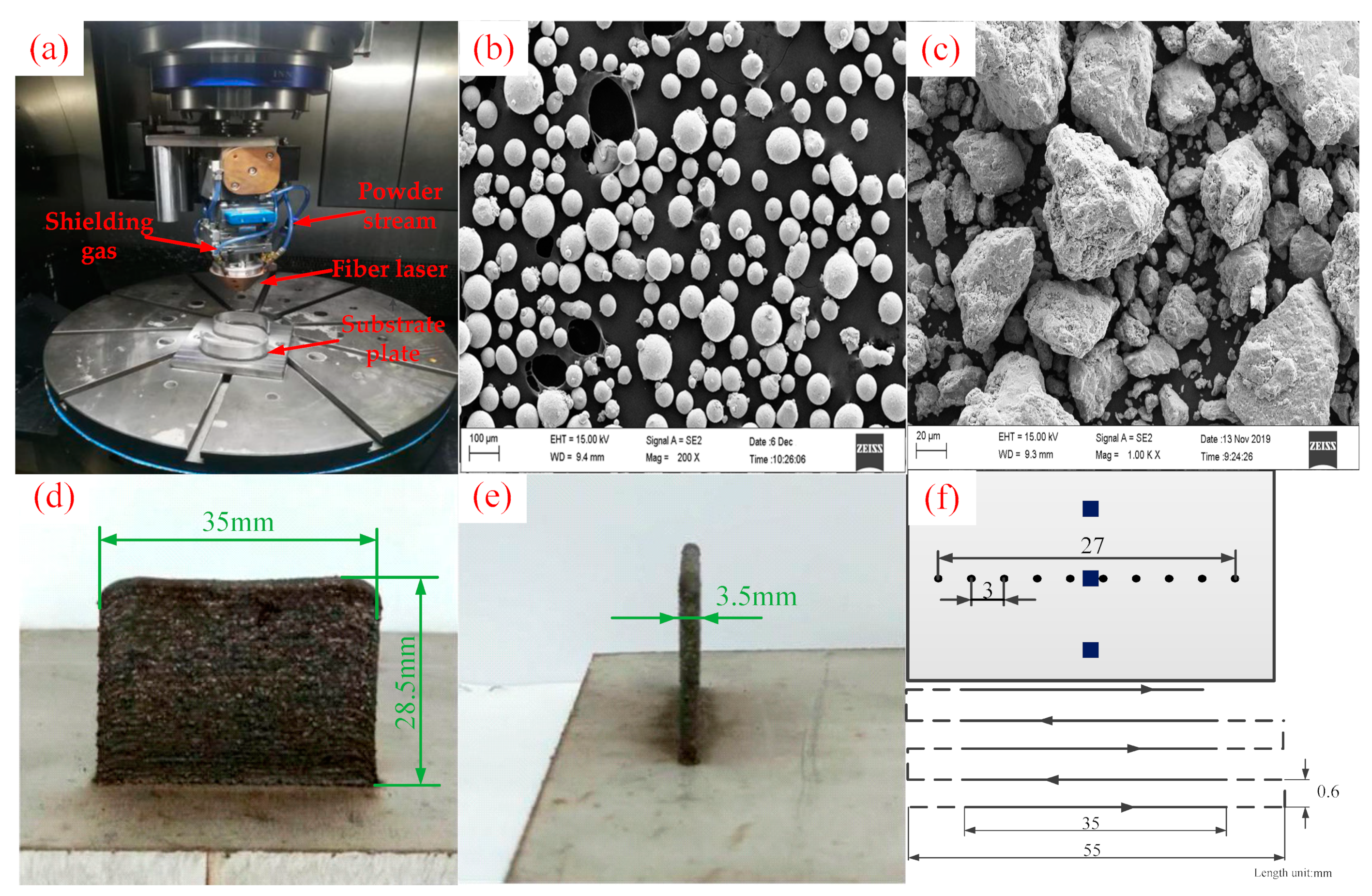
2.2. Laser Cladding Experiment Method
3. Results and Discussion
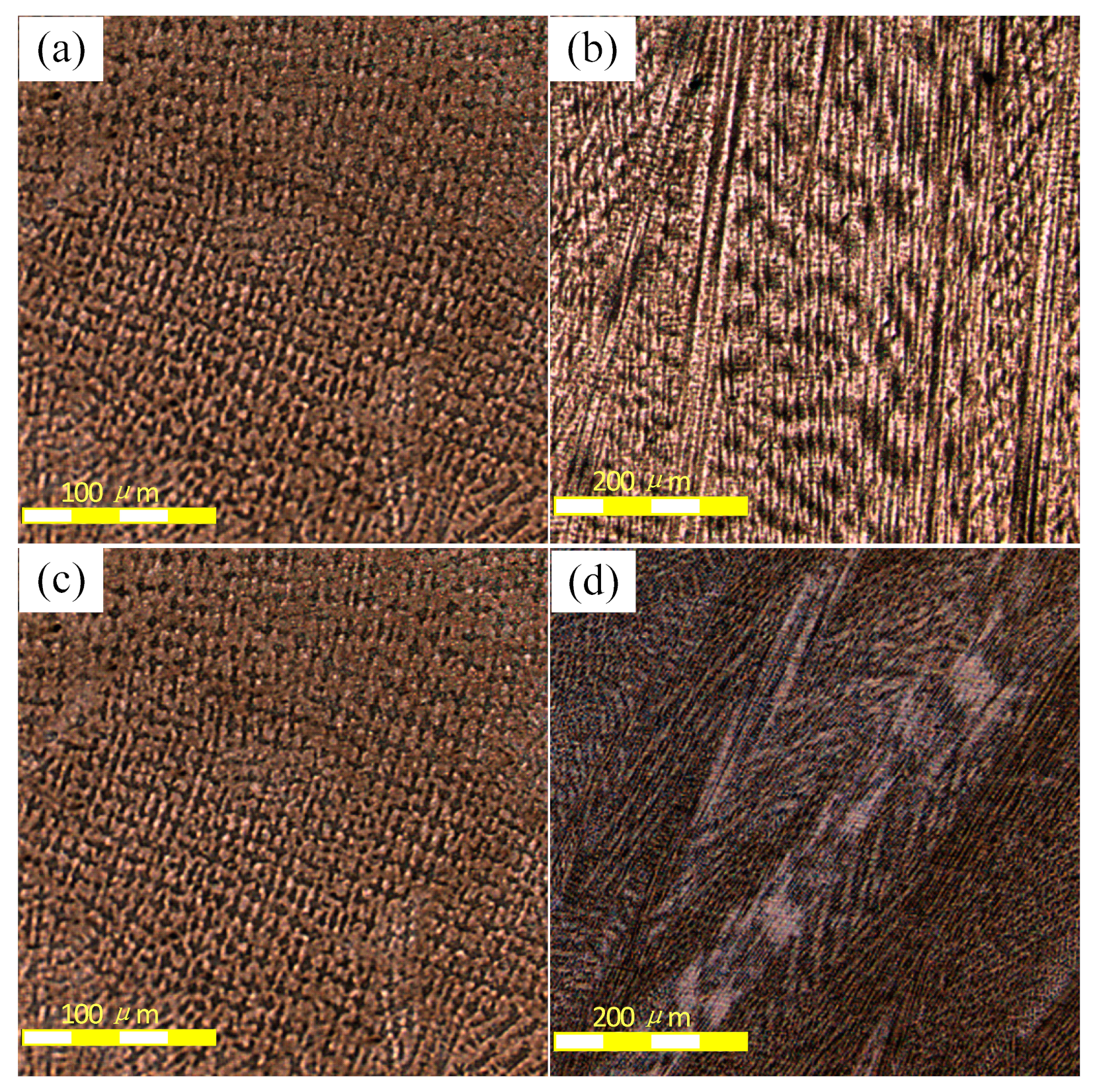
3.1. Solidification Structure Characteristics
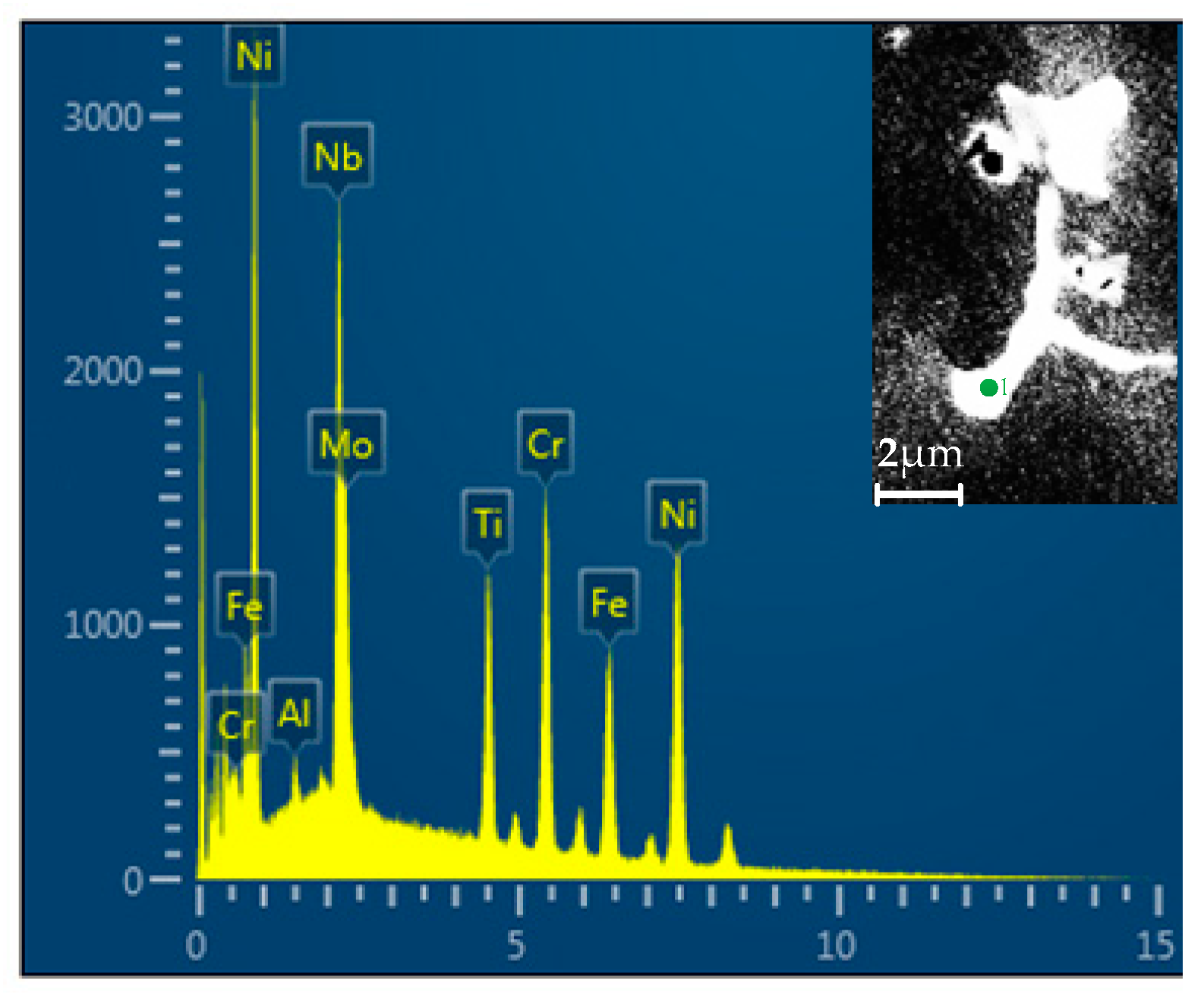
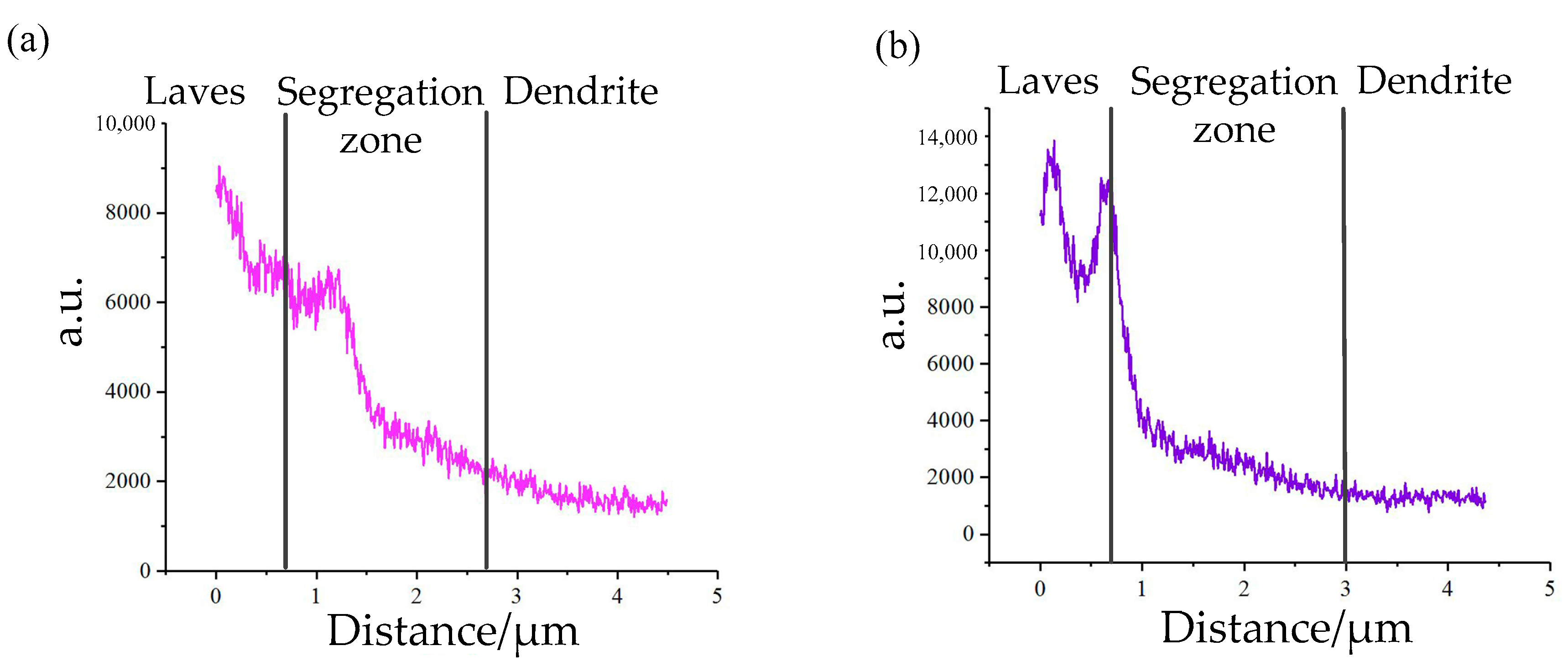
3.2. Influence of V on Element Segregation
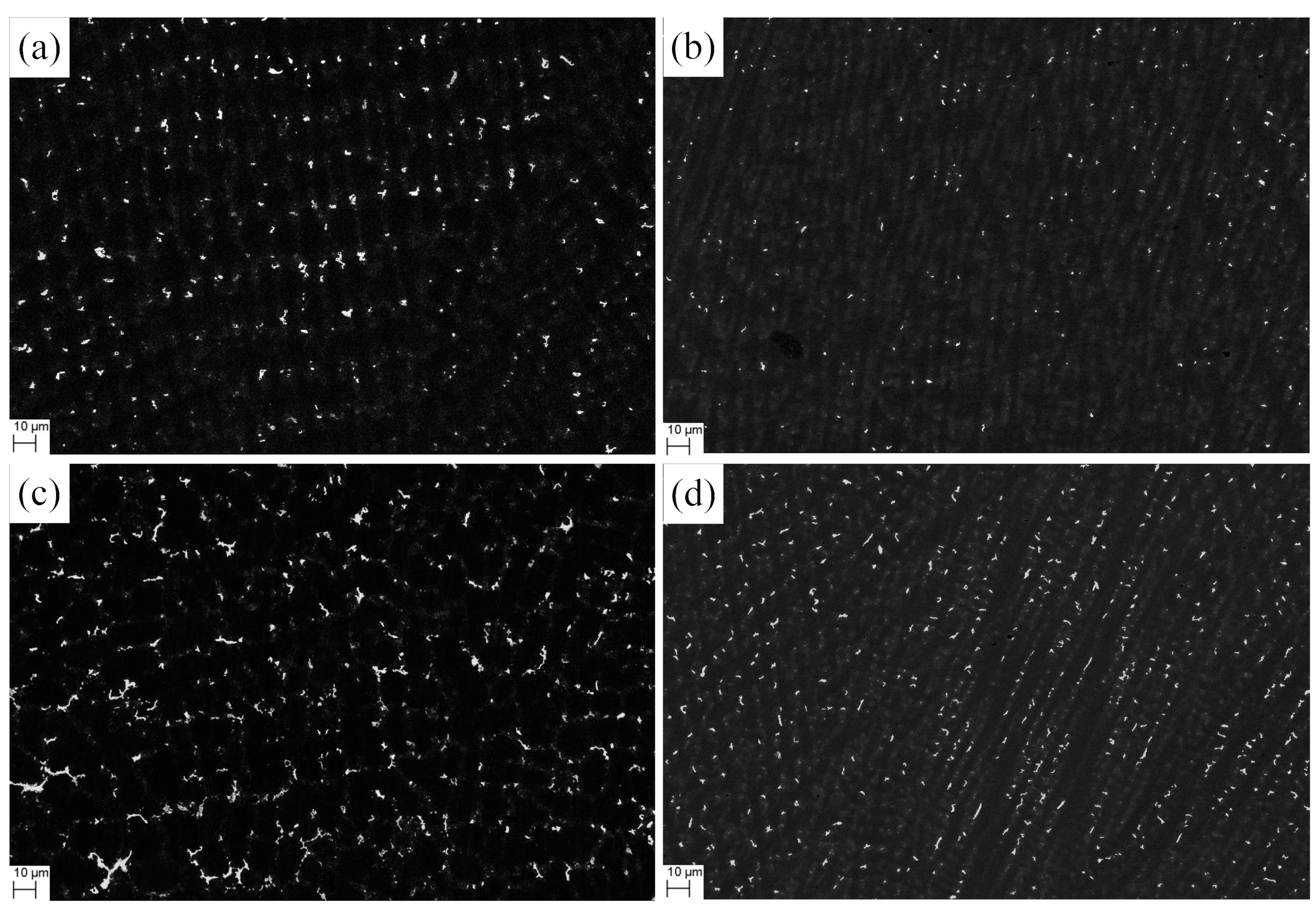
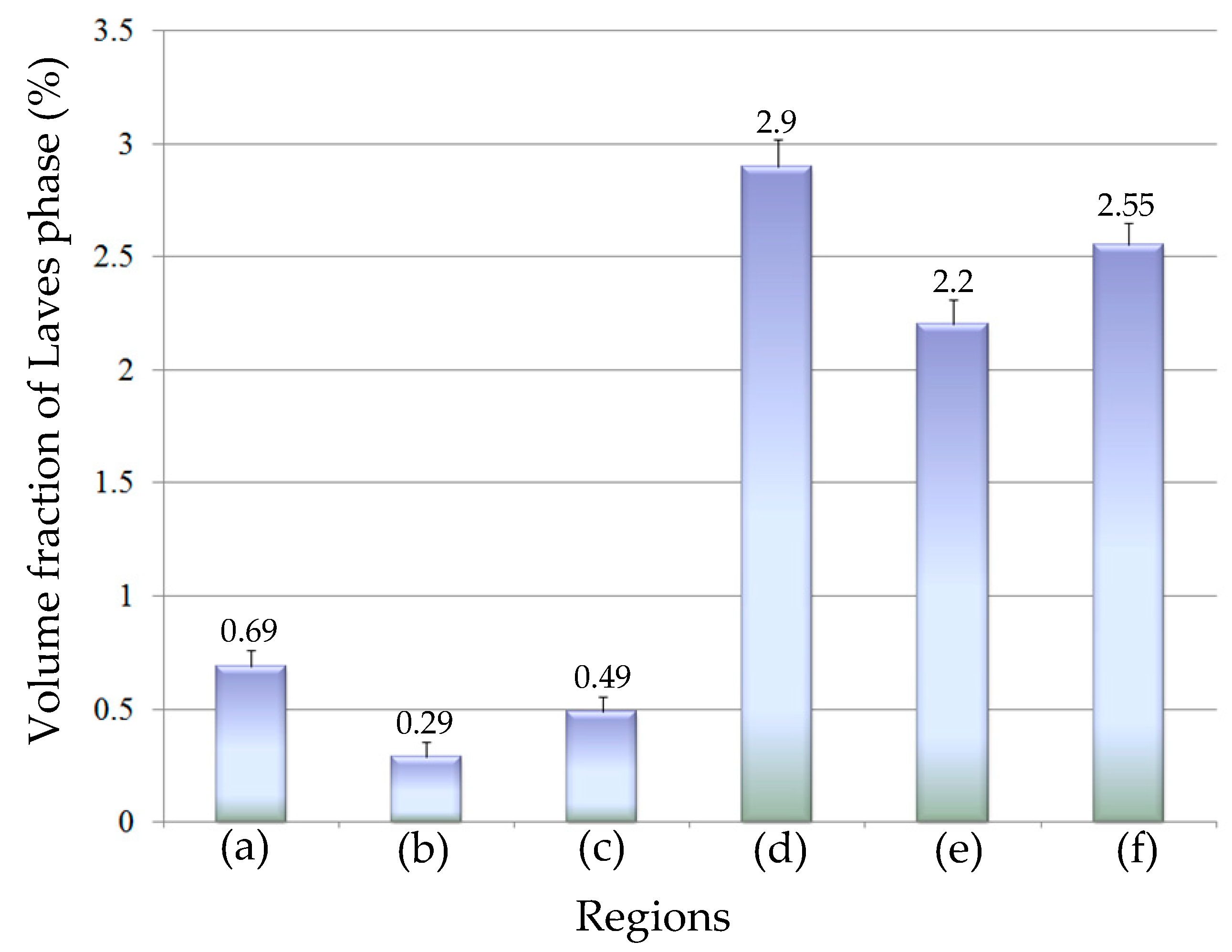
3.3. Influence of V on Laves Phase Formation
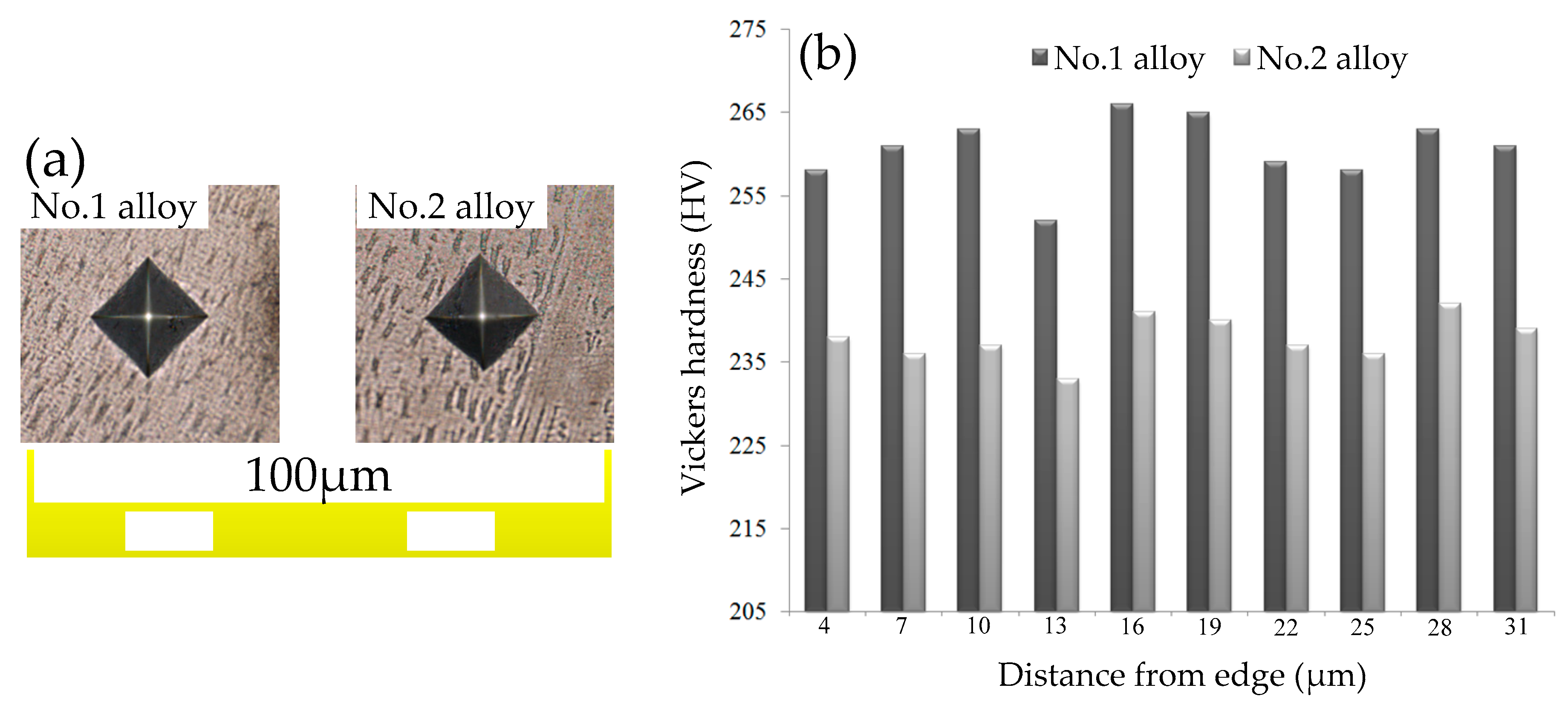
3.4. Influence of V on Hardness of Cladding Layer
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Yu, H.; Chen, C.; Weng, F.; Dai, J. Research and development status of laser cladding on magnesium alloys: A review. Opt. Lasers Eng. 2017, 93, 195–210. [Google Scholar] [CrossRef]
- Sexton, L.; Lavin, S.; Byrne, G.; Kennedy, A. Laser cladding of aerospace materials. J. Mater. Process. 2002, 122, 63–68. [Google Scholar] [CrossRef]
