Industrial Ceramic Brick Drying in Oven by CFD
Abstract
:1. Introduction
- Liquid diffusion: occurs due to the concentration gradient and dominates the drying of grains and ceramic bricks [14];
- Vapor diffusion: occurs due to the partial vapor pressure gradient and is present during brick drying [14];
- Capillary diffusion: refers to the flow of liquid through capillary channels and on the solid surface due to the molecular attraction between the liquid and the solid. This mechanism can occur without heating, resulting only from pressure difference [14];
- Effusion flow (Knudsen): is only important under high-vacuum conditions, as in lyophilization processes, where the product is frozen under vacuum conditions and the formed ice crystals are sublimed [14].
2. Methodology
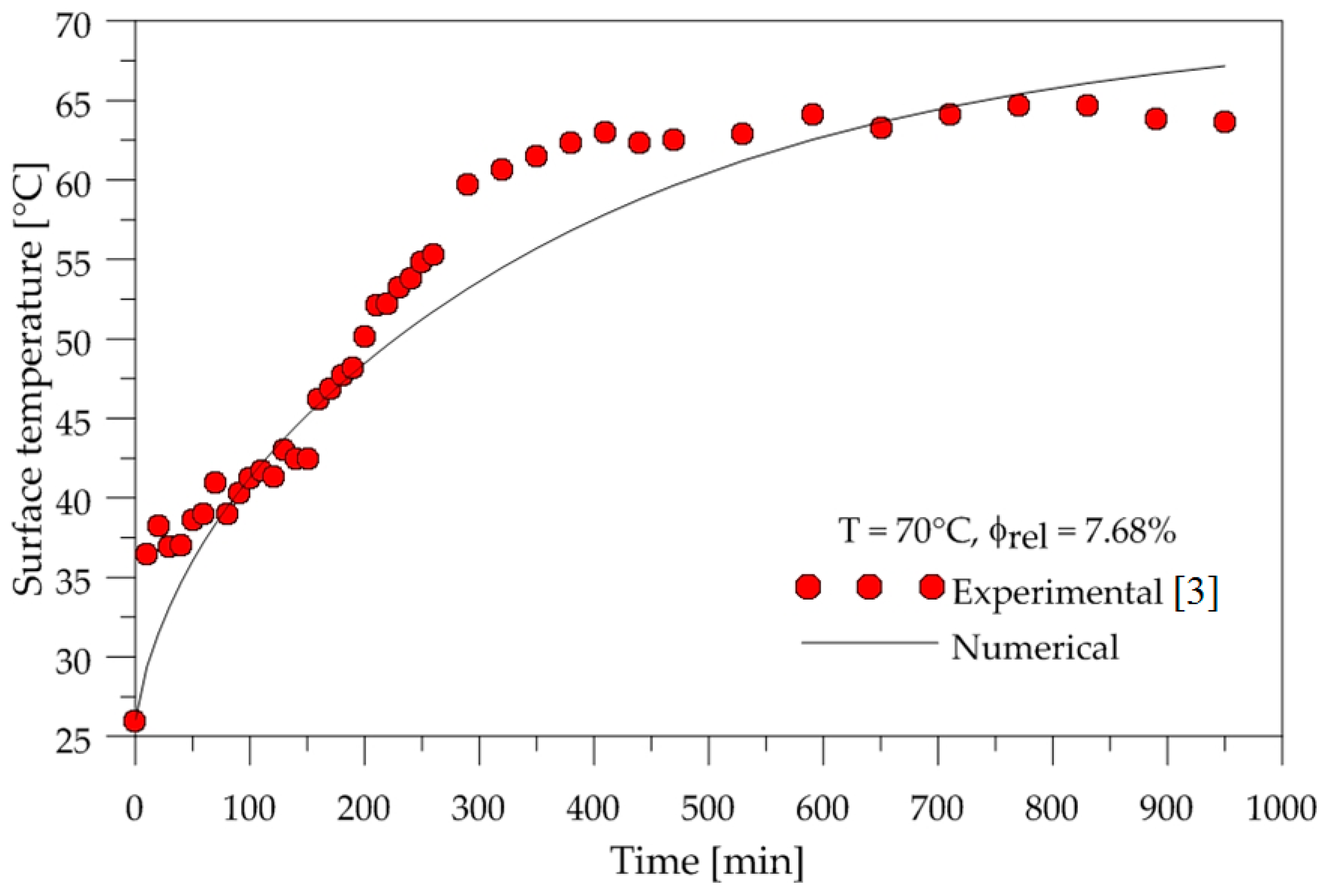
2.1. Experimental Procedure
2.2. Mathematical Procedure
2.2.1. The Geometry and Mesh
2.2.2. Governing Equations
- Solid phase
- Boundary conditions:
- ○
- At the interface: conservative interface flux. Details will be given about this later.
- Initial conditions:
- ○
- Brick temperature, Tbrick,0.
- ○
- Water mass in the brick, mwater,0.
- Gaseous phase
- Boundary conditions:
- ○
- Airflow in the oven inlet, ;
- ○
- Vapor flow rate in the kiln inlet, ;
- ○
- At the interface between the solid and fluid phases was used conservative interface flux;
- ○
- In the oven outlet was used an average pressure of 101,325 Pa.
