Mechanical Properties and Fire Resistance of Magnesium-Cemented Poplar Particleboard
Abstract
:1. Introduction
2. Materials and Methods
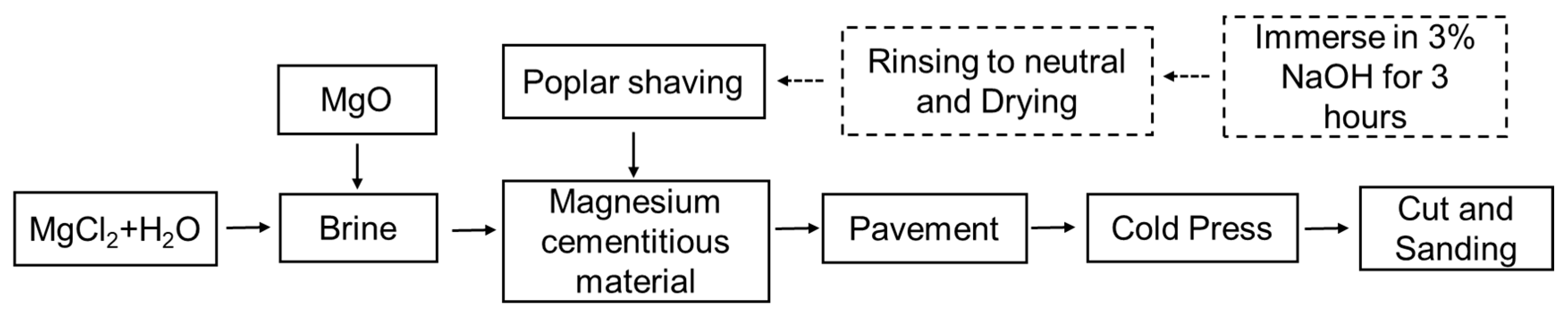
2.1. The Preparation of MCPB
2.2. Mechanical Property Test
2.3. Thickness Swelling (TS) Test
2.4. Characterization
3. Results
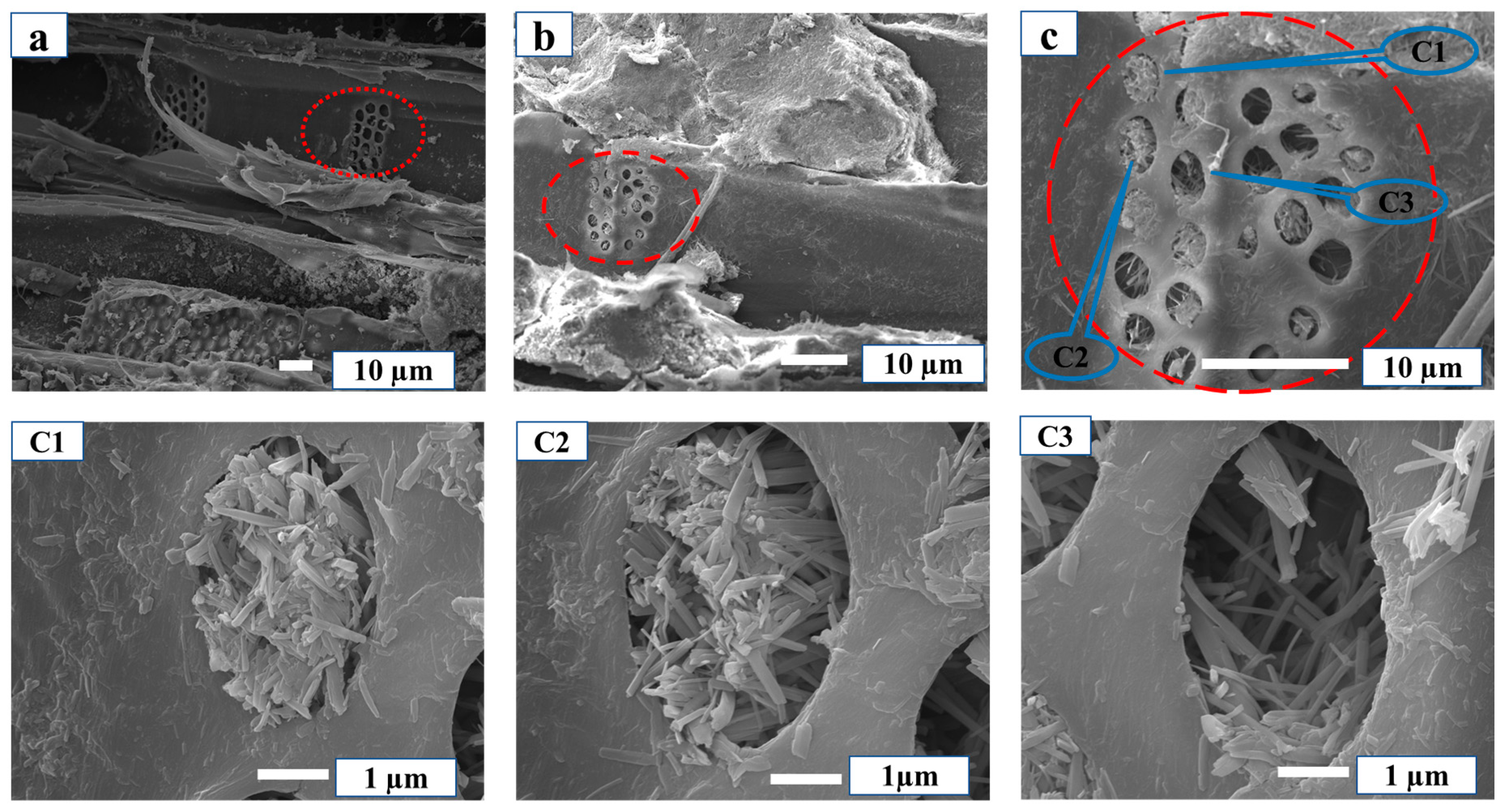
3.1. Physical Combination of MCPB
3.2. Chemical Combination of MCPB
3.3. Mechanical Properties and Water Resistance of MCPB
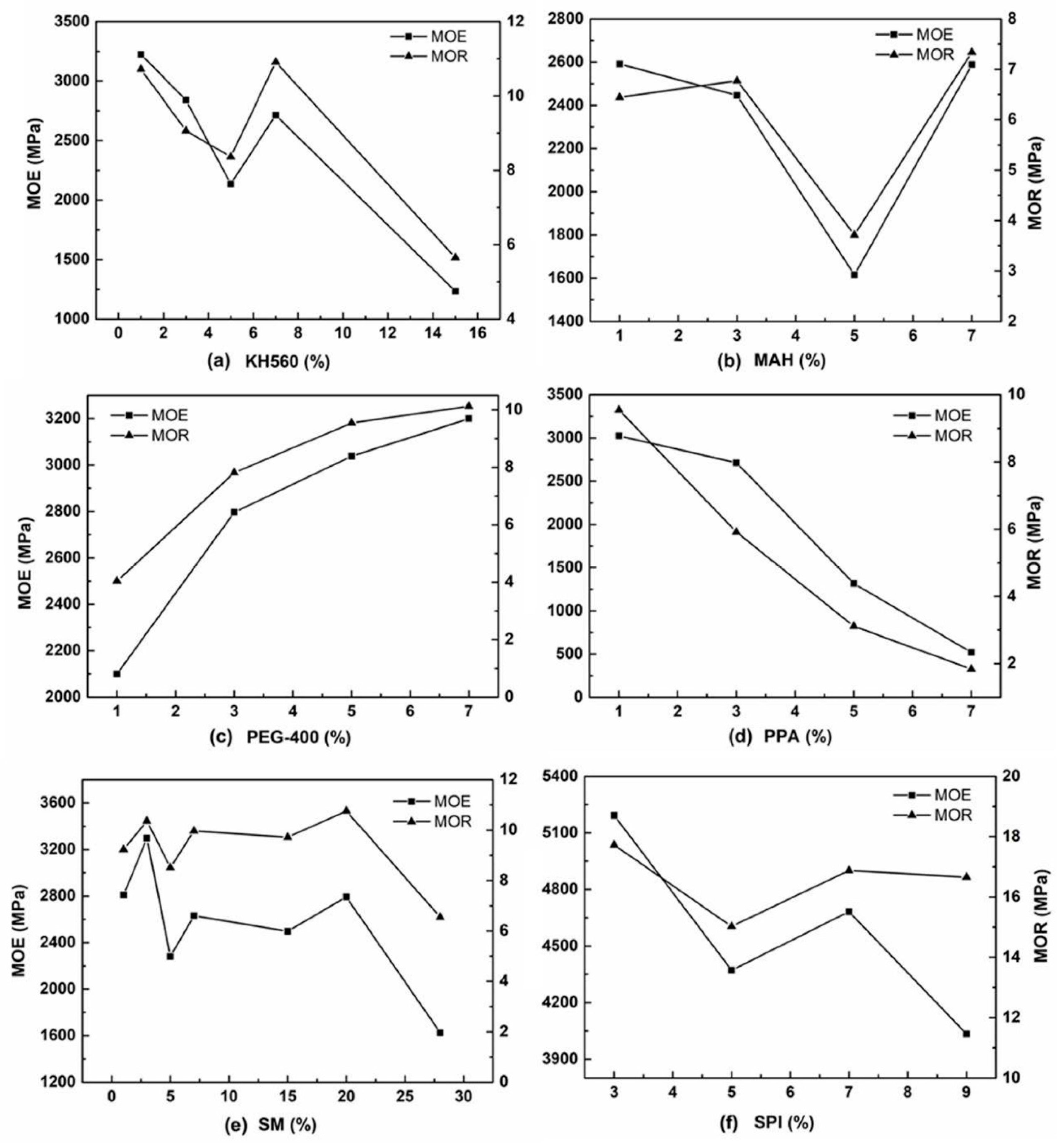
3.3.1. Mechanical Test-MOR and MOE
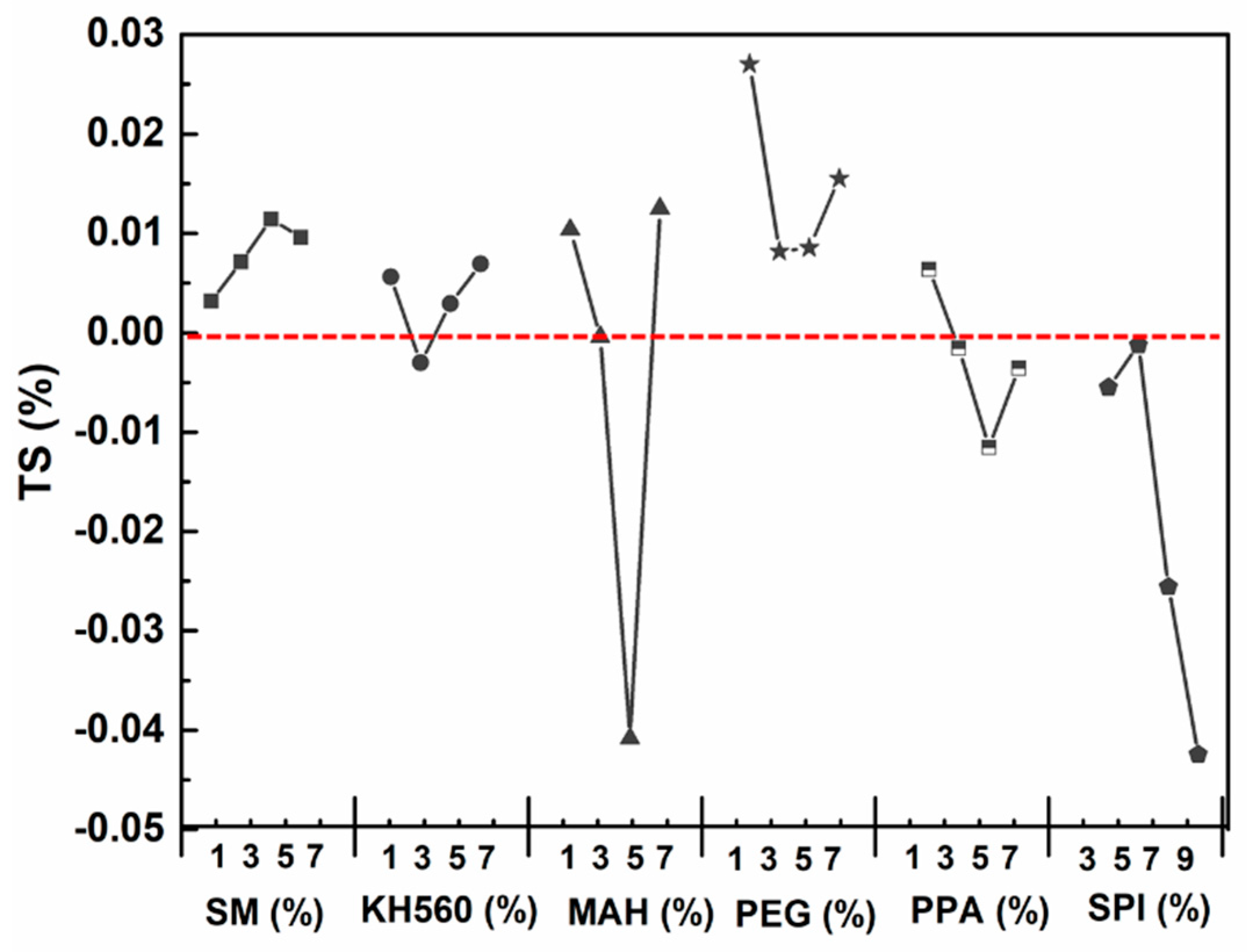
3.3.2. Water Resistance—Thickness Swelling (TS) after 24 h
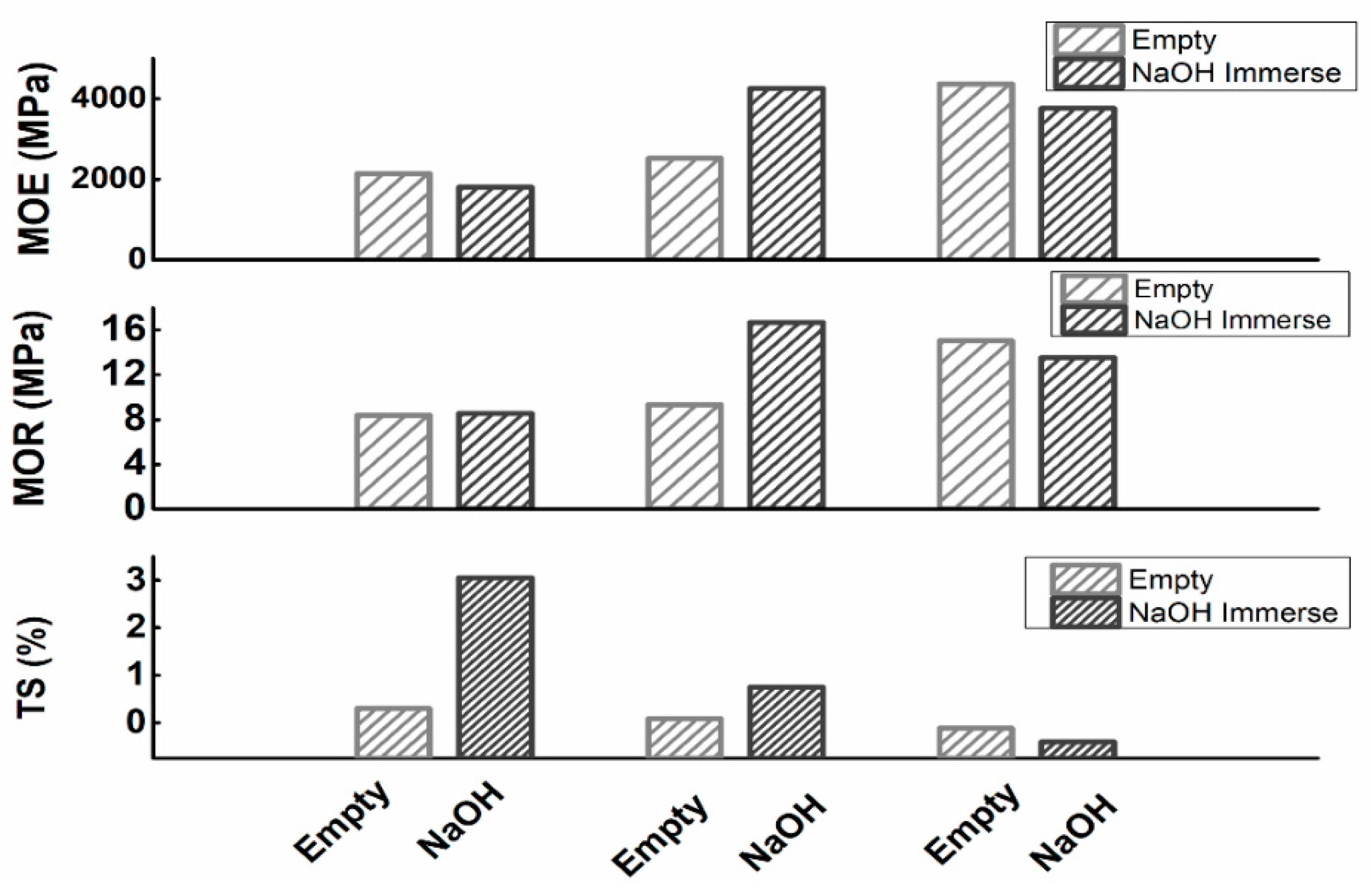
