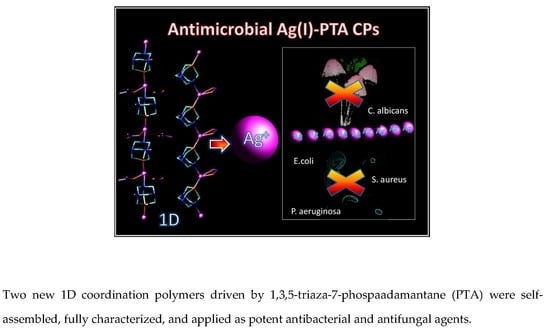
New Microbe Killers: Self-Assembled Silver(I) Coordination Polymers Driven by a Cagelike Aminophosphine
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Antibacterial and Antifungal Activity Studies
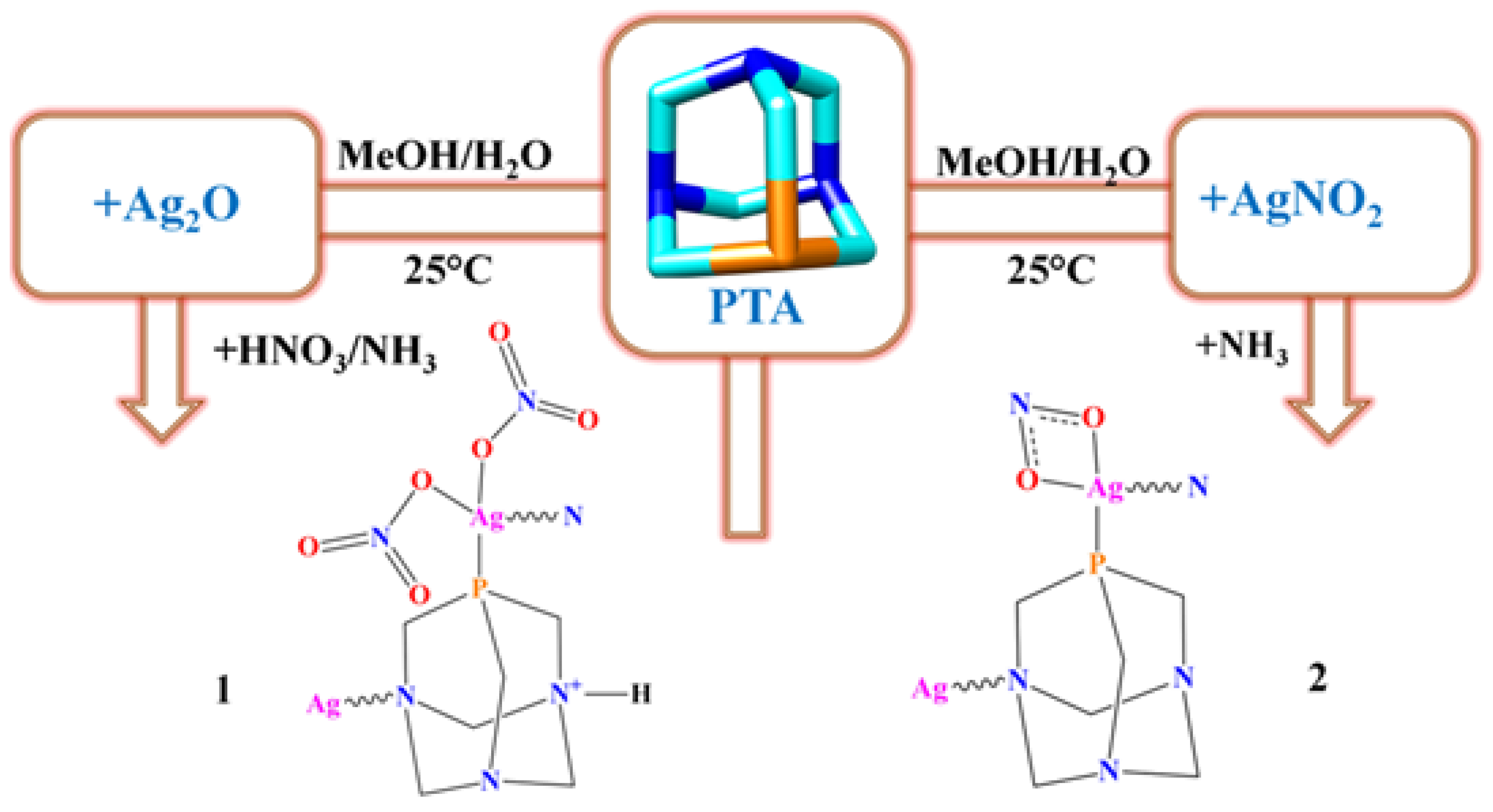
2.3. Synthesis and Analytical Data
2.4. X-Ray Crystallography
3. Results and Discussion
3.1. Synthesis and Characterization of 1 and 2
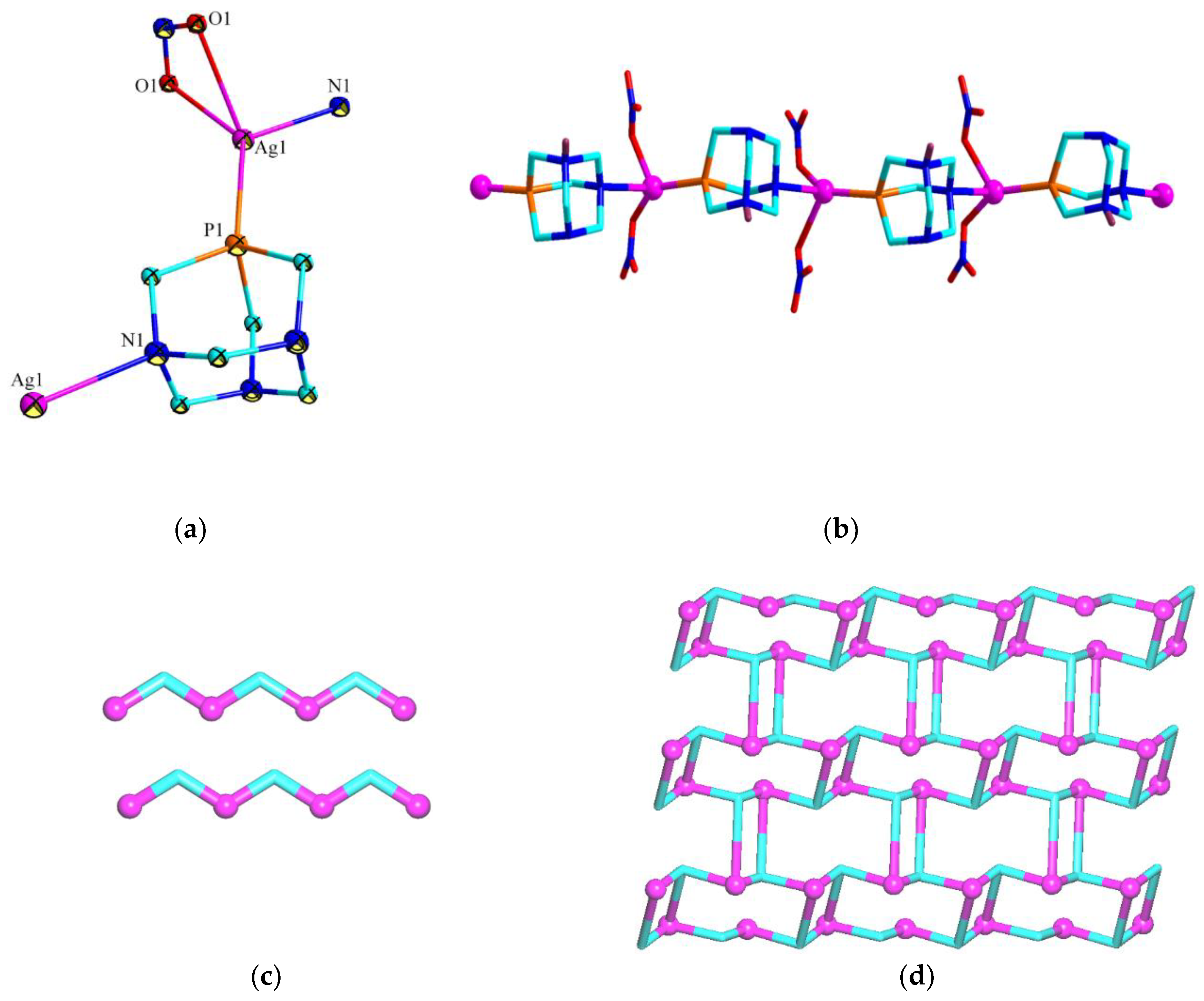
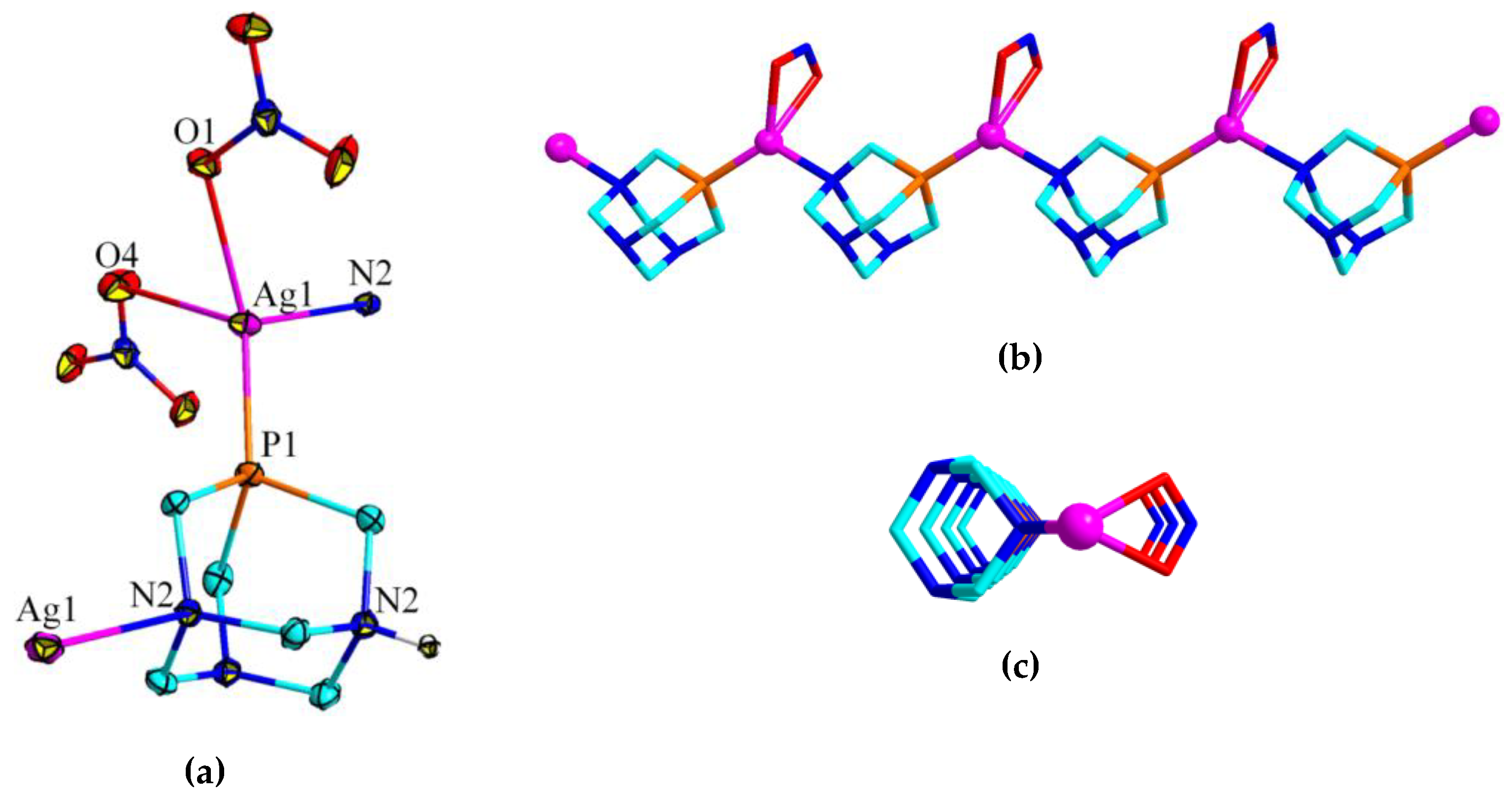
3.2. Crystal Structures of 1 and 2
3.3. Antimicrobial Activity of 1 and 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pettinari, C.; Tabacaru, A.; Galli, S. Coordination Polymers and Metal–Organic Frameworks Based on Poly (Pyrazole)-Containing Ligands. Coord. Chem. Rev. 2016, 307, 1–31. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [PubMed]
- Wencewicz, T.A. New Antibiotics from Nature’s Chemical Inventory. Bioorg. Med. Chem. 2016, 24, 6227–6252. [Google Scholar] [CrossRef] [PubMed]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Vimbela, G.V.; Ngo, S.M.; Freze, C.; Yang, L.; Stout, D.A. Antibacterial Properties and Toxicity from Metallic Nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Sava, D.F.; Eubank, J.F.; Adil, K.; Guillerm, V. Zeolite-Like Metal-Organic Frameworks (Zmofs): Design, Synthesis, and Properties. Chem. Soc. Rev. 2015, 44, 228–249. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, H.C. Recent Progress in the Synthesis of Metal-Organic Frameworks. Sci. Technol. Adv. Mate. 2015, 16, 054202. [Google Scholar] [CrossRef]
