1. Introduction
Early on, nitrogen was introduced into iron-based materials. Its main purpose was to improve corrosion resistance and the mechanical properties of materials, such as surface hardness, wear resistance, fatigue strength, etc. [
1,
2,
3]. In 1972, Kim and Takahashi discovered for the first time that α″-Fe
16N
2 has a “giant magnetization” phenomenon [
4]. Since then, many researchers have moved from studying its mechanical properties to studying its magnetic properties. According to the ratio of nitrogen, the Fe–N system includes iron saturated with nitrogen α-Fe(N), α′-Fe-N martensite, and several Fe–N interstitial compounds with nitrogen ordering (ξ-Fe
2N, ε-Fe
3N, γ′-Fe
4N, and α″-Fe
16N
2) [
5]. FeN and Fe
2N are weakly magnetic, and α″-Fe
16N
2 is more magnetic. However, because α″-Fe
16N
2 is metastable, it is difficult to synthesize and has poor thermodynamic stability. Above 200 °C, it decomposes into α-Fe and γ′-Fe
4N, which greatly limits its application. Although the magnetism of Fe
3N is not as good as that of α″-Fe
16N
2 and α-Fe (the magnetization is 220 emu/g, and the magnetic polarization is about 2.2 T), it has better thermodynamic stability than that of α″-Fe
16N
2 and can be preserved at room temperature. Compared with pure iron, it has the advantage of good oxidation resistance. It also has good corrosion resistance and mechanical properties, high saturation magnetization, high magnetic moment, magnetic permeability, and low coercivity. Hence, it has become a hot topic for research [
6,
7,
8].
At present, the preparation methods of preparing ε-Fe
3N generally include magnetron sputtering [
9,
10], gas reduction-nitriding [
11,
12], the solid-gas reaction method [
3,
13,
14,
15], the sol-gel method [
16,
17,
18], etc. Solid-gas nitriding is the main method, where pure iron powders are reacted with ammonia or a mixed atmosphere of ammonia and hydrogen under a certain temperature to synthesize iron nitride. As an example, Lian et al. and Ren et al. used pure Fe powder as an iron source and got ε-Fe
3N [
13,
14] using the solid-gas nitriding method. They held it for 3–7 h under certain temperature conditions and then cooled it with liquid nitrogen or natural cooling. ε-Fe
3N was first synthesized using the solid-gas reaction method by Zhenghe Hua et al. and Wenxu Yin et al. after preparing the precursor with the sol-gel method [
15,
16]. Wu et al. used a chemical co-precipitation method to prepare the Fe
3O
4 nanoparticles, then the dried Fe
3O
4 nanoparticles were treated in an H
2 + NH
3 mixed gas flow (with a volume ratio of H
2/NH
3 of 1:2) at 400–900 °C for 6 h to get ε-Fe
3N [
5]. Kurian and Gajbhiye firstly decomposed the ferric citrate (Aldrich, 99.9%) to fine particles of α-Fe
2O
3, then the nitridation of these particles was carried out in a quartz tubular furnace under flowing NH
3 gas at 873 K for 5.5 h to get ε-Fe
3N [
3]. It is evident that the existing Fe
3N powder preparation technology has problems, such as a long nitriding time, a complicated process, high equipment requirements, and low efficiency. These seriously restrict the development of ε-Fe
3N powder preparation technology.