- Initial conditions:
- ○
- Oven air temperature, Tkiln,0;
- ○
- Oven water vapor mass t, mvapor,0;
- Interface of the fluid and solid phases
2.2.3. Physical-Chemical Properties
2.2.4. Simulation Data
3. Results and Discussion
3.1. Heating and Drying Kinetics of the Brick
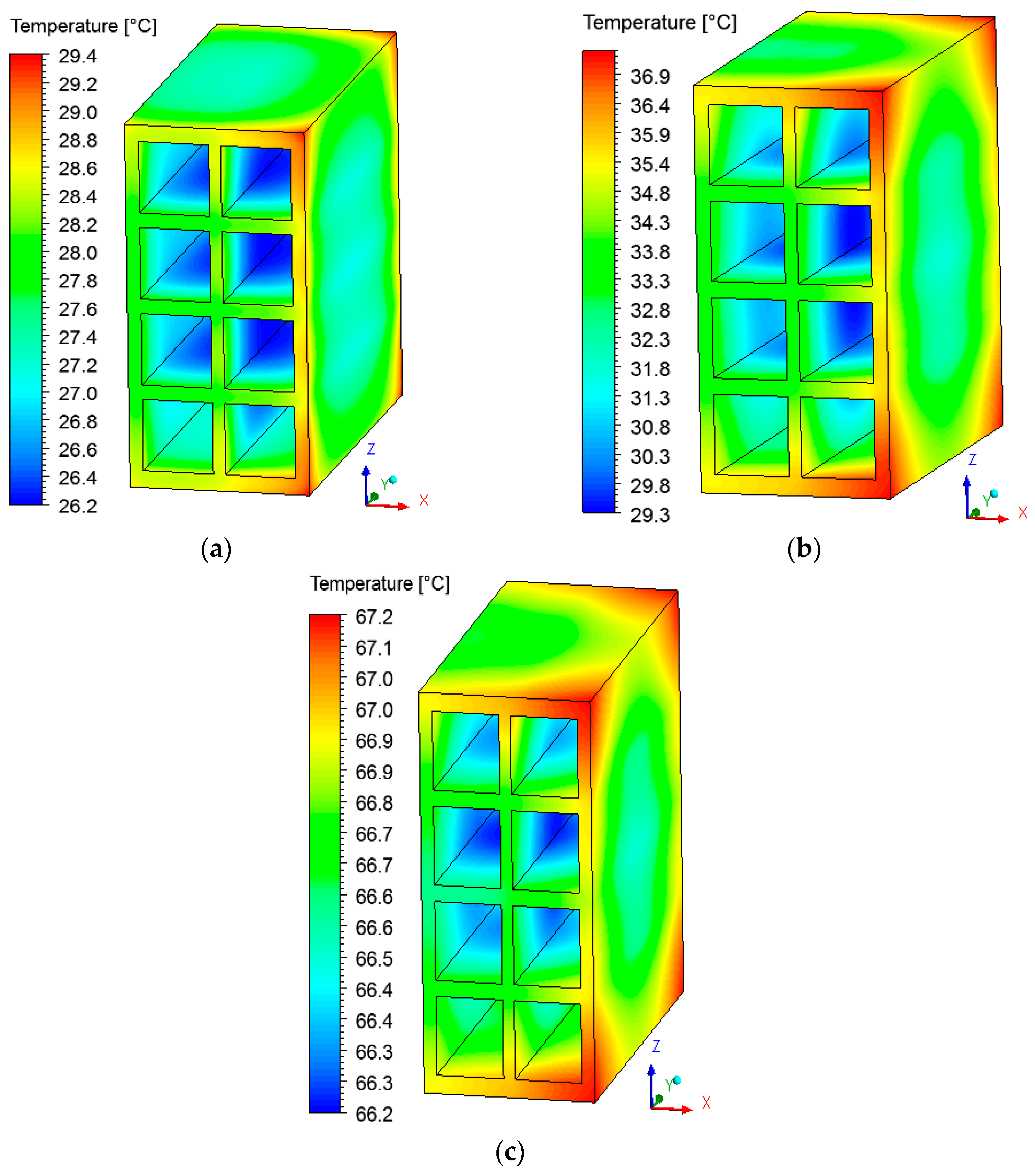
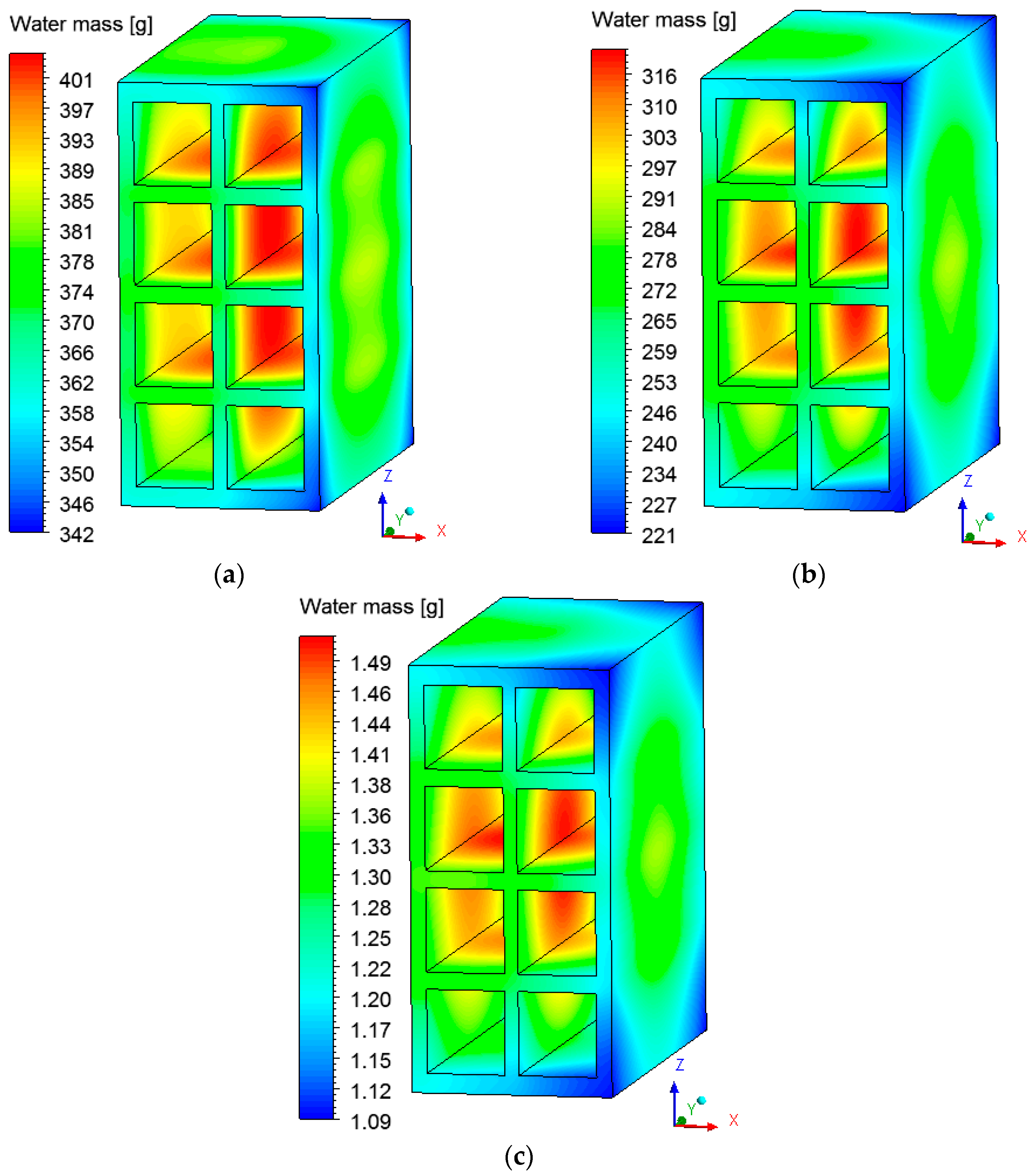