- Mokadem, S.; Bezençon, C.; Hauert, A.; Jacot, A.; Kurz, W. Laser repair of superalloy single crystals with varying substrate orientations. Metall. Mater. Trans. 2007, 38, 1500–1510. [Google Scholar] [CrossRef]
- Ram, G.D.J.; Reddy, A.V.; Rao, K.P.; Reddy, G.M. Improvement in stress rupture properties of Inconel 718 gas tungsten arc welds using current pulsing. J. Mater. 2005, 40, 1497–1500. [Google Scholar] [CrossRef]
- Reddy, G.M.; Murthy, C.S.; Rao, K.S.; Rao, K.P. Improvement of mechanical properties of Inconel 718 electron beam welds—Influence of welding techniques and postweld heat treatment. Int. J. Adv. Manuf. Technol. 2009, 43, 671–680. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, Z.F.; Liu, P.; Gu, Y.F. Effect of processing parameters on microstructure of laser solid forming Inconel 718 superalloy. Opt. Laser Technol. 2018, 98, 409–415. [Google Scholar] [CrossRef]
- Han, D.W.; Sun, W.R.; Yu, L.X. Effects of molybdenum on segregation and diffusion of niobium during the solidification and homogenization of IN718 alloy. Heat Treat. 2018, 33, 6–12. [Google Scholar]
- Miao, Z.; Shan, A.; Wu, Y. Effects of P and B addition on as-cast microstructure and homogenization parameter of Inconel 718 alloy. Nonferr. Met. Soc. China 2012, 22, 318–323. [Google Scholar] [CrossRef]
- Xin, X.; Zhang, W.H.; Yu, L.X. Effects of Co on the solidification and precipitation behaviors of IN 718 alloy. Mater. Sci. Forum 2015, 816, 613–619. [Google Scholar] [CrossRef]
- Li, Y.M.; Liu, H.J. Effect of Zr addition on precipitates in K4169 superalloy. Res. Dev. 2012, 9, 6–10. [Google Scholar]