3.3.3. Effect on the Mechanical Properties of NaOH Treatment
3.4. Thermal Stability and Fire Resistance of MCPB
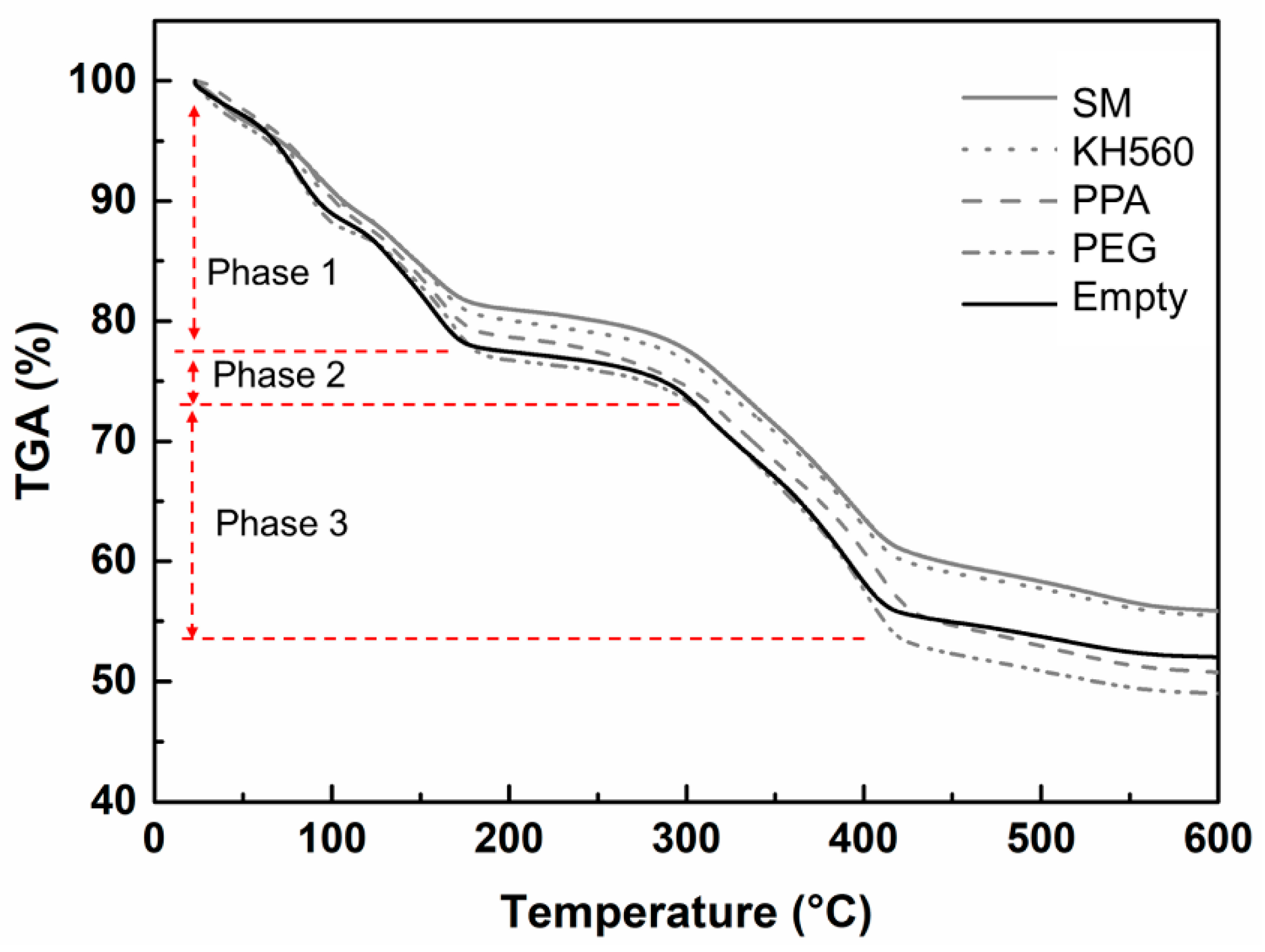
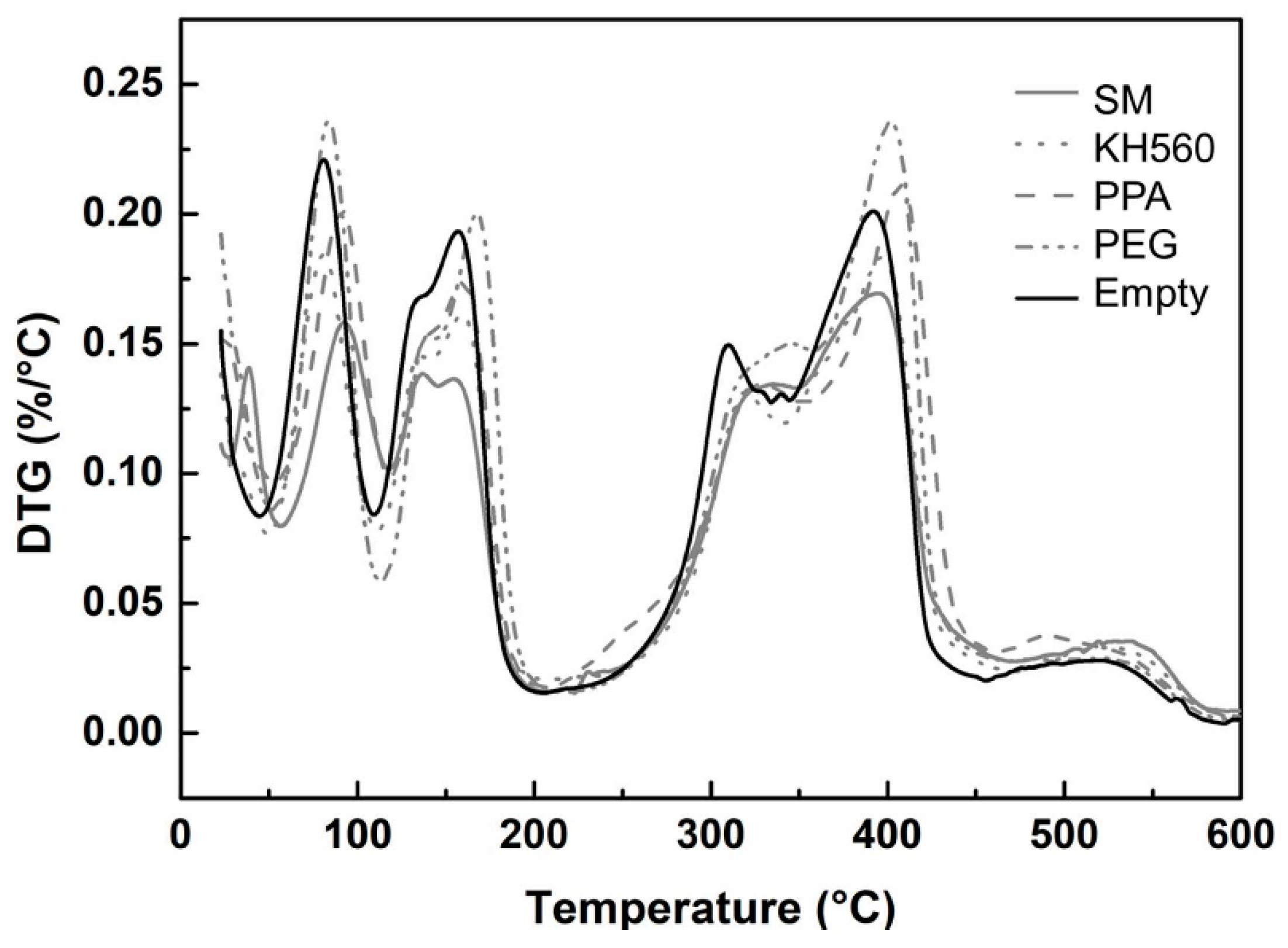
3.4.1. Thermal Stability
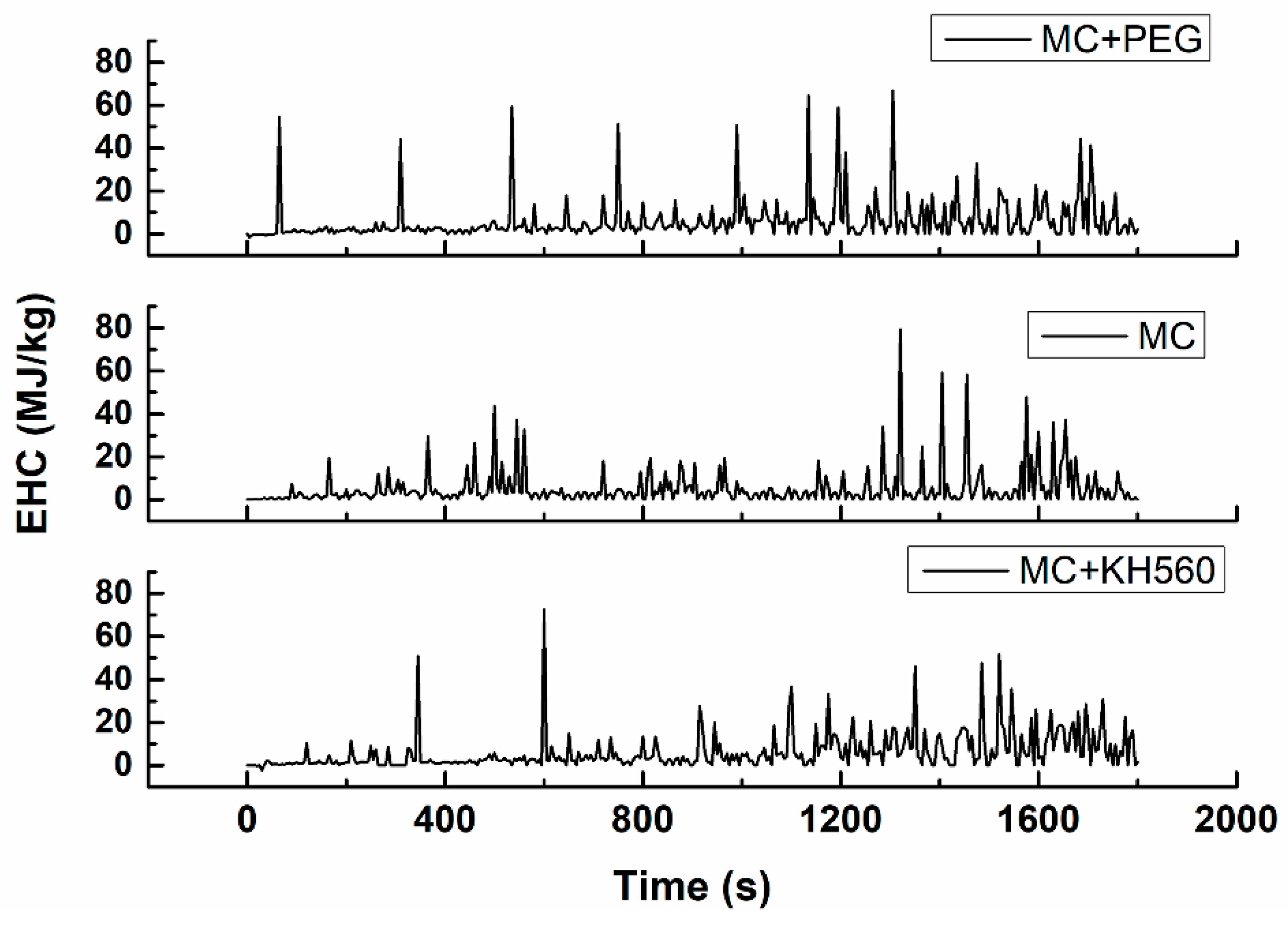
3.4.2. Fire Resistance—Results of CONE
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pia, S.; Johannes, K.; Wolfgang, G.; Wolfgang, K.; Johann, M.; Roland, M.; Hendrikus, W. Technological performance of formaldehyde-free adhesive alternatives for particleboard industry. Int. J. Adhes. Adhes. 2019, 94, 99–131. [Google Scholar]