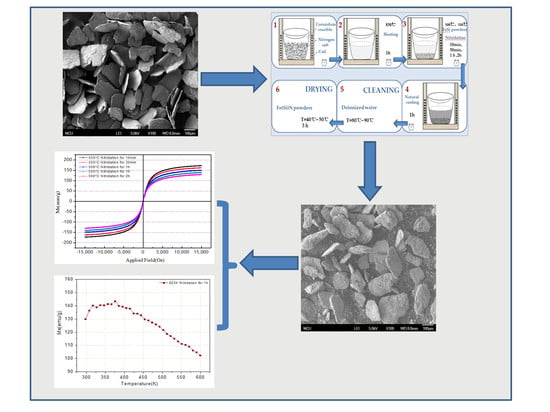
In view of this, we reported a new preparation technology for powder nitriding—the salt bath nitriding reaction method. This preparation technology has characteristics of simplicity, high effectiveness, operability, and a low demand for experimental equipment. In this paper, the ε-Fe(Si)3N powder, with excellent magneto-caloric effects and soft magnetic properties, was successfully synthesized using a rapid and efficient nitriding method with a controlled treatment process. The flaky FeSi alloy powder was used to replace pure iron powder as the iron source, and it was immersed in the molten atomic liquid system containing N for the reaction. Fe(Si)3N powders with excellent soft magnetic properties and temperature dependence were successfully prepared by controlling the nitriding process to achieve a fast and efficient nitriding method. The ε-Fe(Si)3N powder can be applied in a magnetic powder core inductor with a high-frequency circuit because its excellent soft magnetic properties and excellent temperature dependency enable this material to be applied to electronic components under special conditions.
In this paper, two issues of the nitriding of powder were successfully solved. The first is that the alloy powders were difficult to fully nitrify, and the nitriding efficiency was low. Flaky FeSi alloy powders after high-energy ball milling not only possess a large surface area, but also high surface activity. This greatly enlarges the contact area and produces the effect of “double-sided simultaneous nitriding” during nitriding. Thus, the full nitriding time of the powders is greatly shortened, and the efficiency of the nitriding process is greatly improved. Secondly, the Si element increases the entropy values of the powder and reduces the difficulty of the nitriding reaction.
3. Study of the Nitridation Mechanism
In order to investigate the effect of the salt bath nitriding reaction preparation process on the nitriding of powder, this paper studied the influences of nitriding temperature (i.e., 500 or 550 °C) and nitriding time (i.e., 10 min, 30 min, 1 h, or 2 h) on powder nitride. The phase structures of the flaky FeSi powder before and after nitridation are shown in
Figure 3.
Figure 3 shows the original flaky FeSi powder which was successfully prepared by medium frequency melting and high-energy vibration ball milling and the nitriding of samples at different temperatures and times. It can be seen in
Figure 3b–d that the peak of the α-Fe phase still exists in the XRD spectra, indicating that the flake alloy powder nitride prepared at 550 °C for 10 min and 30 min and prepared at 500 °C for 1 h is not enough to make the powder fully nitrided. The XRD spectra shown in
Figure 3e show that when the nitriding temperature of the alloy powder remains unchanged at 500 °C and the nitriding time is prolonged to 2 h, there is no α-Fe phase, and the alloy powder was fully nitrided. When keeping the nitriding time at 1 h and increasing the nitriding temperature to 550 °C, the α-Fe phase was not found, as shown in
Figure 3f. It was evident that prolonging the nitriding time or increasing nitriding temperature was beneficial to the full nitridation of the flaky FeSi alloy powder.
Figure 4 shows the contrast of FeSi alloy powders before and after nitriding. It can be seen from
Figure 4a that the FeSi alloy powder after high-energy vibration ball milling has a sheet-like irregular morphology with a thickness of about 10 μm. This is because the FeSi alloy particles are repeatedly and violently collided, extruded, and welded in high-energy vibration ball milling, which leads to small flat sheet powder forming. As the ball mill continues, the particles undergo plastic deformation, and internal defects (dislocations, vacancies, etc.) increase under the severe impact, which leads to the continuous refinement of the particles into powder of different particle sizes in thin sheets.
The microstructure of the powder at 550 °C nitridation for 1 h was analyzed. The morphology of the powder after nitriding is shown in
Figure 4b. It can be seen from the figure that the shape of the powder particles after nitriding is basically unchanged, but the surface of the powder becomes rough and uneven. This is caused by the reaction and diffusion of nitrogen atoms with the surface of the powder during the nitriding process. When an element penetrates from the metal surface to the inside through diffusion, an intermediate phase (which could be another solid solution) may be formed in the metal surface layer as the diffusion proceeds if the content of the diffusion element exceeds the solubility of the base metal. This phenomenon in which a new phase is formed by diffusion is called reaction-diffusion or phase change diffusion [
19].
