Hydrogen Absorption in Pd–Ag Systems: A TPD and Electrical Resistivity Study
Abstract
:1. Introduction
2. Experimental Methods
2.1. The Activation of Pd–Ag Foils
2.2. Absorption/Desorption Tests and Resistivity Measurements
3. Results and Discussion
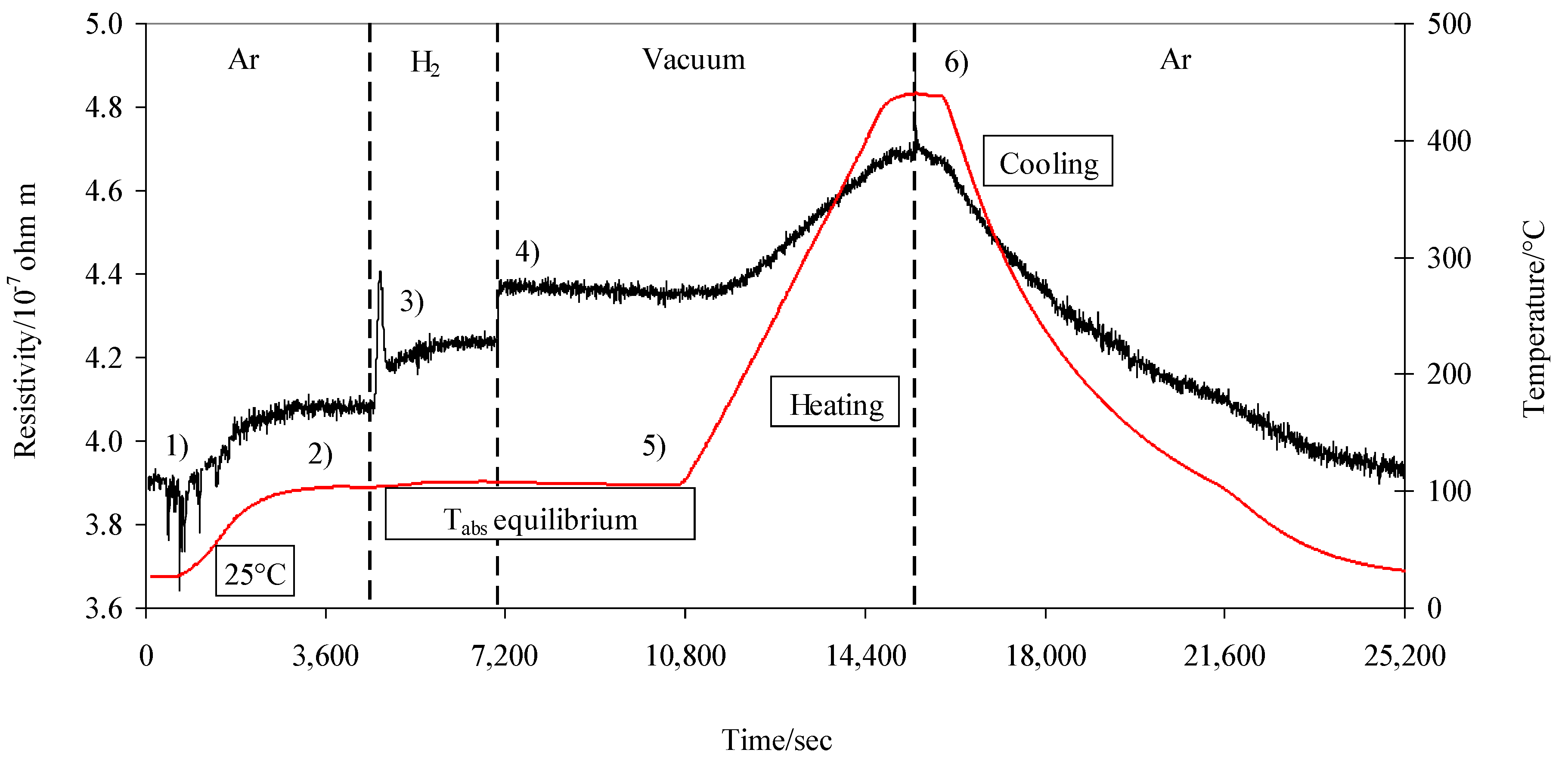
3.1. Resistivity in A Pd–Ag–Hn (n = H/Me) System in Argon and Hydrogen Atmosphere
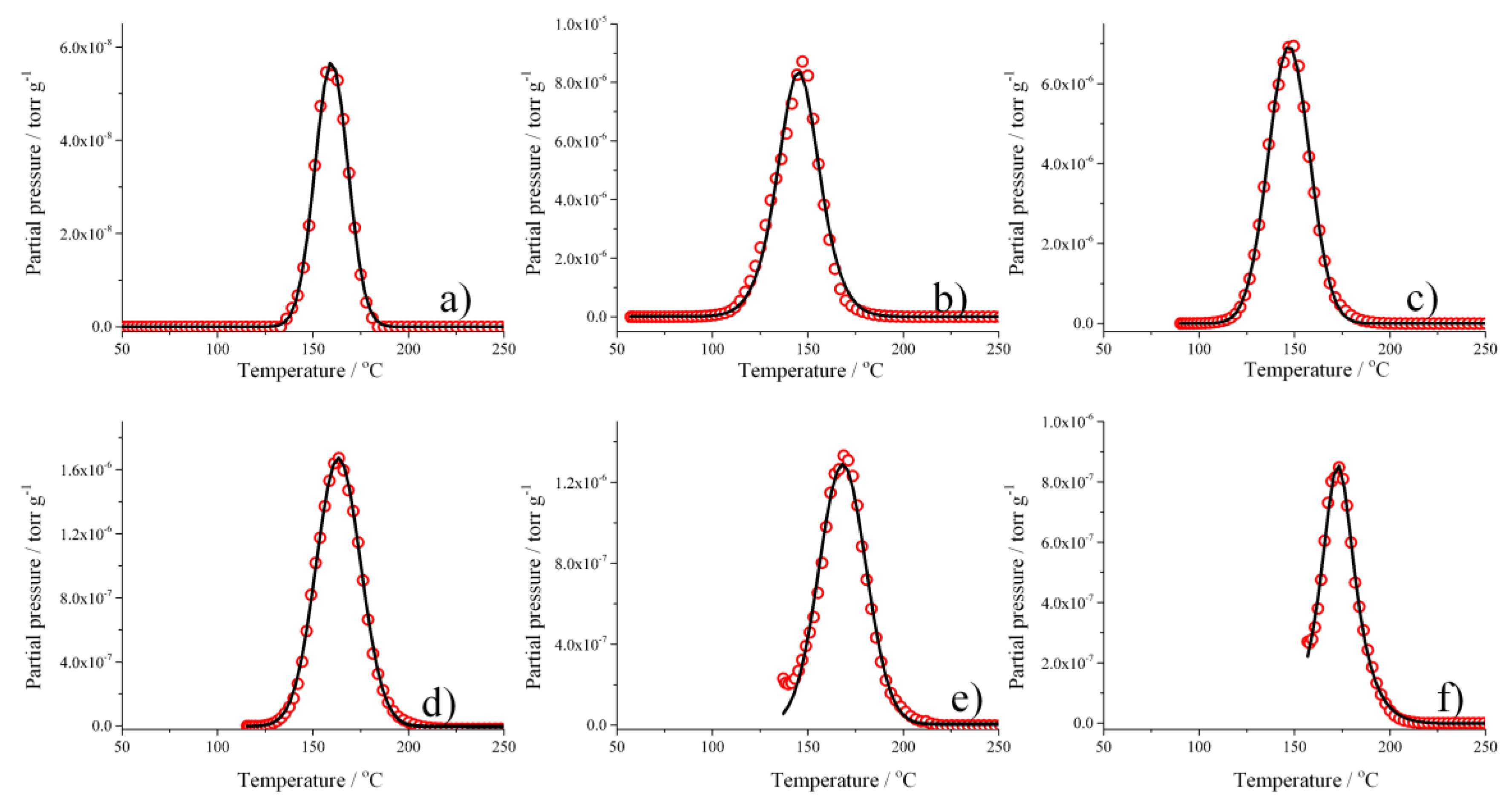
3.2. Thermal Desorption of Hydrogen from A Pd–Ag Alloy.
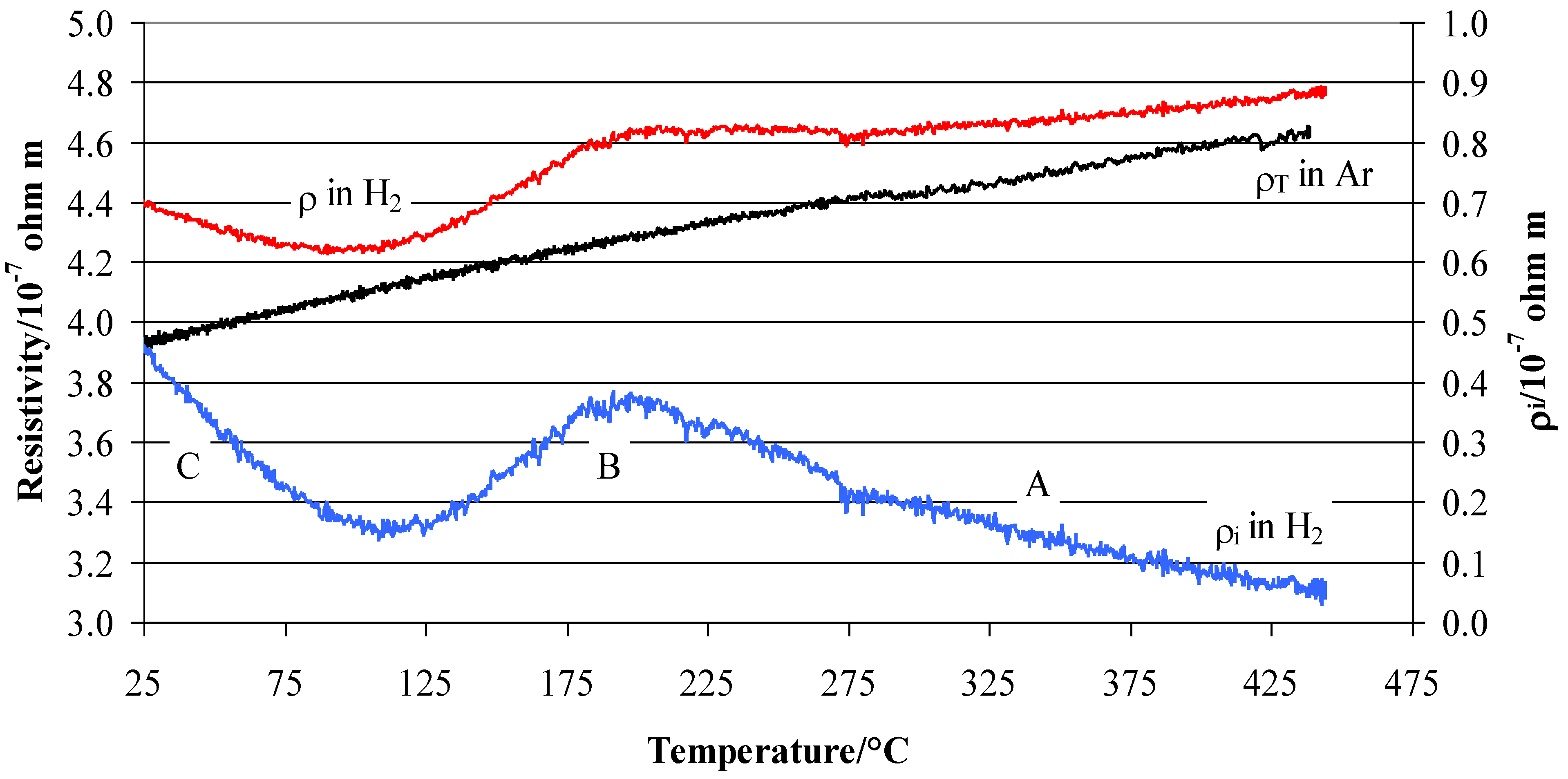
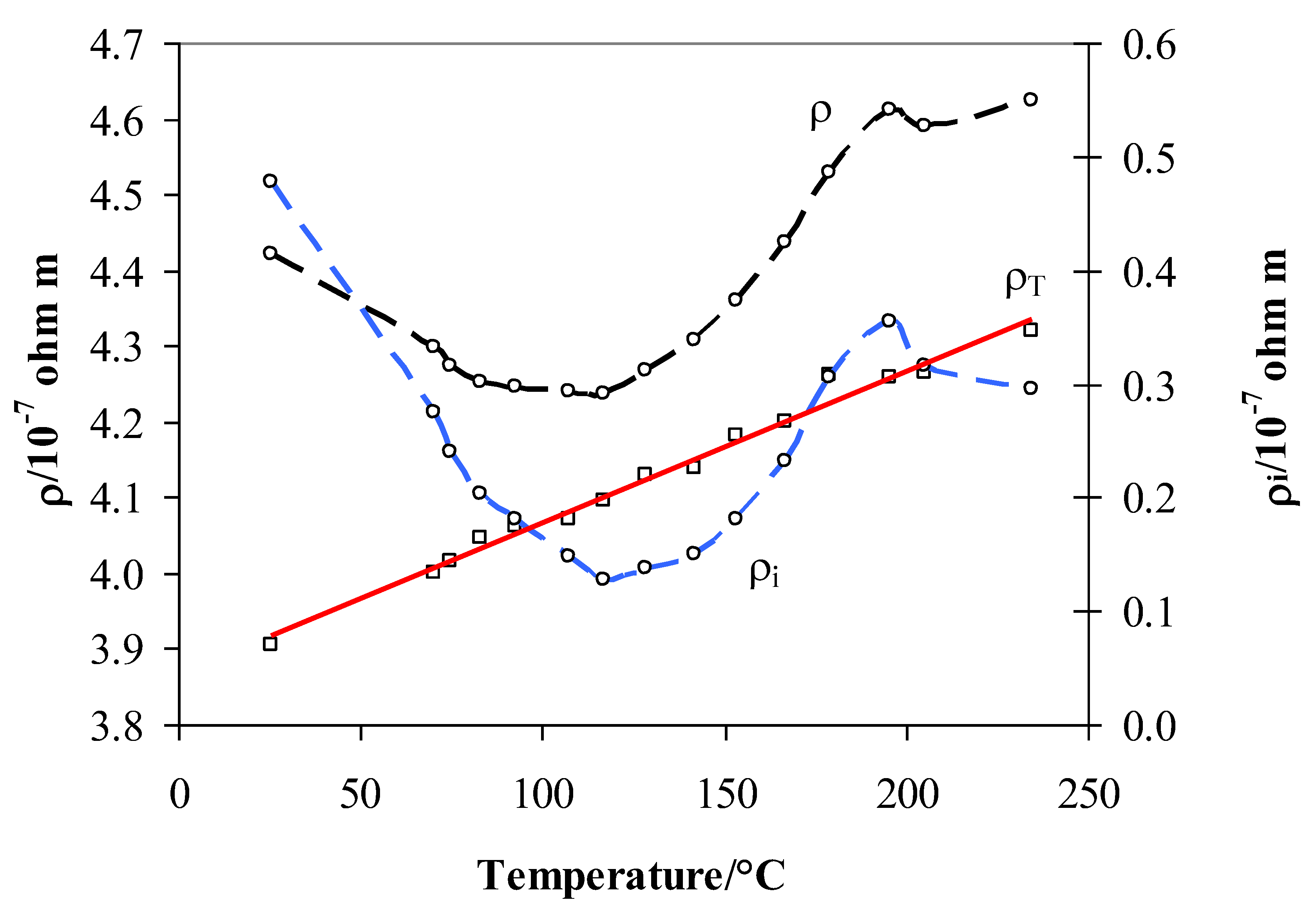
3.3. Resistivity of Pd0.79–Ag0.21 in Hydrogen and a Vacuum and Its Link to Hydride Forms
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Darling, A.S. Thermal and Electrolytic Palladium Alloy Diffusion Cells. Platin. Met. Rev. 1963, 7, 126–129. [Google Scholar]
