Surface Modification of Poly(lactic-co-glycolic acid) Microspheres with Enhanced Hydrophilicity and Dispersibility for Arterial Embolization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of PLGA Microspheres
2.2.2. Preparation of PLGA@PDA Core-Shell Microspheres
2.2.3. Preparation of PLGA@PDA/PEI Core-Shell Microspheres
2.2.4. Materials Characterizations
2.2.5. Evaluation of Hydrophilicity
2.2.6. Cytotoxicity Assay in Vitro
3. Results and Discussion
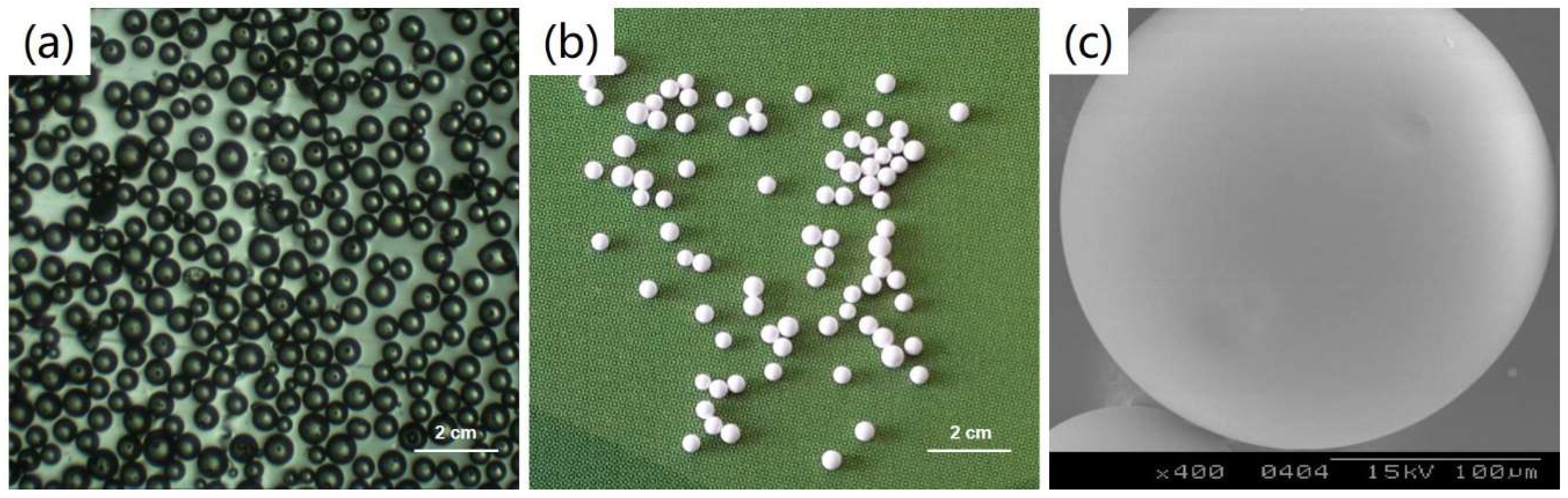
3.1. Morphological Characterization of PLGA Microspheres
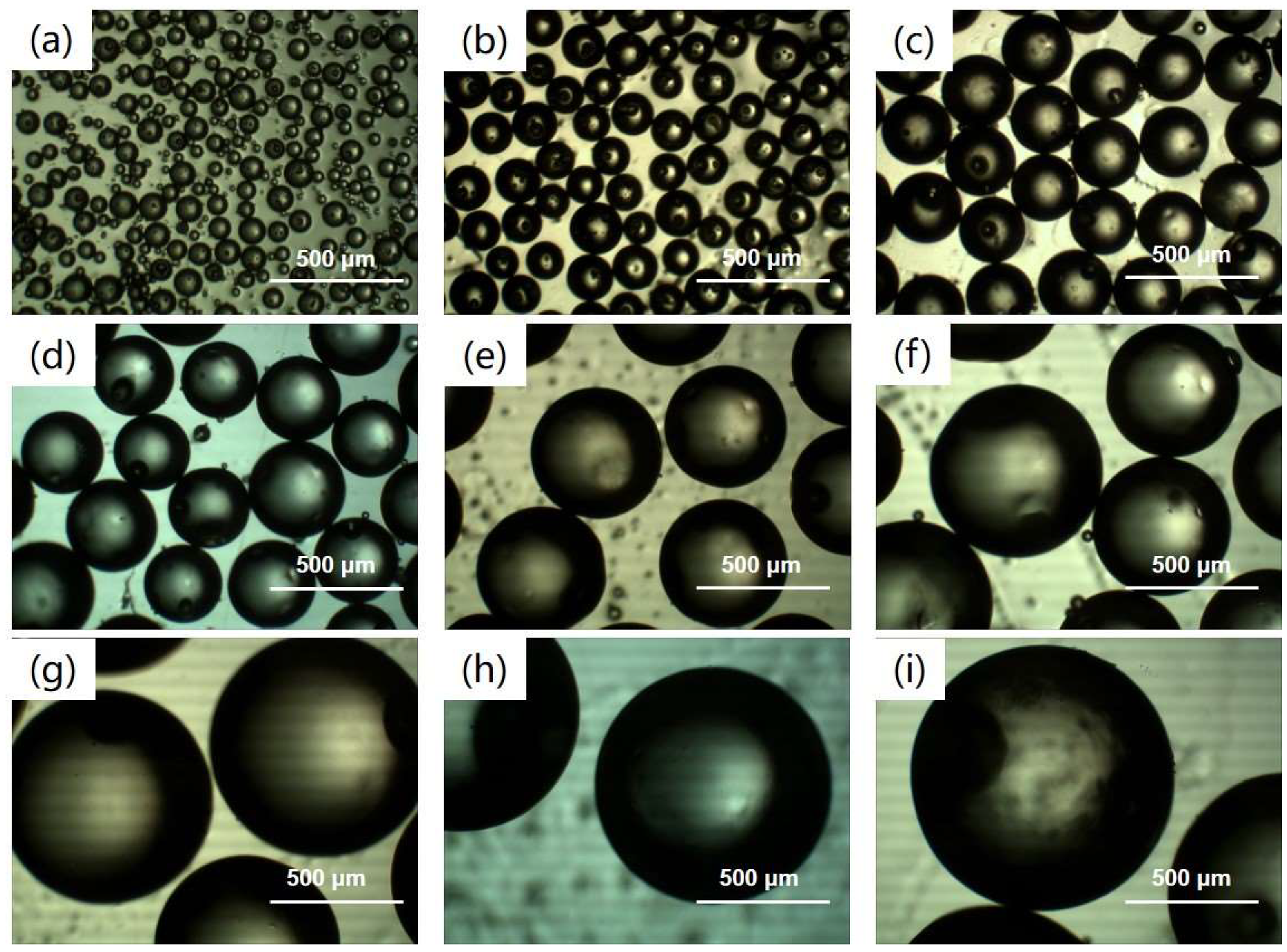
3.2. Surface Morphology Analysis of Modified PLGA Microspheres
3.3. Solubility in the Dichloromethane (DCM) of Modified PLGA Microspheres
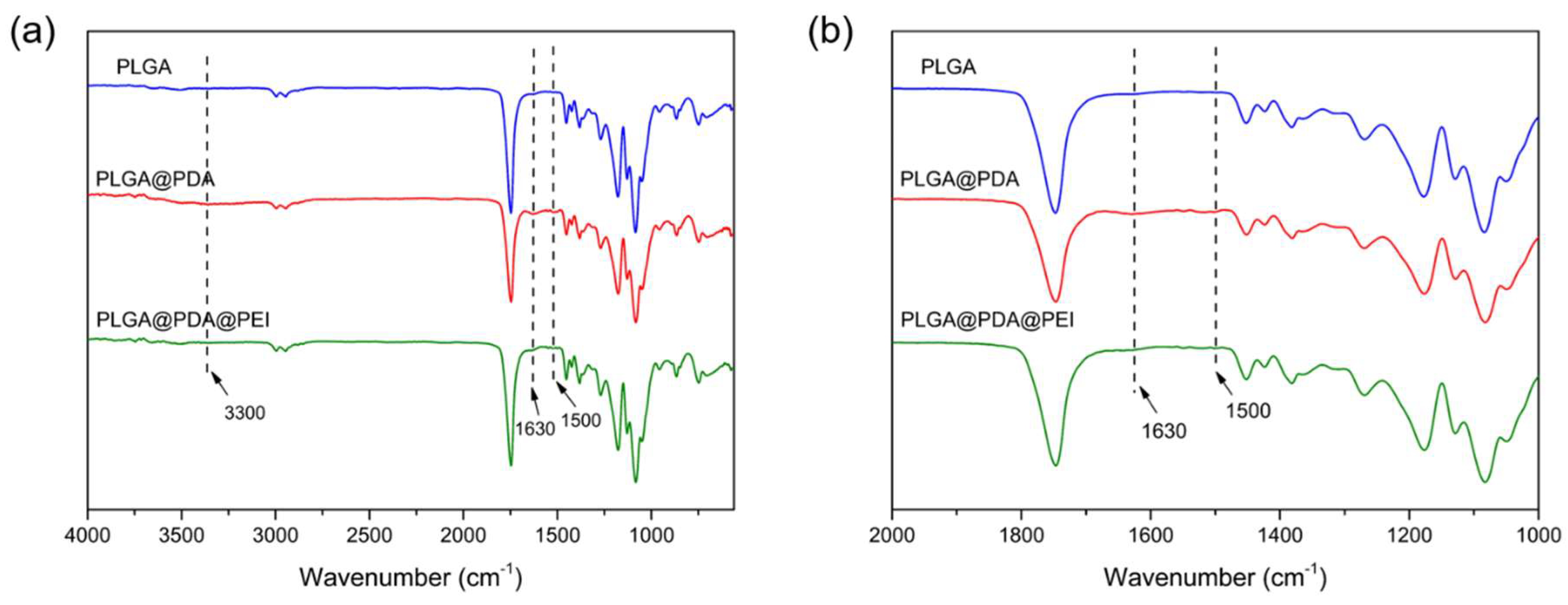
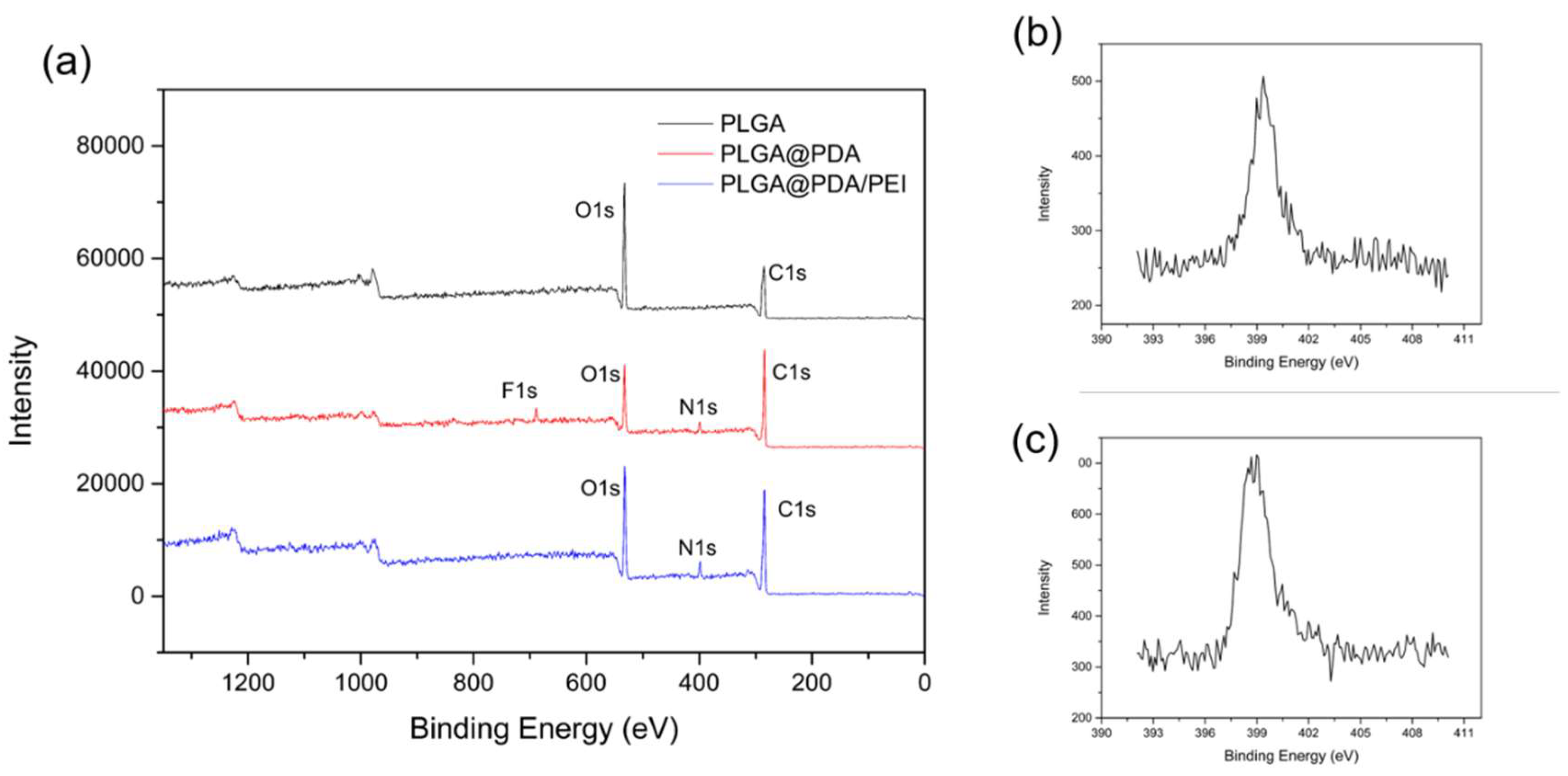
3.4. FT-IR and XPS Spectra Analysis of Modified PLGA Microspheres
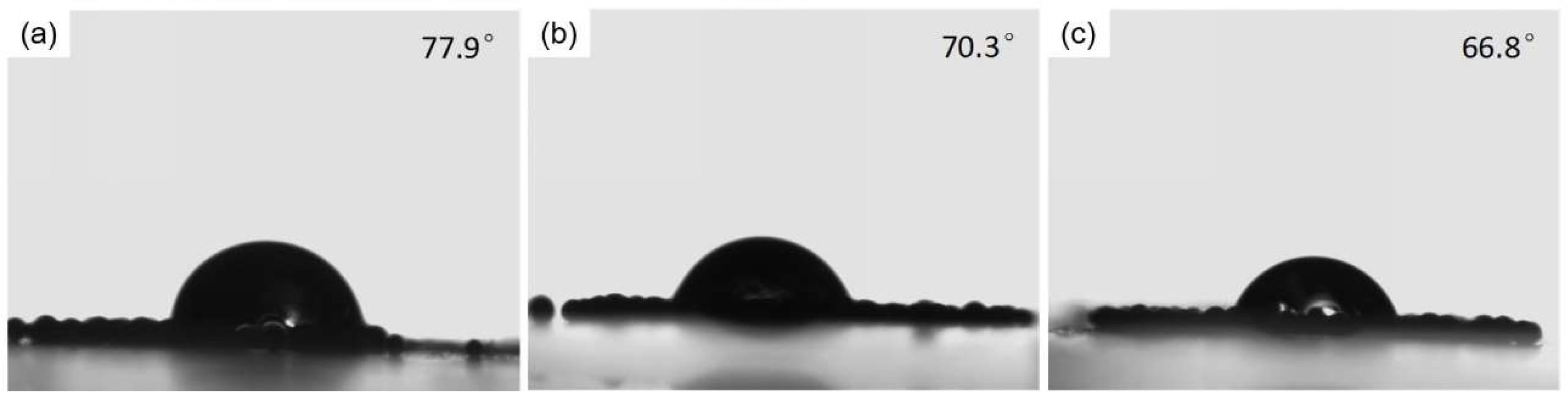
3.5. The Water Contact Angles of Modified PLGA Microspheres
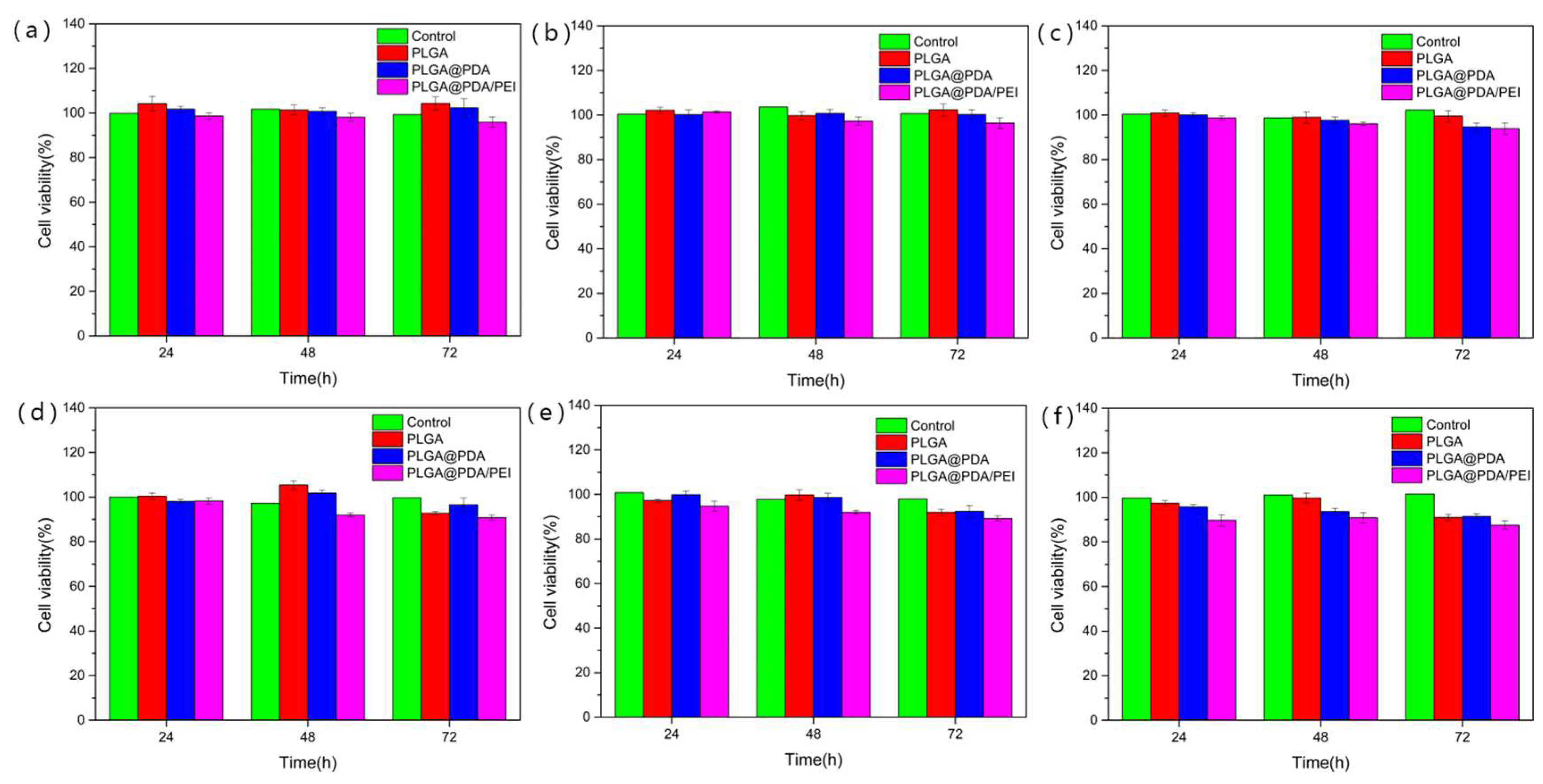
3.6. In-Vitro Cytotoxicity Assessment of Modified PLGA Microspheres
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Du, W.L.; Niu, S.S.; Xu, Z.R.; Xu, Y.L. Preparation, Characterization, and Adsorption Properties of Chitosan Microspheres Crosslinked by Formaldehyde for Copper (II) from Aqueous Solution. J. Appl. Polym. Sci. 2009, 111, 2881–2885. [Google Scholar] [CrossRef]