- Cai, H.; Huang, Y.L.; Li, D. Biological Metal-Organic Frameworks: Structures. Host-Guest Chemistry and Bio-Applications. Coord. Chem. Rev. 2019, 378, 207–221. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, M.; Xie, Z. Nanoscale Metal-Organic Frameworks for Drug Delivery: A Conventional Platform with New Promise. J. Mater. Chem. B 2018, 6, 707–717. [Google Scholar] [CrossRef]
- Ahmed, I.; Jhung, S.H. Composites of Metal–Organic Frameworks: Preparation and Application in Adsorption. Mater. Today 2014, 17, 136–146. [Google Scholar] [CrossRef]
- Imaz, I.; Rubio-Martinez, M.; An, J.; Sole-Font, I.; Rosi, N.L.; Maspoch, D. Metal-Biomolecule Frameworks. Chem. Comm. 2011, 47, 7287–7302. [Google Scholar] [CrossRef] [PubMed]
- Janiak, C.; Vieth, J.K. MOFs, MILs and More: Concepts, Properties and Applications for Porous Coordination Networks (PCNs). New. J. Chem. 2010, 34, 2366–2388. [Google Scholar] [CrossRef]
- Perry IV, J.J.; Perman, J.A.; Zaworotko, J. Design and Synthesis of Metal-Organic Framework Rusing Metal-Organic Polyhedra as Supramolecular Building Blocks. Chem. Soc. Rev. 2009, 38, 1400–1471. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R.; Turner, D.R.; Neville, S.M. Coordination Polymers: Design, Analysis and Application; Royal Society of Chemistry: London, England, 2009; p. 76. [Google Scholar]
- Jaros, S.W.; Sokolnicki, J.; Wołoszyn, A.; Haukka, M.; Kirillov, A.M.; Smolenski, P. Novel 2D Coordination Network Built from Hexacopper(I)-Iodide Clusters and Cagelike Aminophosphine Blocks for Reversible “Turn-On” Sensing of Aniline. J. Mater. Chem. C 2018, 6, 1670–1678. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B.; Shikler, R.; Nabha, S.; Caruso, U. Highly Efficient Dicyano-Phenylenevinylene Fluorophore as Polymer Dopant or Zinc-Driven Self-Assembling Building Block. Inorg. Chem. Commun. 2019, 104, 145–149. [Google Scholar] [CrossRef]