3.2. Heat and Mass Fields inside the Brick
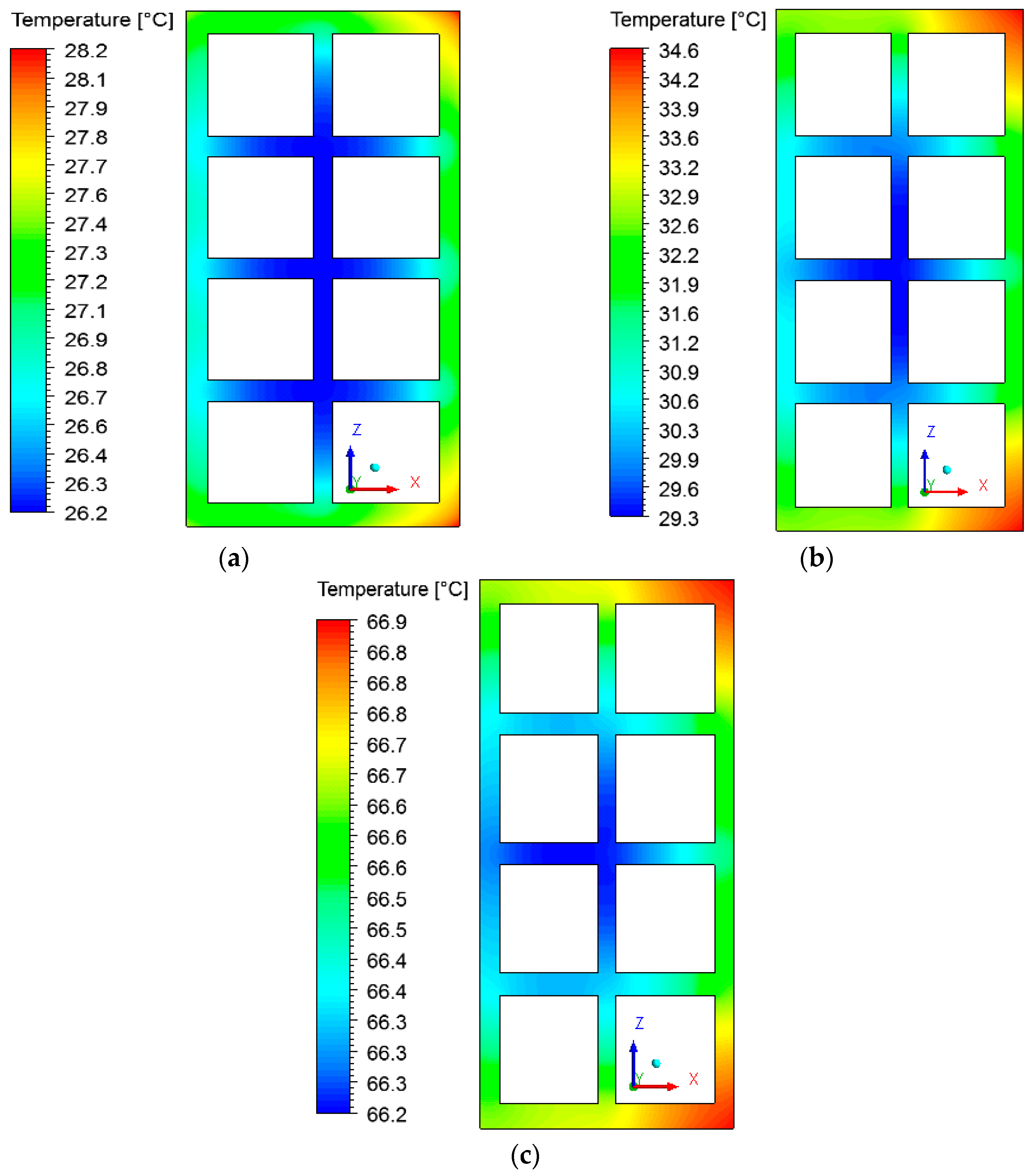
3.3. Heat and Mass Fields on the Surface of the Brick