- Yoshida, S.; Kikuchi, S.; Kanehashi, S.; Okamoto, K.; Ogino, K. Microfluidic Fabrication of Morphology-Controlled Polymeric Microspheres of Blends of Poly(4-butyltriphenylamine) and Poly(methyl methacrylate). Materials 2018, 11, 582. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, K.; Duran, R.; Denys, A.; Bize, P.E.; Borchard, G.; Jordan, O. Drug-eluting embolic microspheres for local drug delivery-State of the art. J. Control. Release 2017, 262, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Porcu, E.P.; Salis, A.; Rassu, G.; Maestri, M.; Galafassi, J.; Bruni, G.; Gavini, E. Engineered polymeric microspheres obtained by multi-step method as potential systems for transarterial embolization and intraoperative imaging of HCC: Preliminary evaluation. Eur. J. Pharm. Biopharm. 2017, 117, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Piao, J.F.; Peng, W.P.; Wang, Y.; Zhang, B.; Gong, X.Q.; Chang, J. Simple and Sensitive Quantification of MicroRNAs via PS@Au Microspheres-Based DNA Probes and DSN-Assisted Signal Amplification Platform. ACS Appl. Mater. Interfaces 2018, 10, 3324–3332. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.Z.; Ouyang, X.L.; Cai, C.Q.; Xu, W.S.; Chen, C.Y.; Chen, X.M. Surface-imprinted microspheres prepared by a template-oriented method for the chiral separation of amlodipine. J. Sep. Sci. 2017, 40, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Saralidze, K.; Koole, L.H.; Knetsch, M.L.W. Polymeric Microspheres for Medical Applications. Materials 2010, 3, 3537–3564. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Kawarada, Y.; Yano, T. Spontaneous rupture of hemangioma of the liver: Treatment with transcatheter hepatic arterial embolization. Am. J. Gastroenterol. 1991, 86, 1645–1649. [Google Scholar]
- Lencioni, R.; de Baere, T.; Soulen, M.C.; Rilling, W.S.; Geschwind, J.F.H. Lipiodol Transarterial Chemoembolization for Hepatocellular Carcinoma: A Systematic Review of Efficacy and Safety Data. Hepatology 2016, 64, 106–116. [Google Scholar] [CrossRef]
- Ozyer, U. Transcatheter Arterial Embolization with N-Butyl-2-Cyanoacrylate in the Management of Spontaneous Hematomas. Cardiovasc. Interv. Radiol. 2017, 40, 41–49. [Google Scholar] [CrossRef]
- Poursaid, A.; Jensen, M.M.; Huo, E.; Ghandehari, H. Polymeric materials for embolic and chemoembolic applications. J. Control. Release 2016, 240, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.Q.; Chen, H.Y.; Zhou, H.J.; Zhou, X.H.; Xu, H. Preparation of Tea Tree Oil/Poly(styrene-butyl methacrylate) Microspheres with Sustained Release and Anti-Bacterial Properties. Materials 2018, 11, 12. [Google Scholar] [CrossRef]
- Xie, Y.; Ma, X.X.; Liu, X.J.; Long, Q.M.; Wang, Y.; Yao, Y.W.; Cai, Q. Carrier-Free Microspheres of an Anti-Cancer Drug Synthesized via a Sodium Catalyst for Controlled-Release Drug Delivery. Materials 2018, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Bee, S.L.; Hamid, Z.A.A.; Mariatti, M.; Yahaya, B.H.; Lim, K.; Bee, S.T.; Sin, L.T. Approaches to Improve Therapeutic Efficacy of Biodegradable PLA/PLGA Microspheres: A Review. Polym. Rev. 2018, 58, 495–536. [Google Scholar] [CrossRef]
- Zhu, Z.; Min, T.T.; Zhang, X.J.; Wen, Y.Q. Microencapsulation of Thymol in Poly(lactide-co-glycolide) (PLGA): Physical and Antibacterial Properties. Materials 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Zheng, X.; Li, H.; He, Y.; Yuan, M.; Shen, M.; Yang, R.; Yang, C. Preparation and In Vitro Release of Total Alkaloids from Alstonia Scholaris Leaves Loaded mPEG-PLA Microspheres. Materials 2019, 12, 1457. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; Lopez, J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 26. [Google Scholar] [CrossRef]