- Jaros, S.W.; Guedes Da Silva, M.F.C.; Florek, M.; Conceiçao Oliveira, M.; Smolenski, P.; Pombeiro, A.J.L.; Kirillov, A.M. Aliphatic Dicarboxylate Directed Assembly of Silver(I)-1,3,5-Triaza-7-Phosphaadamantane Coordination Networks: Topological Versatility and Antimicrobial Activity. Cryst. Growth Des. 2014, 14, 5408–5417. [Google Scholar] [CrossRef]
- Jaros, S.W.; Smolenski, P.; Guedes Da Silva, M.F.C.; Florek, M.; Krol, J.; Staroniewicz, Z.; Pombeiro, A.J.L.; Kirillov, A.M. New Silver BioMOFs Driven by 1,3,5-Triaza-7-Phosphaadamantane-7-Sulfide (PTA=S): Synthesis, Topological Analysis and Antimicrobial Activity. CrystEngComm 2013, 15, 8060–8064. [Google Scholar] [CrossRef]
- Smolenski, P.; Jaros, S.W.; Pettinari, C.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Vitali, L.A. New Water-Soluble Polypyridine Silver(I) Derivatives of 1,3,5-Triaza-7-Phosphaadamantane (PTA) with Remarkable Antimicrobial and Antiproliferative Activity. Dalton. Trans. 2013, 42, 6572–6581. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Wieczorek, S.W.; Guedes Da Silva, M.F.C.; Sokolnicki, J.; Smolenski, P.; Pombeiro, A.J.L. Crystal Engineering with 1,3,5-Triaza-7-Phosphaadamantane (PTA): First PTA-Driven 3D Metal-Organic Frameworks. CrystEngComm 2011, 13, 6329–6333. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Wieczorek, S.W.; Lis, A.; Guedes Da Silva, M.F.C.; Florek, M.; Krol, J.; Staroniewicz, Z.; Smolenski, P.; Pombeiro, A.J.L. 1,3,5-Triaza-7-Phosphaadamantane-7-Oxide (PTA=O): New Diamondoid Building Block for Design of 3D Metal-Organic Frameworks. Cryst. Growth Des. 2011, 11, 2711–2716. [Google Scholar] [CrossRef]