The nitriding process of flaky FeSi powders is a reaction-diffusion process where flaky FeSi powders are immersed into a solution containing the N atom system. Firstly, the active N atoms in the system react with the surface of the alloy powder at a high temperature. The nitriding reaction process is as follows:
At the same time as the reaction, the N atoms diffuse through the defects that were generated during the high-energy ball milling of the powder. Secondly, the N atoms in the system are excited by the temperature and the system compound. If the chemical potential of N in the solution of the system is high enough, then the nitrogen atoms gradually diffuse from the surface of the powder into the interior of the powder.
According to the Arrhenius-type equation [
19], the diffusion coefficient (D) is related to the activation energy.
where D
0 is the pre-exponential term, Q is the activation energy (in units of cal/mol) for the species under consideration, R is the gas constant, and T is the absolute temperature (K).
During the nitridation reaction, the diffusion of nitrogen atoms is mainly affected by temperature, defects, and N atomic chemical potential or
[
19]. The kinetics of the process of diffusion is strongly dependent on temperature. When the temperature of the nitrification system increases, the thermal energy that was supplied to the diffusing atoms permits the atoms to overcome the activation energy barrier, and they more easily to move to new sites. Therefore, the flux of atoms increases, as well. The XRD spectra also showed that the nitriding effect was better when the nitriding reaction time was shortened from 2 to 1 h and the nitriding temperature was raised from 500 to 550 °C. The saturation magnetization of the product was slightly higher than that of the product nitrided at 500 °C for 2 h, which was 139 emu/g. The detailed test results of the saturation magnetization of powders under different nitridation conditions are shown in
Table 1.
The chemical potential of the N atom in the nitrogen salt system indicates the driving force for diffusion. Under the action of the thermal activation energy, the N atoms in the system maintain a high enough chemical potential and diffuse, in depth, continuously and divergently along the defects in the powder. This continues until the chemical potential gradient is 0 in the nitrided layer and there is no driving force for diffusion. The diffusion of nitrogen atoms cannot continue, and the nitriding of powders is complete.
The nitrogen content was analyzed by using an electron probe microanalyzer (EPMA), which used a focused electron beam irradiated onto a small area of the surface of the sample to excite characteristic X-rays of the Fe element and the N element wavelength (energy) in the sample.
Figure 5a,b depicts micro-area distribution maps of the Fe and N elements in the ε-Fe(Si)
3N powder, respectively. In
Figure 5, it is evident that the iron content is relatively large and well-distributed, and the Fe element cluster enrichment region is substantially the same as the N element cluster barren region. Combined with the above XRD pattern, it can be concluded that the Fe element takes the N element as the nucleation core and forms the ε-Fe(Si)
3N phase in the nitriding process.
The thermal decomposition of nitrides should be considered as the weakened metal N bond broken by heat motion and N atoms combined into the strong nitrogen molecule. In order to better understand the thermal stability of nitriding powders, the thermal stability test was carried out using a synchronous thermal analyzer. Thermogravimetric analysis (TG) is a thermal analysis technique for measuring the relationship between the mass of the sample and the temperature change.
Figure 6 shows the thermal analysis curve of the ε-Fe(Si)
3N powder.
It can be seen from
Figure 6 that the weight of ε-Fe(Si)
3N powders changes gradually at first, then loses weight sharply. Eventually, it tended to become stable with the increase of temperature. When the ε-Fe(Si)
3N powders were raised from room temperature to 478.8 °C, their weights did not change. The turning point temperature of weightlessness was 478.8 °C. From the turning point temperature to 553.5 °C, its weightlessness ratio was 1%, then the weight decreased sharply. When the temperature was raised to 654.8 °C, the weightlessness ratio was about 6.5%, and the TG curve began to stabilize. Finally, the weightlessness of the powders was almost complete, and the TG curve showed a steady trend at a temperature of 697.8 °C. This is because the nitrogen in the ε-Fe(Si)
3N powders gradually formed nitrogen gas and escaped with the increase of temperature, which made the weight of the powders slowly decrease. The results show that the properties of the powders themselves basically did not change under the working environment before the turning point temperature (478.8 °C) of weightlessness. Because the weight was basically undergoing no loss, no nitrogen atom formed nitrogen gas to escape and made chemical structure changes in Fe(Si)
3N.