- Pellis, A.; Silvestrini, L.; Scaini, D.; Coburn, J.M.; Gardossi, L.; Kaplan, D.L.; Guebitz, G.M. Enzyme-catalyzed functionalization of poly(l-lactic acid) for drug delivery applications. Process Biochem. 2017, 59, 77–83. [Google Scholar] [CrossRef]
- Notta-Cuvier, D.; Odent, J.; Delille, R.; Murariu, M.; Lauro, F.; Raquez, J.M.; Dubois, P. Tailoring polylactide (PLA) properties for automotive applications: Effect of addition of designed additives on main mechanical properties. Polym. Test. 2014, 36, 1–9. [Google Scholar] [CrossRef]
- Xu, Y.H.; Kim, C.S.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug-polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1692–1716. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Samanta, A.; Srivastava, R.K.; Hakkarainen, M. Nano-Graphene Oxide Functionalized Bioactive Poly(lactic acid) and Poly(epsilon-caprolactone) Nanofibrous Scaffolds. Materials 2018, 11, 14. [Google Scholar]
- Sabee, M.; Kamalaldin, N.A.; Yahaya, B.H.; Hamid, Z.A.A. Characterization and In Vitro Study of Surface Modified PLA Microspheres Treated with NaOH. J. Polym. Mater. 2016, 33, 191–200. [Google Scholar]