- Jaros, S.W.; Guedes Da Silva, M.F.C.; Florek, M.; Smolenski, P.; Pombeiro, A.J.L.; Kirillov, A.M. Silver(I)-1,3,5-Triaza-7-Phosphaadamanatane Coordination Polymers Driven by Substituted Glutarate and Malonate Building Blocks: Self-Assembly Synthesis, Structural Features, and Antimicrobial Properties. Inorg. Chem. 2016, 55, 5886–5894. [Google Scholar] [CrossRef] [PubMed]
- Jaros, S.W.; Guedes Da Silva, M.F.C.; Krol, J.; Oliveira, M.C.; Smolenski, P.; Pombeiro, A.J.L.; Kirillov, A.M. Bioactive Silver-Organic Networks Assembled from 1,3,5-Triaza-7-Phosphaadamantane and Flexible Cyclohexanecarboxylate Blocks. Inorg. Chem. 2016, 55, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Aziz, A.S.; Agatemor, C.; Etkin, N. Antimcrobial Resistance Challenged with Metal-Based Antimicrobial Macromolecules. Biomaterials 2017, 118, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Jaremko, L.; Kirillov, A.M.; Smolenski, P.; Pombeiro, A.J.L. Engineering Coordination and Supramolecular Copper-Organic Networks by Aqueous Medium Self-Assembly with 1,3,5-Triaza-7-Phosphaadamantane (PTA). Cryst. Growth Des. 2009, 9, 3006–3010. [Google Scholar] [CrossRef]
- Jaremko, L.; Kirillov, A.M.; Smolenski, P.; Lis, T.; Pombeiro, A.J.L. Extending the Coordination Chemistry of 1,3,5-Triaza-7-Phosphaadamantane (PTA) to Cobalt Centres: First Examples of Co-PTA Complexes and of a Metal Complex with the PTA Oxide Ligand. Inorg. Chem. 2008, 47, 2922–2924. [Google Scholar] [CrossRef] [PubMed]
- Kirillov, A.M.; Smolenski, P.; Guedes Da Silva, M.F.C.; Pombeiro, A.J.L. The First Copper Complexes Bearing the 1,3,5-Triaza-7-Phosphaadamantane (PTA) Ligand. Eur. J. Inorg. Chem. 2007, 2007, 2686–2692. [Google Scholar] [CrossRef]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017; p. 7. [Google Scholar]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015; p. 28. [Google Scholar]
