Influence of UiO-66(Zr) Preparation Strategies in Its Catalytic Efficiency for Desulfurization Process
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization Methods
2.3. Synthesis of UiO-66(Zr) Samples
2.3.1. Solvothermal Syntheses, UiO-66(Zr)-S
2.3.2. Microwave Assisted Synthesis, UiO-66(Zr)-MW
2.4. Oxidative Desulfurization Studies
3. Results and Discussion
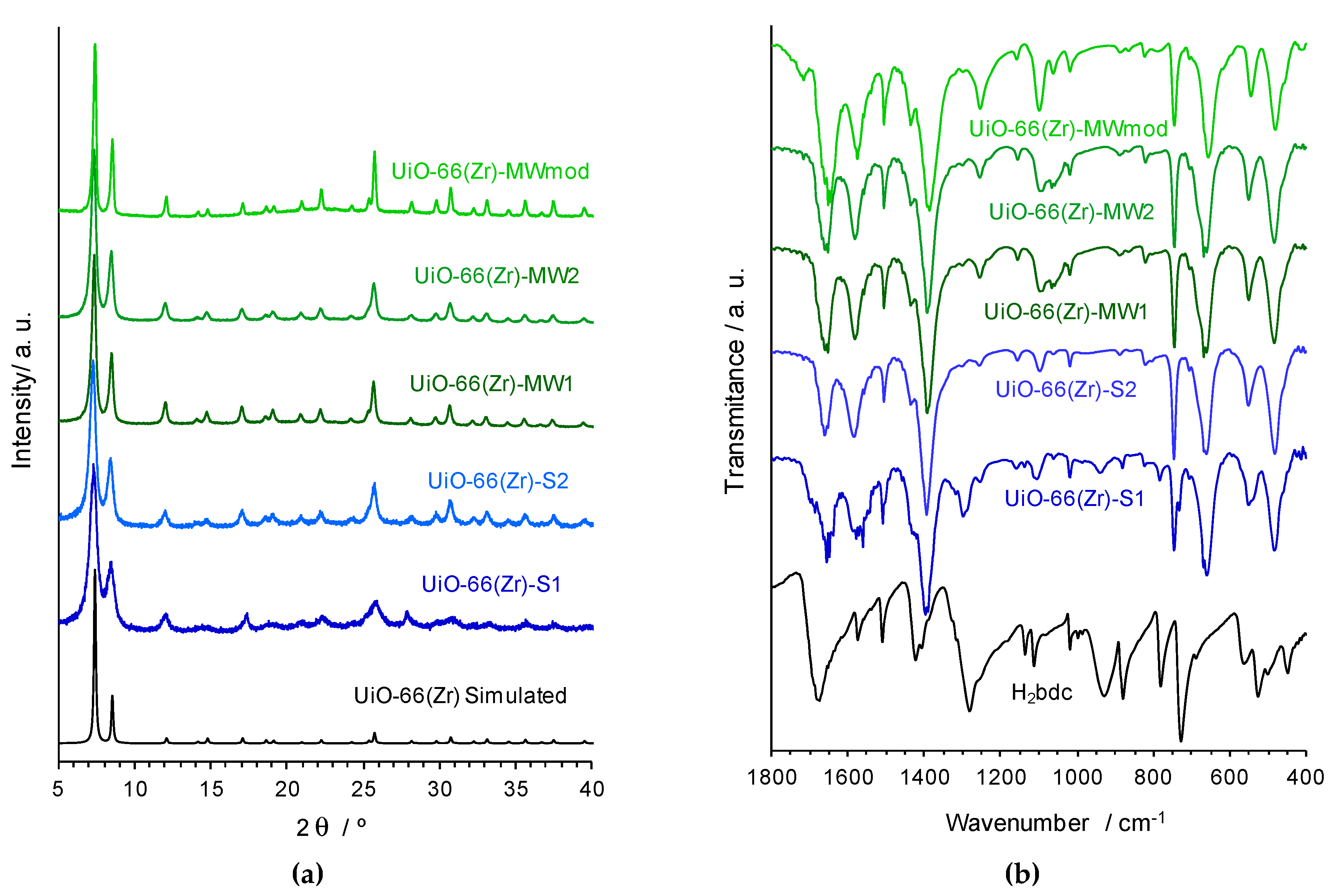
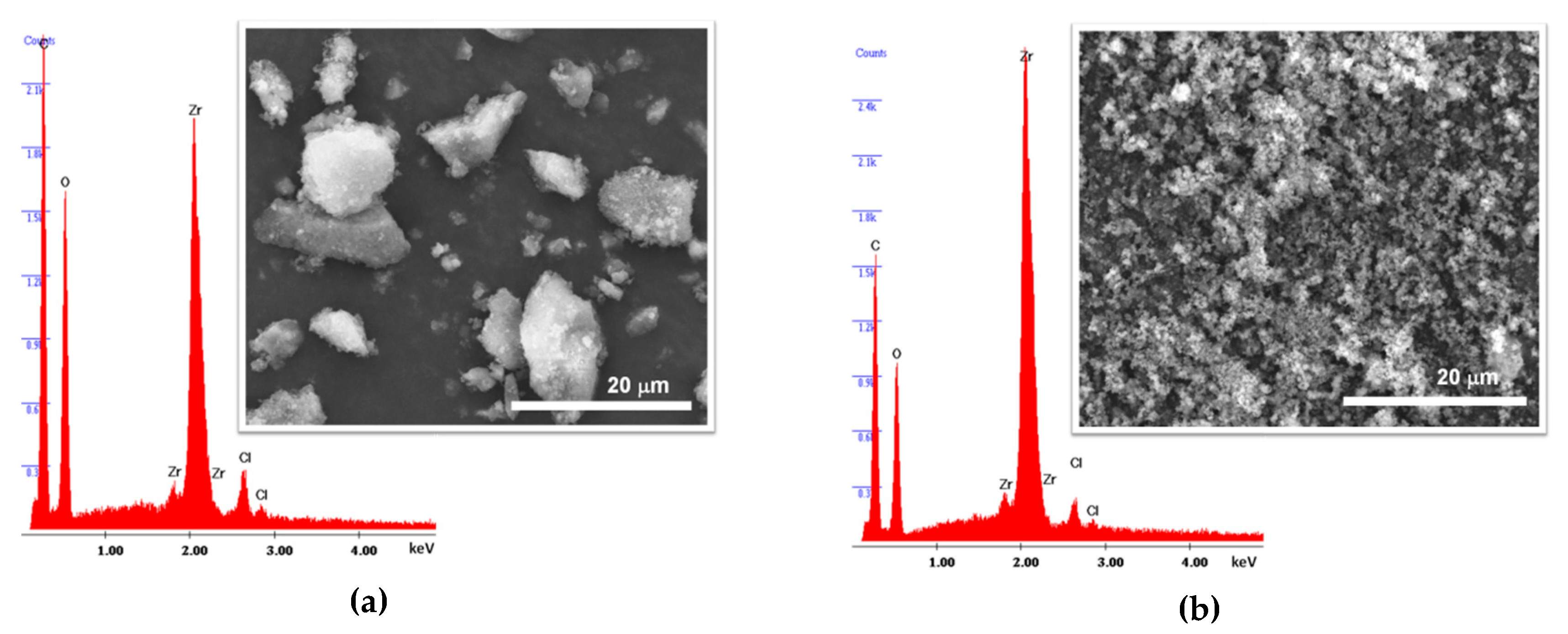
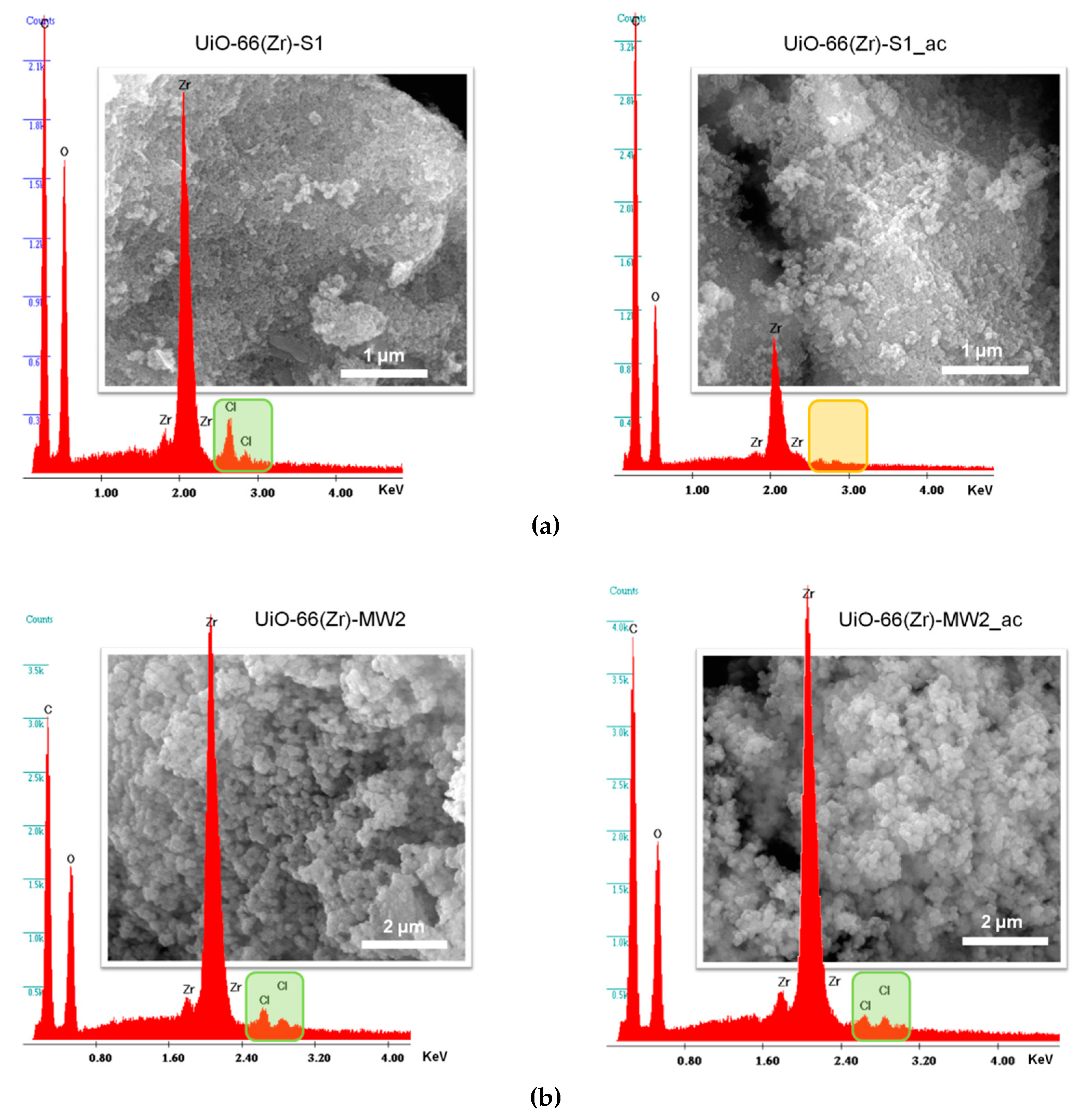
3.1. MOF Materials Characterization
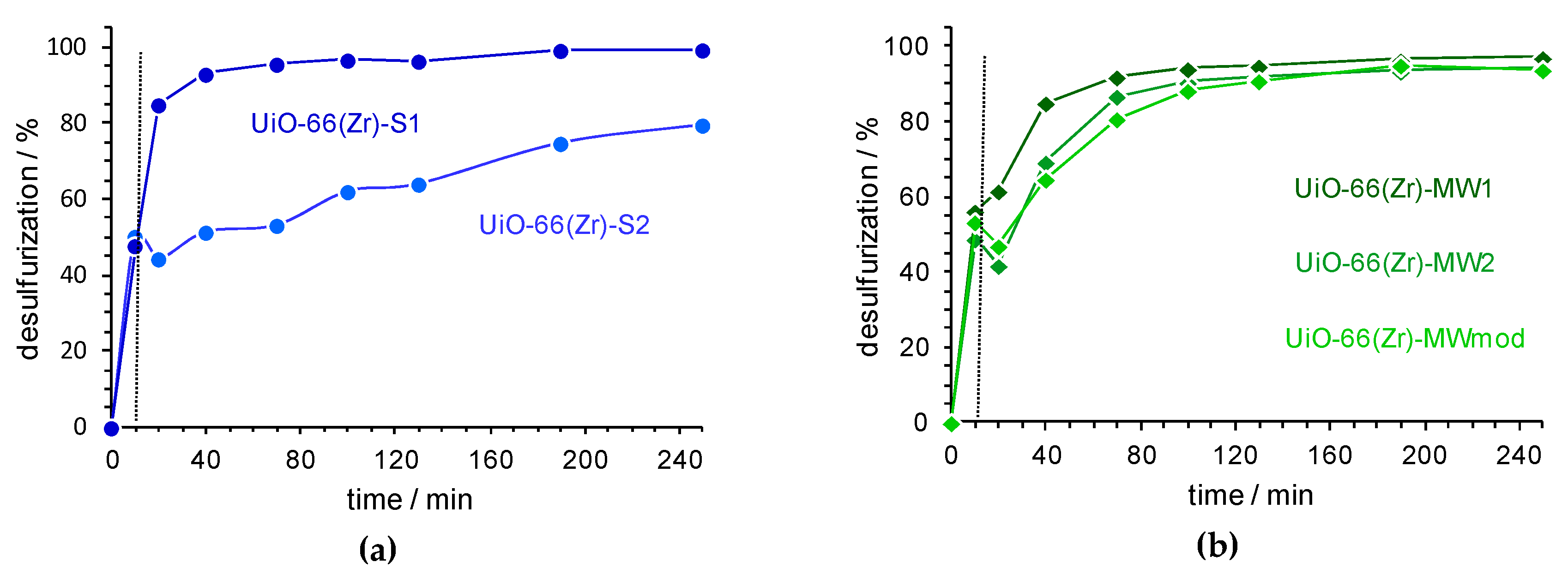
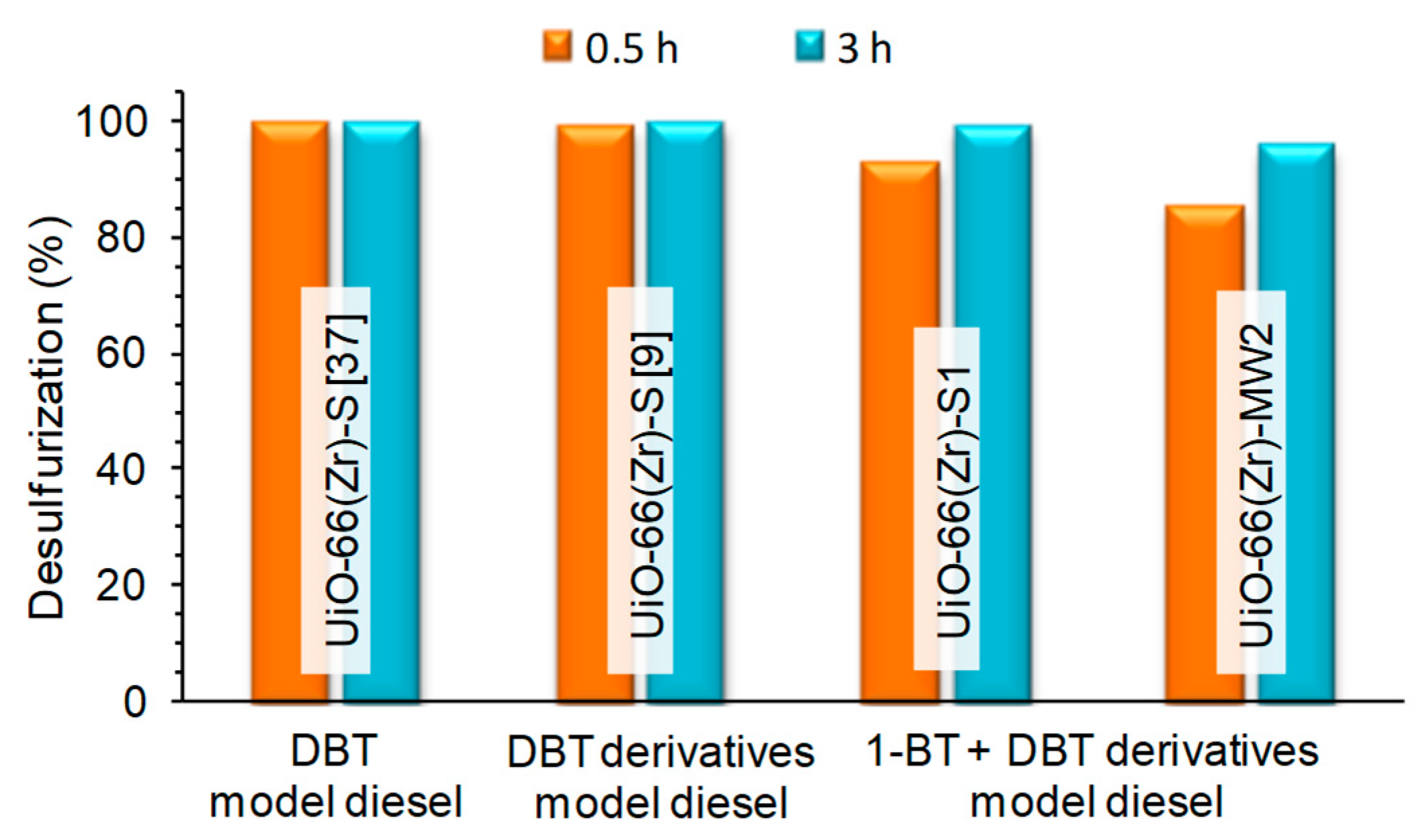
3.2. Extractive and Oxidative Desulfurization
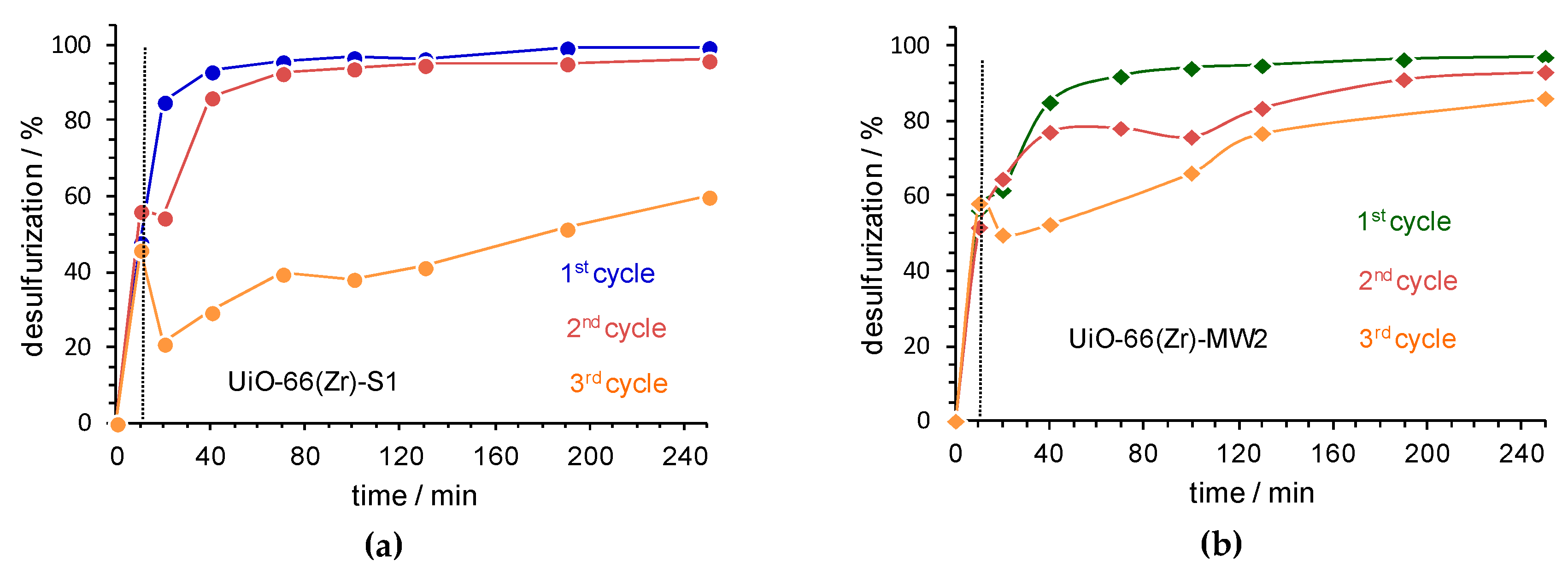
3.3. Catalysts Stability
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fox, E.B.; Liu, Z.-W.; Liu, Z.-T. Ultraclean fuels production and utilization for the twenty-first century: Advances toward sustainable transportation fuels. Energy Fuels 2013, 27, 6335–6338. [Google Scholar] [CrossRef]
- Srivastava, V.C. An evaluation of desulfurization technologies for sulfur removal from liquid fuels. RSC Adv. 2012, 2, 759–783. [Google Scholar] [CrossRef]