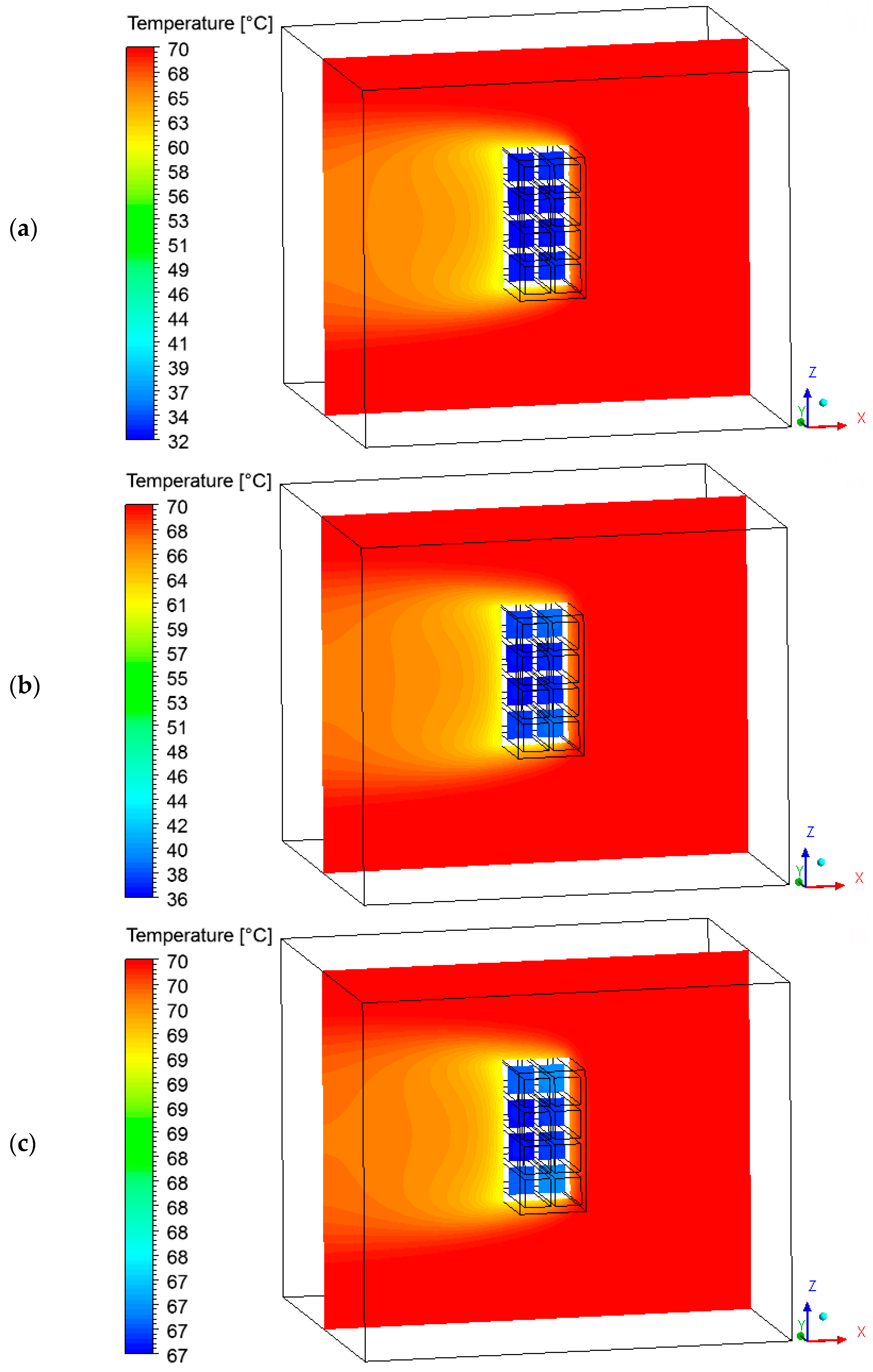
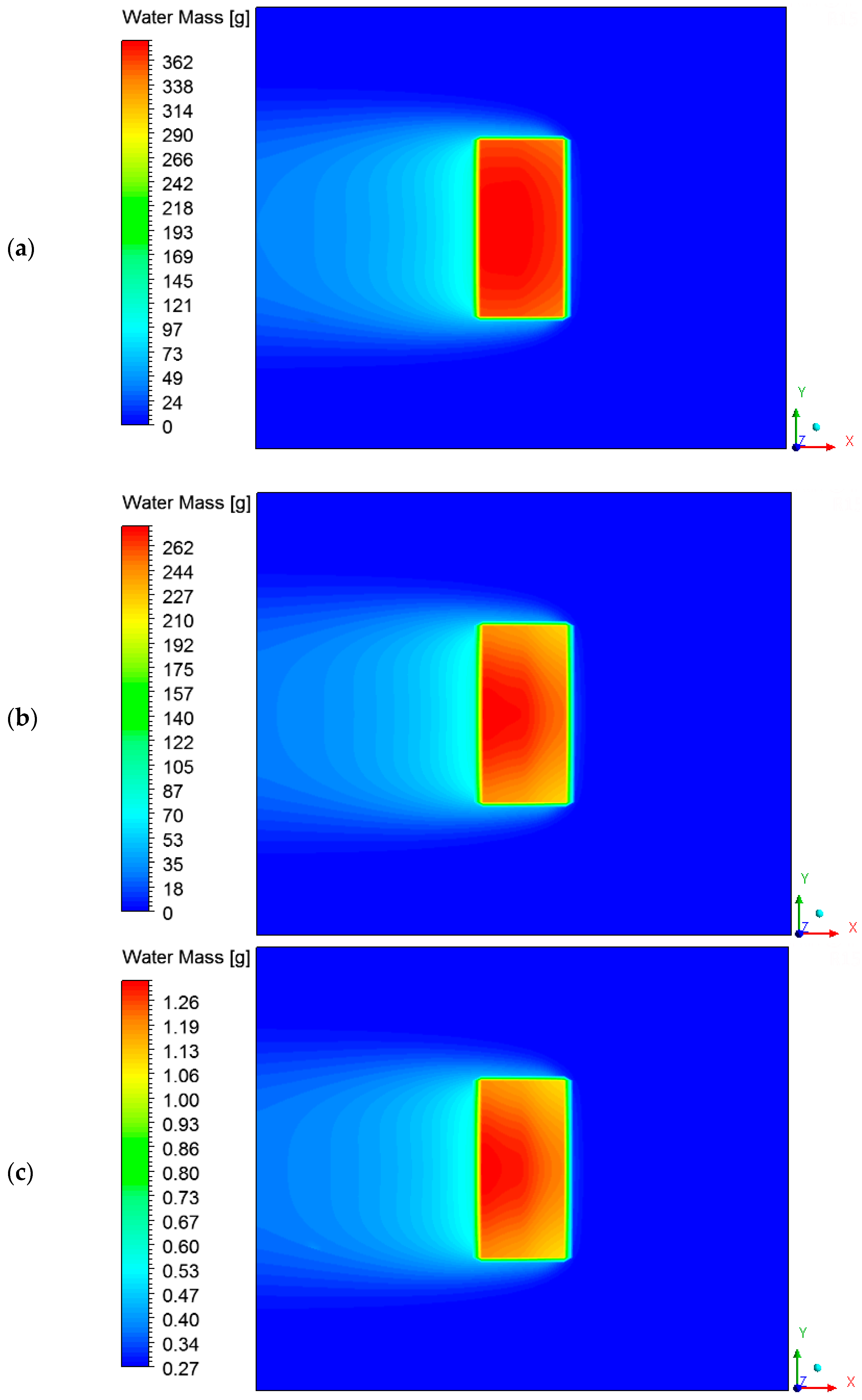
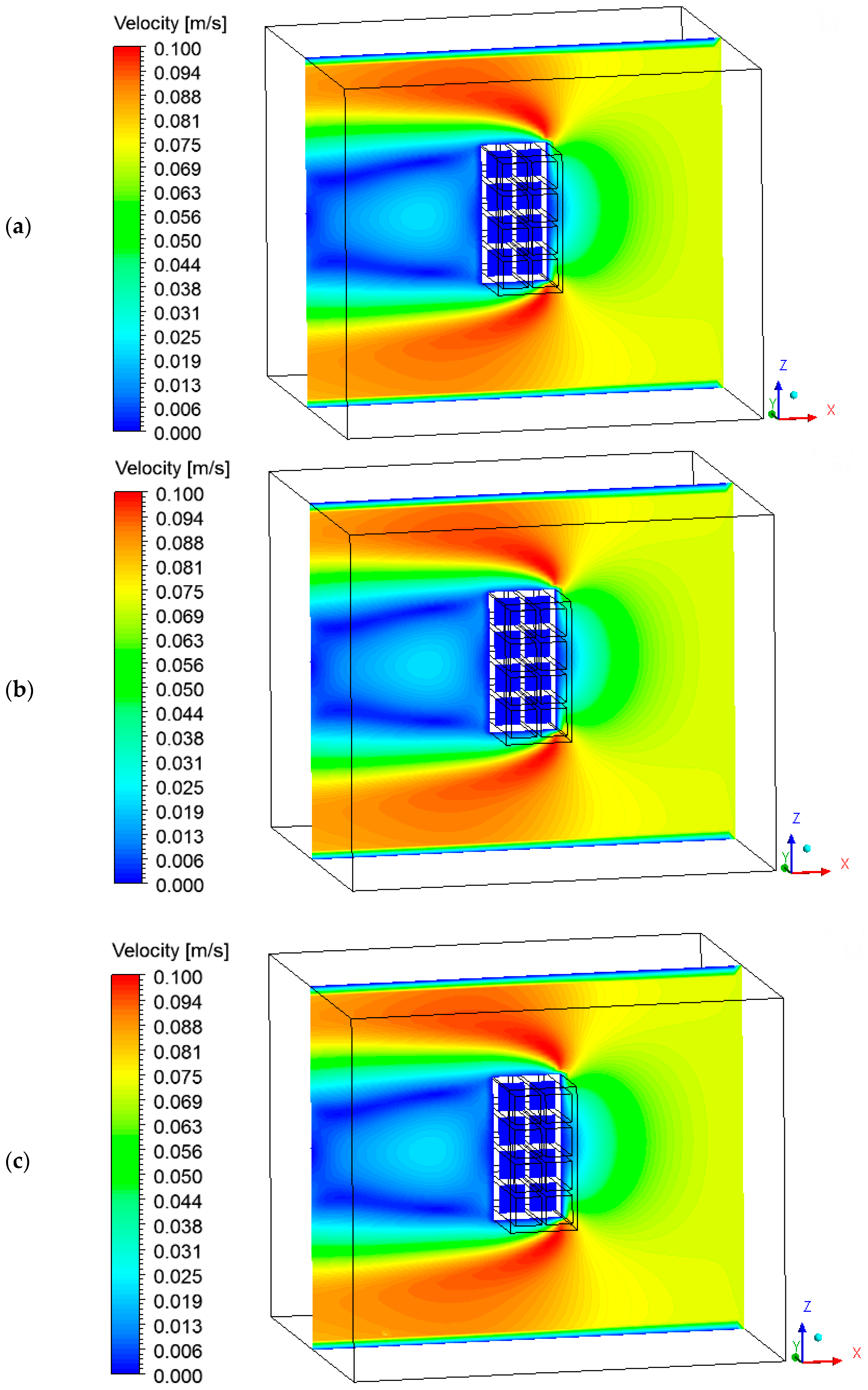
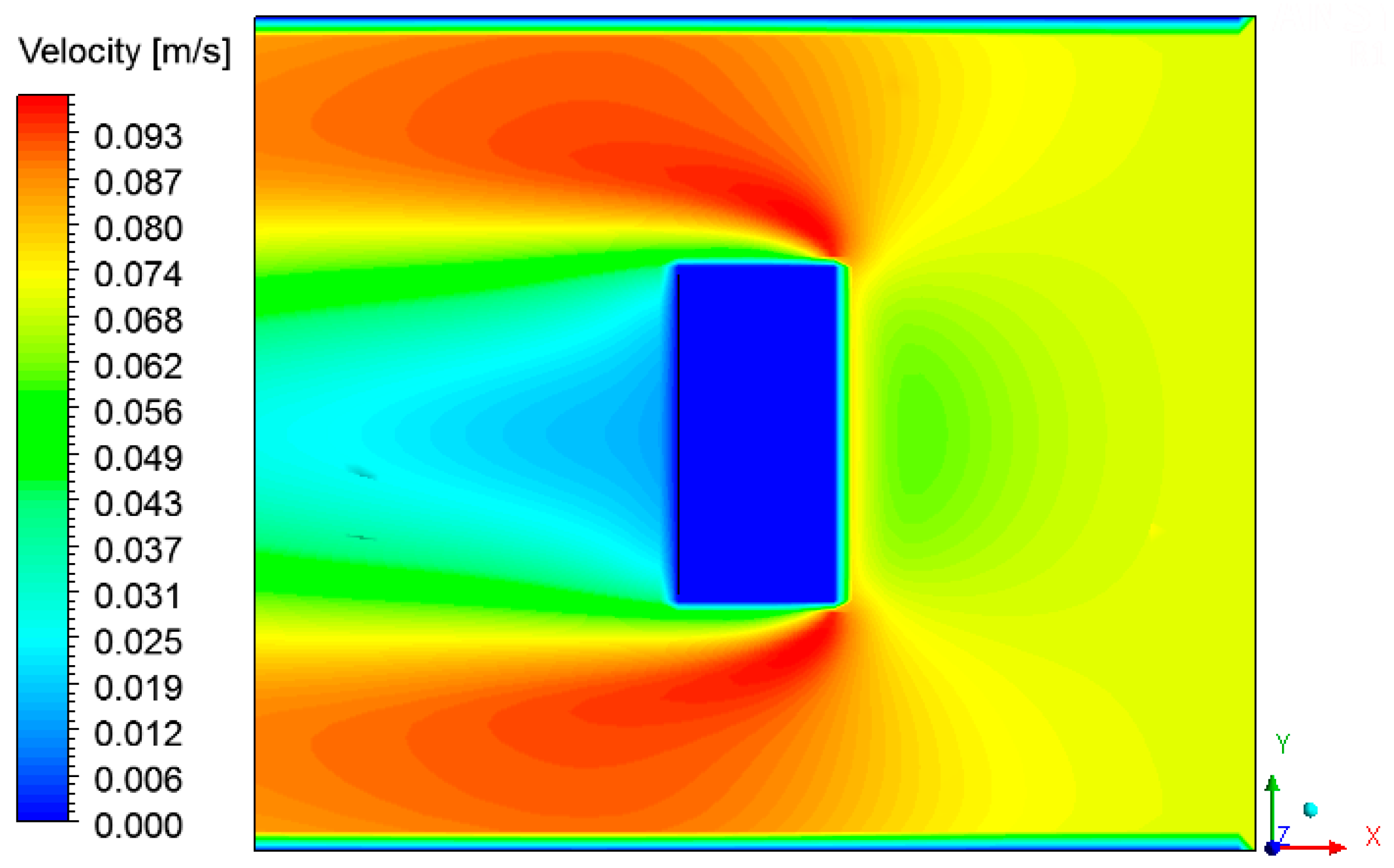