- Medrano, J.A.; Fernandez, E.; Melendez, J.; Parco, M.; Tanaka, D.A.P.; van Sint Annaland, M.; Gallucci, F. Pd-based metallic supported membranes: High-temperature stability and fluidized bed reactor testing. Int. J. Hydrog. Energy 2018, 41, 8706–8718. [Google Scholar] [CrossRef]
- SShu, J.; Grandjean, B.P.A.; Van Neste, A.; Kaliaguine, S. Catalytic palladium-based membrane reactors: A review. Can. J. Chem. Eng. 1991, 69, 1036–1060. [Google Scholar] [CrossRef]
- Okazaki, J.; Tanaka, D.A.P.; Tanco, M.A.L.; Wakui, Y.; Mizukami, F.; Suzuki, T.M. Hydrogen permeability study of the thin Pd–Ag alloy membranes in the temperature range across the α–β phase transition. J. Membr. Sci. 2006, 282, 370–374. [Google Scholar] [CrossRef]
- Tosti, S.; Borgognoni, F.; Santucci, A. Electrical resistivity, strain and permeability of Pd–Ag membrane tubes. Int. J. Hydrog. Energy 2010, 35, 7796–7802. [Google Scholar] [CrossRef]
- Lewis, F.A.; Kandasamy, K.; McNicholl, R.A.; Tong, X.Q. Hydrogen pressure-hydrogen content and electrical resistance-hydrogen content relationships of palladium and palladium alloy-hydrogen systems. Int. J. Hydrog. Energy 1995, 20, 369–372. [Google Scholar] [CrossRef]
- McNicholl, R.A.; Lewis, F.A. Hydrogen contents and electrical resistivity of palladium—Silver alloys. Int. J. Altern. Energy Ecol. 2004, 3, 32–33. [Google Scholar]
- Carson, A.W.; Lewis, A.; Schurter, H. Relationships between the Hydrogen Content and Electrical Resistance of Palladium + Silver Alloys. Trans. Faraday Sec. 1967, 43, 1447–1452. [Google Scholar] [CrossRef]