- Liao, M.H.; Wan, P.B.; Wen, J.R.; Gong, M.; Wu, X.X.; Wang, Y.G.; Zhang, L.Q. Wearable, Healable, and Adhesive Epidermal Sensors Assembled from Mussel-Inspired Conductive Hybrid Hydrogel Framework. Adv. Funct. Mater. 2017, 27, 11. [Google Scholar] [CrossRef]
- Kao, C.T.; Chen, Y.J.; Ng, H.Y.; Lee, A.K.X.; Huang, T.H.; Lin, T.F.; Hsu, T.T. Surface Modification of Calcium Silicate via Mussel-Inspired Polydopamine and Effective Adsorption of Extracellular Matrix to Promote Osteogenesis Differentiation for Bone Tissue Engineering. Materials 2018, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- Zhan, Y.Q.; Wan, X.Y.; He, S.J.; Yang, Q.B.; He, Y. Design of durable and efficient poly(arylene ether nitrile)/bioinspired polydopamine coated graphene oxide nanofibrous composite membrane for anionic dyes separation. Chem. Eng. J. 2018, 333, 132–145. [Google Scholar] [CrossRef]
- Zhao, J.; Fang, C.H.; Zhu, Y.W.; He, G.W.; Pan, F.S.; Jiang, Z.Y.; Wang, B.Y. Manipulating the interfacial interactions of composite membranes via a mussel-inspired approach for enhanced separation selectivity. J. Mater. Chem. A 2015, 3, 19980–19988. [Google Scholar] [CrossRef]
- Zhan, Z.W.; Zhang, X.X.; Huang, J.Y.; Huang, Y.; Huang, Z.W.; Pan, X.; Wu, C.B. Improved Gene Transfer with Functionalized Hollow Mesoporous Silica Nanoparticles of Reduced Cytotoxicity. Materials 2017, 10, 11. [Google Scholar] [CrossRef]