- Babich, I.V.; Moulijn, J.A. Science and technology of novel processes for deep desulfurization of oil refinery streams: A review. Fuel 2003, 82, 607–631. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Hayyan, M.; Hashim, M.A.; Hayyan, A. The role of ionic liquids in desulfurization of fuels: A. review. Renew. Sustain. Energy Rev. 2017, 76, 1534–1549. [Google Scholar] [CrossRef]
- Campos-Martin, J.M.; Capel-Sanchez, M.C.; Perez-Presas, P.; Fierro, J.L.G. Oxidative processes of desulfurization of liquid fuels. J. Chem. Technol. Biotechnol. 2010, 85, 879–890. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.N.; He, H.J.; Cheng, Y.; Yang, C.P.; Zeng, G.M.; Qiu, L. Performances, kinetics and mechanisms of catalytic oxidative desulfurization from oils. RSC Adv. 2016, 6, 103253–103269. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Kong, L.H.; Lu, H. Deep oxidative desulfurization catalyzed by Ti-based metal-organic frameworks. Fuel 2018, 219, 103–110. [Google Scholar] [CrossRef]
- Gomez-Paricio, A.; Santiago-Portillo, A.; Navalon, S.; Concepcion, P.; Alvaro, M.; Garcia, H. MIL-101 promotes the efficient aerobic oxidative desulfurization of dibenzothiophenes. Green Chem. 2016, 18, 508–515. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Ribeiro, S.O.; Karmaoui, M.; Valenca, R.; Ribeiro, J.C.; de Castro, B.; Cunha-Silva, L.; Balula, S.S. Production of ultra-deep sulfur-free diesels using a sustainable catalytic system based on UiO-66(Zr). Chem. Commun. 2015, 51, 13818–13821. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.-C. Recent advances in gas storage and separation using metal–organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, M.; Wang, H.; Zeng, G.; Huang, D.; Cheng, M.; Liu, Y.; Xue, W.; Wang, Z. The application of different typological and structural MOFs-based materials for the dyes adsorption. Coord. Chem. Rev. 2019, 380, 471–483. [Google Scholar] [CrossRef]
- Yi, F.-Y.; Chen, D.; Wu, M.-K.; Han, L.; Jiang, H.-L. Chemical sensors based on metal–organic frameworks. ChemPlusChem. 2016, 81, 675–690. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, H.; Lei, F.; Liang, M.; Qian, X.; Shen, P.; Xu, H.; Chen, Z.; Gao, J.; Yao, J. Benzimidazole-functionalized Zr-UiO-66 nanocrystals for luminescent sensing of Fe3+ in water. J. Solid State Chem. 2017, 245, 160–163. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, X.-F.; Li, Z.-Q.; Wu, F. A new sensor based on amino-functionalized zirconium metal-organic framework for detection of Cu2+ in aqueous solution. Inorg. Chem. Commun. 2016, 74, 22–25. [Google Scholar] [CrossRef]
- Zhuang, S.; Cheng, R.; Wang, J. Adsorption of diclofenac from aqueous solution using UiO-66-type metal-organic frameworks. Chem. Eng. J. 2019, 359, 354–362. [Google Scholar] [CrossRef]