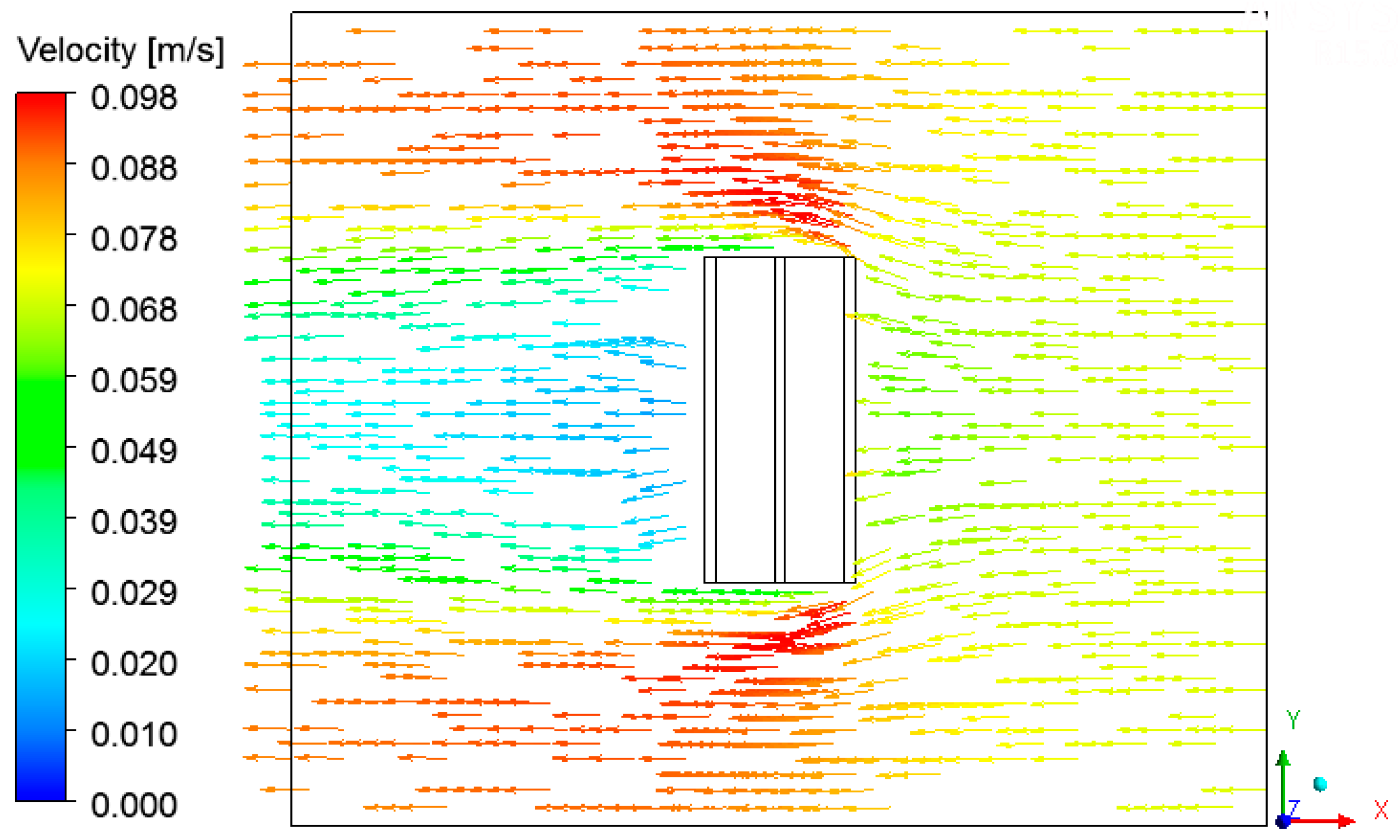
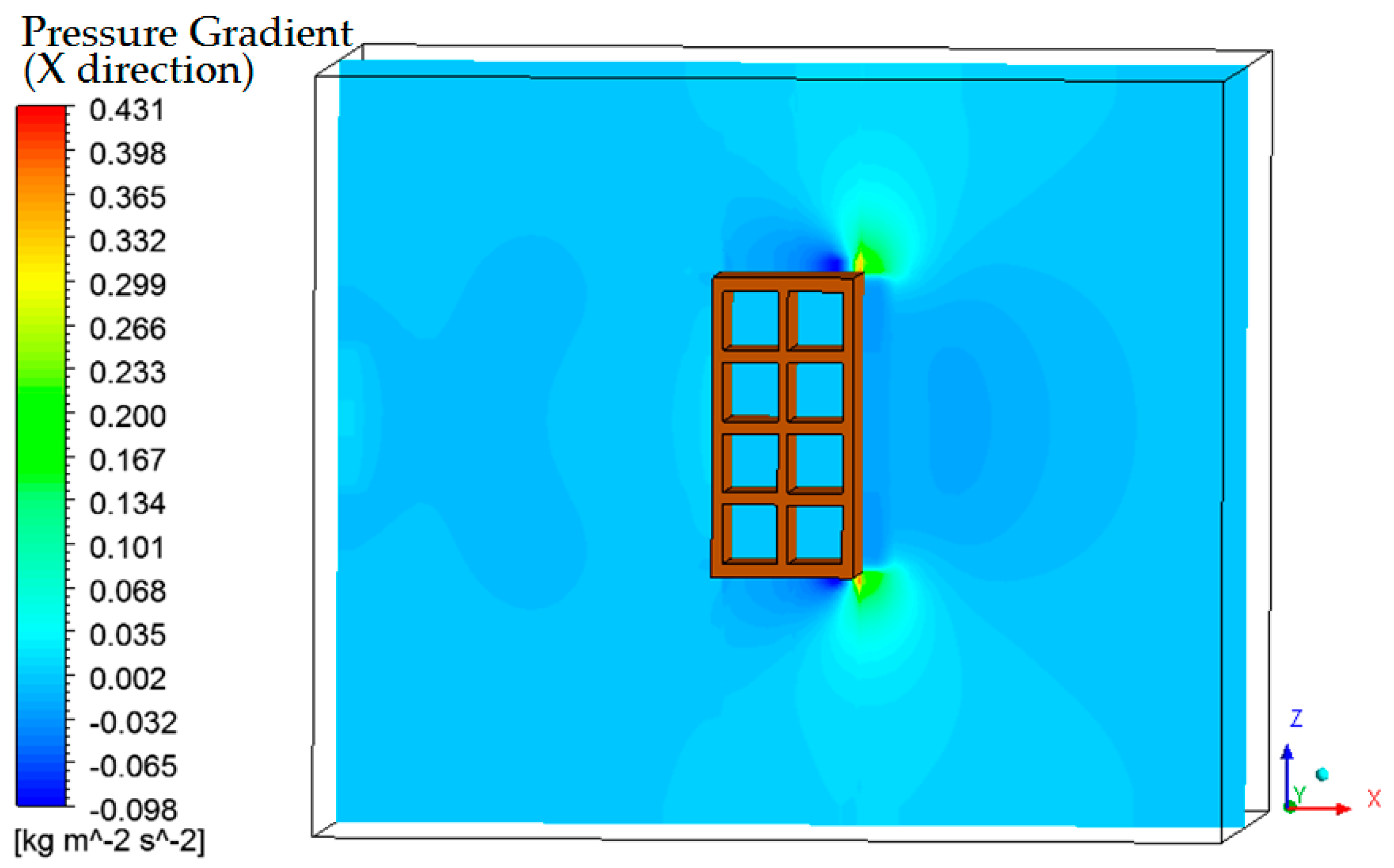
3.4. Heat, Mass, Velocity and Pressure Fields of the Air inside the Oven
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Than the Ceramic is Made? Available online: https://www.minasjr.com.br/do-que-a-ceramica-e-feita/ (accessed on 14 February 2019). (In Portuguese).