- Toth, J.; Garaguly, J.; Petera, L.; Tompa, K. Resistivity changes during hydrogenation of Pd80Ag20 alloy in non-equilibrium circumstances. J. Alloys Compd. 2000, 312, 117–120. [Google Scholar] [CrossRef]
- Brodowsky, H. On the Non-Ideal Solution Behavior of Hydrogen in Metals. Ber. Bunsenges. Phys. Chem. 1972, 76, 740–749. [Google Scholar]
- Pozio, A.; Jovanovic, Z.; Lo Presti, R.; De Francesco, M.; Tosti, S. Pd-Ag hydrogen content and electrical resistivity: Temperature and pressure effect. Int. J. Hydrog. Energy 2012, 37, 7925–7933. [Google Scholar] [CrossRef]
- Baranowski, B. Metal-hydrogen systems at high pressures. In Topics in applied physics. Hydrogen in Metals II; Alefeld, G., Volkl, J., Eds.; Springer-Verlag Berlin Heidelberg: Berlin, Germany, 1978; pp. 157–200. [Google Scholar]
- Szafransky, A.W.; Baranowski, B. The electrical resistance of the Pd-Ag-H system at 25 °C in a wide range of hydrogen pressure. Phys. Stat. Sol. 1972, 9, 435–445. [Google Scholar] [CrossRef]