- Chen, C.; Chen, D.; Xie, S.; Quan, H.; Luo, X.; Guo, L. Adsorption behaviors of organic micropollutants on zirconium metal–organic framework uio-66: Analysis of surface interactions. ACS Appl. Mater. Interfaces 2017, 9, 41043–41054. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Xu, L.; Sun, L.; Du, H. Tuning the optical properties of the zirconium–UiO-66 metal–organic framework for photocatalytic degradation of methyl orange. Inorg. Chem. Commun. 2015, 52, 50–52. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Santiago-Portillo, A.; Asiri, A.M.; Garcia, H. Engineering UiO-66 Metal Organic Framework for Heterogeneous Catalysis. ChemCatChem 2019, 11, 899–923. [Google Scholar] [CrossRef]
- Barbosa, A.D.S.; Juliao, D.; Fernandes, D.M.; Peixoto, A.F.; Freire, C.; de Castro, B.; Granadeiro, C.M.; Balula, S.S.; Cunha-Silva, L. Catalytic performance and electrochemical behaviour of Metal-organic frameworks: MIL-101(Fe) versus NH2-MIL-101(Fe). Polyhedron 2017, 127, 464–470. [Google Scholar] [CrossRef]
- Juliao, D.; Barbosa, A.D.S.; Peixoto, A.F.; Freire, C.; de Castro, B.; Balula, S.S.; Cunha-Silva, L. Improved catalytic performance of porous metal-organic frameworks for the ring opening of styrene oxide. CrystEngComm 2017, 19, 4219–4226. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Barbosa, A.D.S.; Ribeiro, S.; Santos, I.; de Castro, B.; Cunha-Silva, L.; Balula, S.S. Oxidative catalytic versatility of a trivacant polyoxotungstate incorporated into MIL-101(Cr). Catal. Sci. Technol. 2014, 4, 1416–1425. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Silva, P.; Saini, V.K.; Paz, F.A.A.; Pires, J.; Cunha-Silva, L.; Balula, S.S. Novel heterogeneous catalysts based on lanthanopolyoxometalates supported on MIL-101(Cr). Catal. Today 2013, 218, 35–42. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Barbosa, A.D.S.; Silva, P.; Paz, F.A.A.; Saini, V.K.; Fires, J.; de Castro, B.; Balula, S.S.; Cunha-Silva, L. Monovacant polyoxometalates incorporated into MIL-101(Cr): Novel heterogeneous catalysts for liquid phase oxidation. Appl. Catal. A Gen. 2013, 453, 316–326. [Google Scholar] [CrossRef]
- Juliao, D.; Gomes, A.C.; Pillinger, M.; Valença, R.; Ribeiro, J.C.; de Castro, B.; Gonçalves, I.S.; Silva, L.C.; Balula, S.S. Zinc-Substituted Polyoxotungstate@amino-MIL-101(Al)—An Efficient Catalyst for the Sustainable Desulfurization of Model and Real Diesels. Eur. J. Inorg. Chem. 2016, 2016, 5114–5122. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Nogueira, L.S.; Juliao, D.; Mirante, F.; Ananias, D.; Balula, S.S.; Cunha-Silva, L. Influence of a porous MOF support on the catalytic performance of Eu-polyoxometalate based materials: Desulfurization of a model diesel. Catal. Sci. Technol. 2016, 6, 1515–1522. [Google Scholar] [CrossRef]