- Manikandan, S.G.K.; Sivakumar, D.; Rao, K.P. Laves phase in alloy 718 fusion zone—Microscopic and calorimetric studies. Mater. Charact. 2015, 100, 192–206. [Google Scholar] [CrossRef]
- Muroga, T.; Nagasaka, T.; Abe, K.; Chernov, V.M.; Matsui, H.; Smith, D.L. Vanadium alloys—Overview and recent results. J. Nucl. Mater. 2002, 307, 547–554. [Google Scholar] [CrossRef]
- Filipovic, M.; Kamberovic, Z.; Korac, M.; Jordovic, B. Effect of niobium and vanadium additions on the as-cast microstructure and properties of hypoeutectic Fe–Cr–C alloy. ISIJ Int. 2013, 53, 2160–2166. [Google Scholar] [CrossRef]
- Ma, W.; Xie, Y.; Chen, C.; Fukanuma, H.; Wang, J.; Ren, Z.; Huang, R. Microstructural and mechanical properties of high-performance Inconel 718 alloy by cold spraying. J. Alloy. Compd. 2019, 792, 456–467. [Google Scholar] [CrossRef]
- Ahmad, R.; Asmael, M.B.A.; Shahizan, N.R.; Gandouz, S. Reduction in secondary dendrite arm spacing in cast eutectic Al–Si piston alloys by cerium addition. Int. J. Miner. Metall. Mater. 2017, 24, 91–101. [Google Scholar] [CrossRef]
- Miao, Z.; Shan, A.; Wang, W.; Lu, J.; Xu, W.; Song, H.W. Solidification process of conventional superalloy by confocal scanning laser microscope. Trans. Nonferr. Met. Soc. China 2011, 21, 236–242. [Google Scholar] [CrossRef]
- Filipovic, M.; Kamberovic, Z.; Korac, M. Solidification of high chromium white cast iron alloyed with vanadium. Mater. Trans. 2011, 52, 386–390. [Google Scholar] [CrossRef]
- Baker, T.N. Processes, microstructure and properties of vanadium microalloyed steels. Mater. Sci. Technol. 2009, 25, 1083–1107. [Google Scholar] [CrossRef]
- Long, Y.T.; Nie, P.L.; Li, Z.G.; Huang, J.; Xiang, L.I.; Xu, X.M. Segregation of niobium in laser cladding Inconel 718 superalloy. Trans. Nonferr. Met. Soc. China 2016, 26, 431–436. [Google Scholar] [CrossRef]