- Asgedom, A.; Bråtveit, M.; Moen, B. High Prevalence of Respiratory Symptoms among Particleboard Workers in Ethiopia: A Cross-Sectional Study. Int. J. Environ. Res. Public Health. 2019, 16, 2158. [Google Scholar] [CrossRef] [PubMed]
- Robert, K.; Bronisław, P. Fused magnesia-zirconia co-clinker for fired refractories. Ceram. Int. 2017, 43, 14263–14270. [Google Scholar]
- Morteza, N.; Zahra, B.; Rahim, M.G.; Farhad, K. Application of response surface methodology for evaluating particleboard properties made from cotton stalk particles. Wood Mat. Sci. & Eng. 2018, 13, 73–80. [Google Scholar]
- Seyedeh, M.; Aliakbar, E.; Kazem, D.; Asghar, T. Use of amino silane coupling agent to improve physical and mechanical properties of UF-bonded wheat straw (Triticum aestivum L.) poplar wood particleboard. J. For. Res. 2016, 27, 427–431. [Google Scholar]
- Seyedeh, M.; Kazem, D. The influence of silane coupling agent and poplar particles on the wettability, surface roughness, and hardness of UF-bonded wheat straw (Triticum aestivum L.)/poplar wood particleboard. J. For. Res. 2014, 25, 667–670. [Google Scholar]
- Wang, L.; Yu, I.K.M.; Tsang, D.; Li, S.; Li, J.; Poon, C.; Wang, Y.; Dai, J. Transforming wood waste into water-resistant magnesia-phosphate cement particleboard modified by alumina and red mud. J. Clean. Prod. 2017, 168, 452–462. [Google Scholar] [CrossRef]