- Fateen, W.; Khan, F.; O’neill, R.J. Healthcare costs of transarterial chemoembolization in the treatment of hepatocellular carcinoma. J. Hepatocell. Carcinoma 2017, 4, 123–130. [Google Scholar] [CrossRef]




| Samples | Content/% | Relative Content | ||||
|---|---|---|---|---|---|---|
| C | O | N | O/C | N/C | N/O | |
| PLGA | 61.2 | 38.8 | - | 0.634 | 0 | 0 |
| PLGA@PDA | 68.42 | 23.57 | 5.46 | 0.344 | 0.080 | 0.232 |
| PLGA@PDA/PEI | 67.6 | 25.18 | 7.22 | 0.372 | 0.107 | 0.287 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, J.; Ren, J. Surface Modification of Poly(lactic-co-glycolic acid) Microspheres with Enhanced Hydrophilicity and Dispersibility for Arterial Embolization. Materials 2019, 12, 1959. https://doi.org/10.3390/ma12121959
Wang J, Li J, Ren J. Surface Modification of Poly(lactic-co-glycolic acid) Microspheres with Enhanced Hydrophilicity and Dispersibility for Arterial Embolization. Materials. 2019; 12(12):1959. https://doi.org/10.3390/ma12121959
Chicago/Turabian StyleWang, Jiao, Jianbo Li, and Jie Ren. 2019. "Surface Modification of Poly(lactic-co-glycolic acid) Microspheres with Enhanced Hydrophilicity and Dispersibility for Arterial Embolization" Materials 12, no. 12: 1959. https://doi.org/10.3390/ma12121959