- Knorovsky, G.A.; Cieslak, M.J.; Headley, T.J.; Romig, A.D.; Hammetter, W.F. Inconel 718: A solidification diagram. Metall. Mater. Trans. 1989, 20, 2149–2158. [Google Scholar] [CrossRef]
- Chen, M.R.; Lin, S.J.; Yeh, J.W.; Chuang, M.H.; Chen, S.K.; Huang, Y.S. Effect of vanadium addition on the microstructure, hardness, and wear resistance of Al 0.5 CoCrCuFeNi high-entropy alloy. Metall. Mater. Trans. 2006, 37, 1363–1369. [Google Scholar] [CrossRef]
- Sozanska, M.; Maciejny, A.; Dagbert, C.; Galland, J.; Hyspecká, L. Use of quantitative metallography in the evaluation of hydrogen action during martensitic transformations. Mater. Sci. Eng. 1999, 273, 485–490. [Google Scholar] [CrossRef]
- Sui, S.; Chen, J.; Fan, E.; Yang, H.; Lin, X.; Huang, W. The influence of Laves phases on the high-cycle fatigue behavior of laser additive manufactured Inconel 718. Mater. Sci. Eng. 2017, 695, 6–13. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Li, Z.G.; Nie, P.L.; Wu, Y.X. Effect of ultrarapid cooling on microstructure of laser cladding IN718 coating. Surf. Eng. 2013, 29, 414–418. [Google Scholar] [CrossRef]
- Stevens, E.L.; Toman, J.; Chmielus, M. Variation of hardness, microstructure, and Laves phase distribution in direct laser deposited alloy 718 cuboids. Mater. Des. 2017, 119, 188–198. [Google Scholar] [CrossRef] [Green Version]





| Parameters | Laser Power (W) | Scanning Speed (mm/s) | Powder Federate (g·min−1) | Shield Gas Flow (L·min−1) |
|---|---|---|---|---|
| - | 1200 | 8 | 18 | 15 |
| Elements | Ni | Cr | Nb | Mo | Ti | Al | C | Fe | V |
|---|---|---|---|---|---|---|---|---|---|
| No.1 Alloy | 53.1 | 18.43 | 5 | 3.18 | 1.06 | 0.54 | 0.014 | 18.61 | 0.066 |
| No.2 Alloy | 53.2 | 18.28 | 5 | 3.2 | 1.08 | 0.54 | 0.015 | 18.64 | - |
| Alloy | Ti | Cr | Fe | Ni | Nb | Mo |
|---|---|---|---|---|---|---|
| No.1 | 1.64 | 14.14 | 12.95 | 45.04 | 21.24 | 5 |
| No.2 | 2.24 | 13.18 | 16.98 | 34.97 | 26.15 | 6.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Xie, H.; Sun, C.; Zhao, X.; Li, F. Influence of Vanadium on the Microstructure of IN718 Alloy by Laser Cladding. Materials 2019, 12, 3839. https://doi.org/10.3390/ma12233839
Yang K, Xie H, Sun C, Zhao X, Li F. Influence of Vanadium on the Microstructure of IN718 Alloy by Laser Cladding. Materials. 2019; 12(23):3839. https://doi.org/10.3390/ma12233839
Chicago/Turabian StyleYang, Kun, Hualong Xie, Cong Sun, Xiaofei Zhao, and Fei Li. 2019. "Influence of Vanadium on the Microstructure of IN718 Alloy by Laser Cladding" Materials 12, no. 23: 3839. https://doi.org/10.3390/ma12233839