- Juliao, D.; Gomes, A.C.; Pillinger, M.; Cunha-Silva, L.; de Castro, B.; Goncalves, I.S.; Balula, S.S. Desulfurization of model diesel by extraction/oxidation using a zinc-substituted polyoxometalate as catalyst under homogeneous and heterogeneous (MIL-101(Cr) encapsulated) conditions. Fuel Process. Technol. 2015, 131, 78–86. [Google Scholar] [CrossRef]
- Ribeiro, S.; Granadeiro, C.M.; Silva, P.; Paz, F.A.A.; de Biani, F.F.; Cunha-Silva, L.; Balula, S.S. An efficient oxidative desulfurization process using terbium-polyoxometalate@MIL-101(Cr). Catal. Sci. Technol. 2013, 3, 2404–2414. [Google Scholar] [CrossRef]
- Balula, S.S.; Granadeiro, C.M.; Barbosa, A.D.S.; Santos, I.; Cunha-Silva, L. Multifunctional catalyst based on sandwich-type polyoxotungstate and MIL-101 for liquid phase oxidations. Catal. Today 2013, 210, 142–148. [Google Scholar] [CrossRef]
- Han, Y.T.; Liu, M.; Li, K.Y.; Zuo, Y.; Wei, Y.X.; Xu, S.T.; Zhang, G.L.; Song, C.S.; Zhang, Z.C.; Guo, X.W. Facile synthesis of morphology and size-controlled zirconium metal-organic framework UiO-66: The role of hydrofluoric acid in crystallization. CrystEngComm 2015, 17, 6434–6440. [Google Scholar] [CrossRef]