- Leary, K.J.; Michaels, J.N.; Stacy, A.M. Penetration of Hydrogen into Subsurface Sites of Silica-Supported Palladium during Temperature-Programmed Desorption. Langmuir 1988, 4, 1251–1257. [Google Scholar] [CrossRef]
- Baumberger, M.; Stocker, W.; Rieder, K.H. Investigations of the selective population of hydrogen subsurface sites on Pd(110) using He diffraction and thermal-desorption spectroscopy. Appl. Phys. A 1986, 41, 151–156. [Google Scholar] [CrossRef]
- Mott, N.F. The Basis of the Electron Theory of Metals, with Special Reference to the Transition Metals. Proc. Phys. Soc. 1949, 62, 416–422. [Google Scholar] [CrossRef] [Green Version]
- Fort, D.; Harris, I.R. The physical properties of some palladium alloy hydrogen diffusion membrane material. J. Less Common Met. 1975, 41, 313–327. [Google Scholar] [CrossRef]
- Brodowsky, H.; Poeschel, E. Wasserstoff in Pd/Ag-Legierungen. Z. Phys. Chem. NF 1965, 44, 143–159. [Google Scholar] [CrossRef]
- Millet, P.; Lebouin, C.; Decaux, C.; Ngameni, R.; Ranjbari, A.; Guymont, M. Characterization of metal hydrides using pneumato-chemical impedance spectroscopy. Int. J. Hydrog. Energy 2009, 34, 4990–4996. [Google Scholar] [CrossRef]
- Serra, E.; Kemali, M.; Ross, D.K.; Perujo, A. Hydrogen and deuterium in Pd-25 pct Ag alloy: Permeation, diffusion, solubilization, and surface reaction. Met. Mater. Trans. A 1998, 29, 1023–1028. [Google Scholar] [CrossRef]
- Jeemaa, N.; Grandjean, B.P.A.; Kaliaguine, S. Diffusion coefficient of hydrogen in a Pd-Ag Membrane: Effect of hydrogen solubility. Can. J. Chem. Eng. 1995, 73, 405–410. [Google Scholar] [CrossRef]