- Bravo, J.; Bolano, S.; Gonsalvi, L.; Peruzzini, M. Coordination Chemistry of 1,3,5-Triaza-7-Phospaadamantane (PTA) and Derivatives. Part II. The Quest for Tailored Ligands, Complexes and Related Applications. Coord. Chem. Rev. 2010, 254, 555–607. [Google Scholar] [CrossRef]
- Lis, A.; Guedes Da Silva, M.F.C.; Kirillov, A.M.; Smolenski, P.; Pombeiro, A.J.L. Design of Silver(I)-PTA Coordination Polymers Through Controlled N-P-Coordination of 1,3,5-Triaza-7-Phospaadamantene (PTA) with Arylcarboxylates. Cryst. Growth Des. 2010, 10, 5244–5253. [Google Scholar] [CrossRef]
- Phillips, A.D.; Gonsalvi, L.; Romerosa, A.; Vizza, F.; Peruzzini, M. Coordination Chemistry of 1,3,5-Triaza-7-Phospaadamantene (PTA): Transition Metal Complexes and Related Catalytic, Medicinal and Photoluminescent Applications. Coord. Chem. Rev. 2004, 248, 955–993. [Google Scholar] [CrossRef]
- Edwards-Jones, V. The Benefits of Silver in Hygiene, Personal Care and Healthcare. Lett. Appl. Microbiol. 2009, 49, 147–152. [Google Scholar] [CrossRef]
- Gilchrist, M.; Shore, A.C.; Benjamin, N. Inorganic Nitrate and Nitrile and Control of Blood Pressure. Cardiovasc. Res. 2011, 89, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Cammack, R.; Joannou, C.L.; Cui, X.Y.; Martinez, C.T.; Maraj, S.R.; Hughes, M.N. Nitrite and Nitrosyl Compounds in Food Preservations. Biochim. et Biophys. Acta 1999, 1411, 475–488. [Google Scholar] [CrossRef]