- Vermoortele, F.; Bueken, B.; le Bars, G.; van de Voorde, B.; Vandichel, M.; Houthoofd, K.; Vimont, A.; Daturi, M.; Waroquier, M.; van Speybroeck, V.; et al. Synthesis modulation as a tool to increase the catalytic activity of metal-organic frameworks: The unique case of UiO-66(Zr). J. Am. Chem. Soc. 2013, 135, 11465–11468. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.M.; Dong, Q.L.; Wang, Y.; Li, Y.; Deng, S.J.; Zhang, N. Time modulation of defects in UiO-66 and application in oxidative desulfurization. CrystEngComm 2018, 20, 5658–5662. [Google Scholar] [CrossRef]
- Xu, J.H.; Zhao, S.; Chen, W.; Wang, M.; Song, Y.F. highly efficient extraction and oxidative desulfurization system using Na7H2LaW10O36.32H2O in [bmim]BF4 at room temperature. Chem. A Eur. J. 2012, 18, 4775–4781. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.; Barbosa, A.D.S.; Gomes, A.C.; Pillinger, M.; Goncalves, I.S.; Cunha-Silva, L.; Balula, S.S. Catalytic oxidative desulfurization systems based on Keggin phosphotungstate and metal-organic framework MIL-101. Fuel Process. Technol. 2013, 116, 350–357. [Google Scholar] [CrossRef]
- Zhu, W.S.; Huang, W.L.; Li, H.M.; Zhang, M.; Jiang, W.; Chen, G.Y.; Han, C.R. Polyoxometalate-based ionic liquids as catalysts for deep desulfurization of fuels. Fuel Process. Technol. 2011, 92, 1842–1848. [Google Scholar] [CrossRef]