- Julio, C.; Diogo, L.; Gonzalo, M.; Julio, C.; Gilmara, O.; Juliano, F. Manufacture of particleboard based on cement bag and castor oil polyurethane resin. Constr. Build. Mater. 2015, 87, 8–15. [Google Scholar]
- Wan, N.; Mohammed, D.; Muhammad, S.; Boon, J. Mechanical properties and dimensional stability of particleboard fabricated from steam pre-treated banana trunk waste particles. J. Build. Eng. 2019, 26, 2352–7012. [Google Scholar]
- Monteiro, S.; Martins, J.; Magalhães, F.; Carvalho, L. Low Density Wood Particleboards Bonded with Starch Foam-Study of Production Process Conditions. Materials 2019, 12, 1975. [Google Scholar] [CrossRef]
- Paiman, B.; Seng, H.; Nurul, F.; Muhamad, S.; Zaidon, A. A preliminary study on physical and mechanical properties of particleboard made from palm oil-treated rubberwood particles. J. Indian Acad. Wood Sci. 2019, 16, 27–30. [Google Scholar]
- Ulrich, H.; Holger, M.; Carsten, M. Use of alkyl ketene dimer (AKD) for surface modification of particleboard chips. Eur. J. Wood Wood Prod. 2009, 67, 37–45. [Google Scholar]
- Wang, X.; Yu, Y. The compatibility of two common fast-growing species with Portland cement. J. Indian Acad. Wood Sci. 2012, 9, 154–159. [Google Scholar] [CrossRef]
- An, G.; Ronald, S. Wood-Cement Particleboard: Impact Behavior and Potential Application in Crash Barriers. J. Mater. Civ. Eng. 2012, 24, 130–140. [Google Scholar]
- Raúl, E.; Alain, C. Physical and mechanical properties of gypsum particleboard reinforced with Portland cement. Eur. J. Wood Wood Prod. 2011, 69, 247–254. [Google Scholar]
- Li, X.; Zheng, X.; Wu, Y. Study on Hot-Pressing Technology of Cement Bamboo Particleboard. Adv. Mater. Res. 2011, 168–170, 1233–1237. [Google Scholar]
- GB/T 4897-2015. Particleboard; Standardization Administration of China: Beijing, China, 2015.
- GB/T 175-2007. Common Portland Cement; Standardization Administration of China: Beijing, China, 2007.
- GB/T 17657-2013. Test Methods of Evaluating the Properties of Wood-Based Panels and Surface Decorated Wood-Based Panels; Standardization Administration of China: Beijing, China, 2013.