- Lin, L.; Hu, J.Y.; Wu, Y.; Chen, M.; Ou, J.; Yan, W.L. Assessment of the Inhibitory Effect of Sodium Nitrite, Nisin, Potassium Sorbate, and Sodium Lactate on Staphylococcus Aureus Growth and Staphylococcal Enterotoxin a Production in Cooked Pork Sausage Using a Predictive Growth Model. Food Sci. Hum. Wellness 2018, 7, 83–90. [Google Scholar] [CrossRef]
- Spacciapoli, P.; Buxton, D.; Rothstein, P. Antimicrobial Activity of Silver Nitrate against Periodontal. J. Periodont Res. 2001, 36, 108–113. [Google Scholar] [CrossRef]
- Daigle, D.J.; Pepperman, A.B., Jr.; Vail, S.L. Phospaadamantanes. Synthesis of 2-Thia-1,3,5-Triaza-7-Phospadamantane-2-2-Dioxide and Derivatives. J. Heterocycl Chem. 1974, 11, 1085–1086. [Google Scholar] [CrossRef]
- Daigle, D.J. 1,3,5-Triaz-7-Phosphatricyclo[3.3.1.13,7]Decane and Derivatives. Inorg. Synth. 1998, 32, 40. [Google Scholar]
- Grove, D.C.; Randall, W.A. Assay Methods of Antibiotic. A Laboratory Manual; Medical Encyclopedia: New York, NJ, USA, 1955. [Google Scholar]
- Lorian, V. Antibiotics in Laboratory Medicine, 2nd ed.; Williams & Wilkins: Philadelphia, PA, USA, 1986; p. 93. [Google Scholar]
- Balouini, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Arendrup, M.C. Document E.DEF 7.3: Method for the Determination of Broth Dilution of Antifungal agents for Fermentative Yeasts; EUCAST: Copenhagen, Denmark, December 2015. [Google Scholar]
- Cardoso, J.M.S.; Galvao, A.M.; Guerreiro, S.I.; Leitao, J.H.; Suarez, A.C.; Carvalho, M.F.N.N. Antibacterial Activity of Silver Camphorimine Coordination Polymers. Dalton Trans. 2016, 45, 7114–7123. [Google Scholar] [CrossRef]
- Lu, X.; Ye, J.; Sun, Y.; Bogale, R.F.; Zhao, L.; Tian, P.; Ning, G. Ligand Effects on the Structural Dimensionality and Antibacterial Activities of Silver-Based Coordination Polymers. Dalton Trans. 2014, 43, 10104–10113. [Google Scholar] [CrossRef]