- Zheng, H.Q.; Zeng, Y.N.; Chen, J.; Lin, R.G.; Zhuang, W.E.; Cao, R.; Lin, Z.J. Zr-based metal-organic frameworks with intrinsic peroxidase-like activity for ultradeep oxidative desulfurization: Mechanism of H2O2 decomposition. Inorg. Chem. 2019, 58, 6983–6992. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Qi, H.; Zhou, W.; Xu, W.; Sun, Y.Y. Green and scalable synthesis of nitro- and amino-functionalized UiO-66(Zr) and the effect of functional groups on the oxidative desulfurization performance. Inorg. Chem. Front. 2019, 6, 1267–1274. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viana, A.M.; Ribeiro, S.O.; Castro, B.d.; Balula, S.S.; Cunha-Silva, L. Influence of UiO-66(Zr) Preparation Strategies in Its Catalytic Efficiency for Desulfurization Process. Materials 2019, 12, 3009. https://doi.org/10.3390/ma12183009
Viana AM, Ribeiro SO, Castro Bd, Balula SS, Cunha-Silva L. Influence of UiO-66(Zr) Preparation Strategies in Its Catalytic Efficiency for Desulfurization Process. Materials. 2019; 12(18):3009. https://doi.org/10.3390/ma12183009
Chicago/Turabian StyleViana, Alexandre M., Susana O. Ribeiro, Baltazar de Castro, Salete S. Balula, and Luís Cunha-Silva. 2019. "Influence of UiO-66(Zr) Preparation Strategies in Its Catalytic Efficiency for Desulfurization Process" Materials 12, no. 18: 3009. https://doi.org/10.3390/ma12183009