- Paolone, A.; Tosti, S.; Santucci, A.; Palumbo, O.; Trequattrini, F. Hydrogen and Deuterium Solubility in Commercial Pd–Ag Alloys for Hydrogen Purification. Chem. Eng. 2017, 1, 1–9. [Google Scholar] [CrossRef]
- Piccard, C.; Kleppa, O.J.; Boureau, G. High temperature thermodynamics of the solutions of hydrogen in palladium-silver alloys. J. Chem. Phys 1979, 70, 2710–2719. [Google Scholar] [CrossRef]
- Pozio, A.; De Francesco, M.; Jovanovic, Z.; Tosti, S. Pd-Ag hydrogen diffusion cathode for alkaline water electrolysers. Int. J. Hydrog. Energy 2011, 36, 5211–5217. [Google Scholar] [CrossRef]


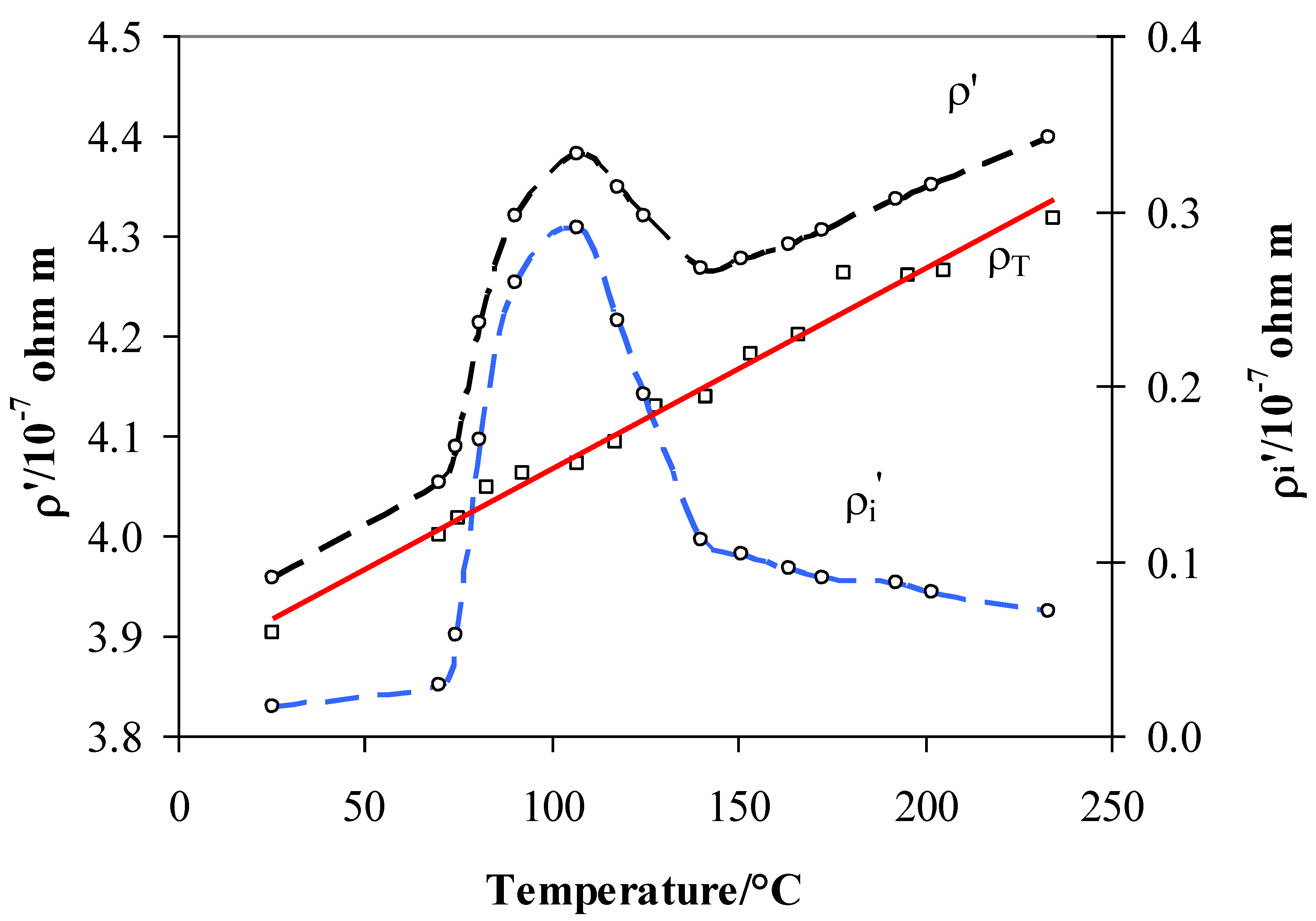
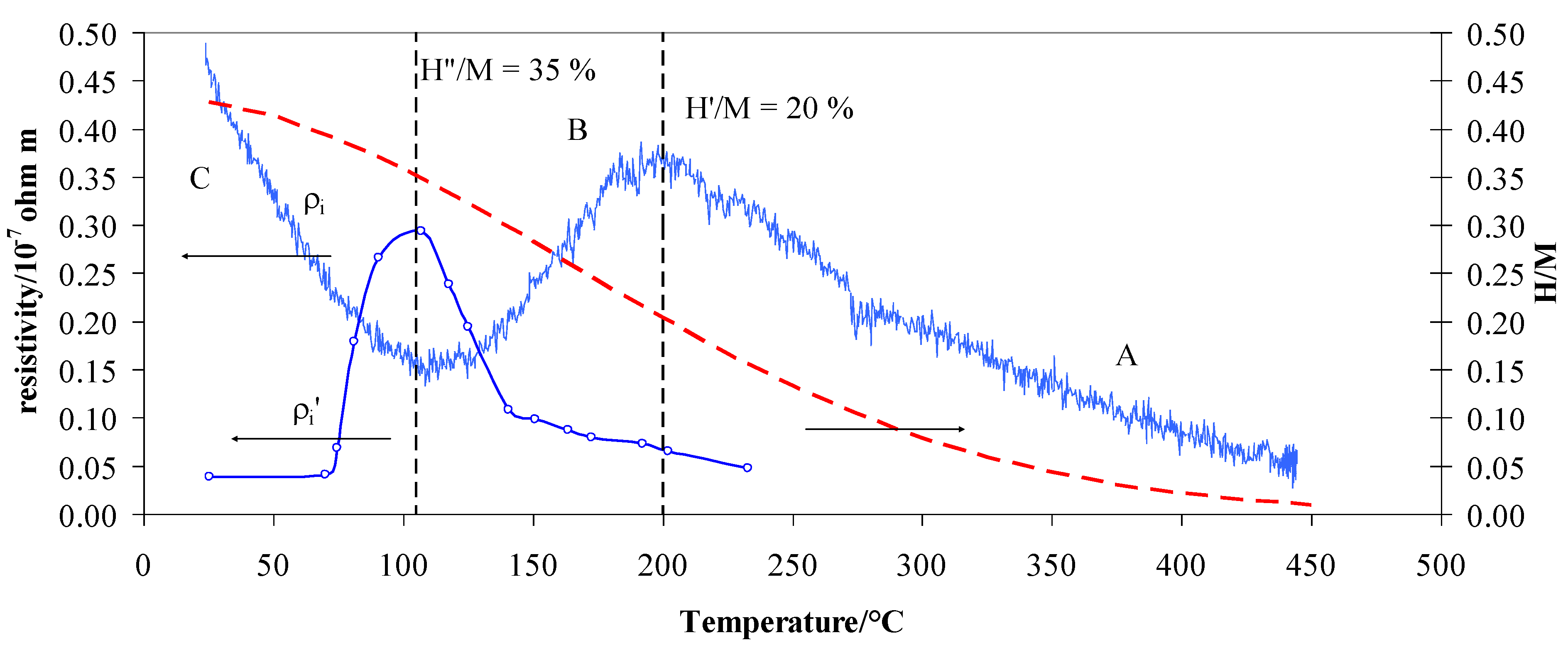
| Temperature/°C | H/M | H Position | Resistivity | Role |
|---|---|---|---|---|
| >200 | 0–0.20 | Octahedral | Increase | d-band filling |
| 200–110 | 0.20–0.39 | Octahedral | Decrease | Number of valence e− increases |
| <110 | >0.39 | Octahedral ↔ Tetrahedral | Increase | Scattering effect |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozio, A.; Jovanovic, Z.; Tosti, S. Hydrogen Absorption in Pd–Ag Systems: A TPD and Electrical Resistivity Study. Materials 2019, 12, 3160. https://doi.org/10.3390/ma12193160
Pozio A, Jovanovic Z, Tosti S. Hydrogen Absorption in Pd–Ag Systems: A TPD and Electrical Resistivity Study. Materials. 2019; 12(19):3160. https://doi.org/10.3390/ma12193160
Chicago/Turabian StylePozio, Alfonso, Zoran Jovanovic, and Silvano Tosti. 2019. "Hydrogen Absorption in Pd–Ag Systems: A TPD and Electrical Resistivity Study" Materials 12, no. 19: 3160. https://doi.org/10.3390/ma12193160