- Young, R.J.; Begg, S.L.; Coghlan, C.J.; McDevitt, C.A.; Sumby, C.J. Antibacterial Coordination Polymers Exploring the Use of Structure and Polymer Incorporation to Tune Silver Ion Release and Antibacterial Activity of Silver Coordination Polymers. Eur. J. Inorg. Chem. 2018, 3512–3518. [Google Scholar] [CrossRef]
| MIC [µg·mL−1] | Normalized MIC [nmol·mL−1] 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Strains | 1 | 2 | AgNO2 | AgNO3 | 1 | 2 | AgNO2 | AgNO3 |
| 1 | P. aeruginosa | 6 | 3 | 3 | 9 | 15 | 10 | 24 | 53 |
| 2 | E. coli | 6 | 4 | 3 | 9 | 15 | 13 | 21 | 53 |
| 3 | S. aureus | 20 | 6 | 5 | 20 | 51 | 19 | 24 | 118 |
| 4 | C. albicans | 40 | 30 | 20 | 40 | 103 | 97 | 118 | 236 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaros, S.W.; Haukka, M.; Florek, M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L.; Kirillov, A.M.; Smoleński, P. New Microbe Killers: Self-Assembled Silver(I) Coordination Polymers Driven by a Cagelike Aminophosphine. Materials 2019, 12, 3353. https://doi.org/10.3390/ma12203353
Jaros SW, Haukka M, Florek M, Guedes da Silva MFC, Pombeiro AJL, Kirillov AM, Smoleński P. New Microbe Killers: Self-Assembled Silver(I) Coordination Polymers Driven by a Cagelike Aminophosphine. Materials. 2019; 12(20):3353. https://doi.org/10.3390/ma12203353
Chicago/Turabian StyleJaros, Sabina W., Matti Haukka, Magdalena Florek, M. Fátima C. Guedes da Silva, Armando J. L. Pombeiro, Alexander M. Kirillov, and Piotr Smoleński. 2019. "New Microbe Killers: Self-Assembled Silver(I) Coordination Polymers Driven by a Cagelike Aminophosphine" Materials 12, no. 20: 3353. https://doi.org/10.3390/ma12203353