- GB/T 17671-1999. Testing Method for Strength of Cement Mortar; Standardization Administration of China: Beijing, China, 1999.
- ISO 5660-1-2002. Reaction-to-FIRE Tests—Heat Release, Smoke Production and Mass Loss Rate; International Organization for Standardization: Geneva, Switzerland, 2002.





| Material | Grade | Manufacturer |
|---|---|---|
| MgO | Industrial | Shi Jiazhuang Tian Yu Magnesium Industry Co., Ltd., Shijiazhuang, China |
| MgCl2 | Industrial | Wuxi Yatai United Chemical Co., Ltd., Wuxi, China |
| H2O | Chemical | Laboratory of Beijing Forestry University |
| Soybean meal (SM) | Food grade | Shandong Yu Wang Ecological Food Co., Ltd., Dezhou, China |
| Silane coupling agent (KH560) | Chemical | Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China |
| Polyacrylic Acid (PAA) | Chemical | Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China |
| Polyethylene glycol (PEG-400) | Chemical | Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China |
| Soybean protein isolate (SPI) | Food grade | Shan Dong Yu Wang Ecological Food Co., Ltd., Dezhou, China |
| Maleic anhydride (MAH) | Chemical | Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China |
| NaOH | Chemical | Tian Jin Da Mao Chemical Reagent Factory |
| Mechanical Test | Chemical Addition | Ratio (%) | ||||
|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 9 | ||
| MOE (MPa) | SM | 2809.08 | 3296.69 | 2280 | 2630.69 | — |
| KH560 | 3224.22 | 2839.84 | 2134.43 | 2713.36 | — | |
| MAH | 2590.7 | 2445.78 | 1614.5 | 2588.31 | — | |
| PEG | 2099.83 | 2796.89 | 3037.93 | 3200 | — | |
| PAA | 3023.78 | 2713.15 | 1316.42 | 520.6 | — | |
| SPI | — | 5192.83 | 4371.5 | 4682.33 | 4033.5 | |
| MOR (MPa) | SM | 9.23 | 10.36 | 8.51 | 9.97 | — |
| KH560 | 10.72 | 9.06 | 8.36 | 10.91 | — | |
| MAH | 6.44 | 6.77 | 3.71 | 7.34 | — | |
| PEG | 4.04 | 7.82 | 9.54 | 10.13 | — | |
| PAA | 9.55 | 5.92 | 3.11 | 1.84 | — | |
| SPI | — | 17.73 | 15.03 | 16.88 | 16.66 | |
| Proportion (%) | TS (%) with Various Chemical Additions | |||||
|---|---|---|---|---|---|---|
| SM | KH560 | MAH | PEG | PAA | SPI | |
| 1 | 0.32 | 0.56 | 1.04 | 2.7 | 0.64 | −0.55 |
| 3 | 0.71 | −0.3 | −0.04 | 0.82 | −0.15 | −0.12 |
| 5 | 1.14 | 0.29 | −4.08 | 0.86 | −1.15 | −2.56 |
| 7 | 0.96 | 0.69 | 1.25 | 1.55 | −0.36 | −4.25 |
| Samples | Parameter | Peak Fitting of C1s | |||
|---|---|---|---|---|---|
| C–C/C=C | C–O | C=O/O–C–O | O–C=O | ||
| NaOH treated | Peak position | 284.8 | 286.4 | 287.9 | 288.8 |
| Peak area | 20443.6 | 12273.5 | 2201.1 | 1421.6 | |
| Empty | Peak position | 284.8 | 286.4 | 287.9 | 288.8 |
| Peak area | 18759.1 | 11867.4 | 1838.2 | 1981.8 | |
| Test Group | HRR (kW/m2) | TSP (m2) | THR (MJ/kg) | CO Release (g/s) | CO2 Release (g/s) |
|---|---|---|---|---|---|
| 26(MC + KH560) | 18 | 0.192 | 29.71 | 0.002 | 0.031 |
| 33(MC + PEG) | 43 | 0.366 | 34.39 | 0.003 | 0.051 |
| 35(MC) | 24 | 0.167 | 39.39 | 0.002 | 0.032 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, N.; Wu, D.; Sun, P.; Liu, H.; Luo, B.; Li, L. Mechanical Properties and Fire Resistance of Magnesium-Cemented Poplar Particleboard. Materials 2019, 12, 3161. https://doi.org/10.3390/ma12193161
Zheng N, Wu D, Sun P, Liu H, Luo B, Li L. Mechanical Properties and Fire Resistance of Magnesium-Cemented Poplar Particleboard. Materials. 2019; 12(19):3161. https://doi.org/10.3390/ma12193161
Chicago/Turabian StyleZheng, Nihua, Danni Wu, Ping Sun, Hongguang Liu, Bin Luo, and Li Li. 2019. "Mechanical Properties and Fire Resistance of Magnesium-Cemented Poplar Particleboard" Materials 12, no. 19: 3161. https://doi.org/10.3390/ma12193161