- Amin, S.K.; El-Sherbiny, S.A.; Abo El-Magd, A.A.M.; Belal, A.; Abadir, M.F. Fabrication of geopolymer bricks using ceramic dust waste. Constr. Build. Mater. 2017, 157, 610–620. [Google Scholar]
- Silva, J.B. Simulation and Experimentation of Drying Ceramic Bricks. Ph.D. Thesis, Federal University of Campina Grande, Campina Grande, Brazil, 2009; 173p. (In Portuguese). [Google Scholar]
- Almeida, G.S.; Silva, J.B.; Silva, C.J.; Swarnakar, R.; Neves, G.A.; Lima, A.G.B. Heat and mass transport in an industrial tunnel dryer: Modeling and simulation applied to hollow bricks. Appl. Therm. Eng. 2013, 55, 78–86. [Google Scholar] [CrossRef]
- Almeida, G.S.; Tavares, F.V.S.; Lima, W.M.P.B.; Lima, A.G.B. Energetic and exergetic analysis of the clay bricks drying in an industrial tunnel dryer. Defect Diffus. Forum 2016, 369, 104–109. [Google Scholar] [CrossRef]
- Cade, M.A.; Nascimento, J.J.S.; Lima, A.G.B. Drying of holed ceramic bricks: An approach by finite-volumes. Matéria 2005, 10, 443–453. (In Portuguese) [Google Scholar]
- Silva, J.B.; Almeida, G.S.; Lima, W.C.P.B.; Neves, G.A.; Lima, A.G.B. Heat and mass diffusion including shrinkage and hygrothermal stress during drying of holed ceramics bricks. Defect Diffus. Forum 2011, 312–315, 971–976. [Google Scholar] [CrossRef]
- Silva, J.B.; Almeida, G.S.; Lima, W.C.P.B.; Neves, G.A.; Lima, A.G.B. Heat and mass transfer and volume variations during drying of industrial ceramic bricks: An experimental investigation. Defect Diffus. Forum 2012, 326, 267–272. [Google Scholar] [CrossRef]
- de Souza Tavares, F.; de Farias Neto, S.R.; Barbosa, E.S.; de Lima, A.G.B.; Silva, C.J.B. Drying of ceramic hollow bricks in an industrial tunnel dryer: A finite volume analysis. Int. J. Multiphysics 2014, 8, 297–312. [Google Scholar] [CrossRef]
- Batista, V.R.; Nascimento, J.J.S.; Lima, A.G.B.D. Drying and burning of massive and cast ceramic bricks including dimensional variations and structural damage. Rev. Eletrônica Mater. E Process 2008, 3, 46–61. (In Portuguese) [Google Scholar]
- Batista, V.R.; Nascimento, J.J.S.; Lima, A.G.B. Drying and volumetric retraction of solid and hollow ceramic bricks: A theoretical and experimental investigation. Rev. Matéria 2009, 14, 1088–1100. (In Portuguese) [Google Scholar]
- Khalili, K.; Bagherian, M.; Khisheh, S. Numerical simulation of drying ceramic using finite element and machine vision. Procedia Technol. 2014, 12, 388–393. [Google Scholar] [CrossRef]
- Nascimento, J.J.S.; Lima, A.G.B. Experimental drying of ceramics bricks including shrinkage. Defect Diffus. Forum 2015, 365, 106–111. [Google Scholar]
- Mujumdar, A.S. Advances in Drying; Hemisphere Publishing Corporation: Washington, DC, USA, 1980; Volume 1, 301p. [Google Scholar]
- Silva, W.P.; Silva, C.M.D.P.S.; Silva, L.D.; Farias, V.S.O. Drying of clay slabs: Experimental determination and prediction by two-dimensional diffusion models. Ceram. Int. 2013, 39, 7911–7919. [Google Scholar] [CrossRef]
- Augier, F.; Coumans, W.J.; Hugget, A.; Kaasschieter, E.F. On the risk of cracking in clay drying. Chem. Eng. J. 2002, 86, 133–138. [Google Scholar] [CrossRef]
- Van Der Zanden, A.J.J.; Schoenmakers, A.M.E.; Kerkhof, P.J.A.M. Isothermal vapour and liquid transport inside clay during drying. Dry. Technol. 1996, 14, 647–676. [Google Scholar] [CrossRef]
- Silva, W.P.; Farias, V.S.O.; Neves, G.A.; Lima, A.G.B. Modeling of water transport in roof tiles by removal of moisture at isothermal conditions. Heat Mass Transf. 2012, 48, 809–821. [Google Scholar] [CrossRef]
- Araújo, M.V.; Santos, R.S.; Silva, R.M.E.; Lima, A.G.B. Drying of industrial hollow ceramic brick: Analysis of the moisture content and temperature parameters. Defect Diffus. Forum 2017, 380, 72–78. [Google Scholar] [CrossRef]
- Brito, M.K.T.; Almeida, D.B.T.; Lima, A.G.B.; Rocha, L.A.; Lima, E.S.; Oliveira, V.A.B. Heat and mass transfer during drying of clay ceramic materials: A Three-dimensional analytical study. Diffus. Found. 2016, 10, 93–106. [Google Scholar] [CrossRef]
- Nascimento, J.J.S.; Neves, G.A.; Belo, F.A.; Lima, A.G.B. Simultaneous Heat and Moisture Transfer and Shrinkage During drying of Ceramic Materials. In Proceedings of the 14th International Drying Symposium, São Paulo, Brazil, 22–25 August 2004; pp. 501–509. [Google Scholar]
- Numerical, Analytical and Experimental Method: Concurrent or Complementary in Engineering? Available online: https://www.esss.co/blog/metodo-numerico-analitico-e-experimental-concorrentes-ou-complementares-na-engenharia/ (accessed on 27 February 2019). (In Portuguese).
- Khan, F.A.; Straatman, A.G. A conjugate fluid-porous approach to convective heat and mass transfer with application to produce drying. J. Food Eng. 2016, 179, 55–67. [Google Scholar] [CrossRef]
- Almeida, G.S. Simulation and Experimentation of Red Ceramic Drying in Industrial Thermal Systems. Ph.D. Thesis, Federal University of Campina Grande, Campina Grande, Brazil, 2009; 189p. (In Portuguese). [Google Scholar]
- Rieger, J.; Weiss, C.; Rummer, B. Modelling and control of pollutant formation in blast stoves. J. Clean. Prod. 2015, 88, 254–261. [Google Scholar] [CrossRef]
- Martín, D.; Aparicio, P.; Galán, E. Accelerated carbonation of ceramic materials. Application to bricks from Andalusian factories (Spain). Constr. Build. Mater. 2018, 181, 598–608. [Google Scholar] [CrossRef]
- ANSYS Help Viewer, version 15.0; ANSYS, Inc.: Canonsburg, PA, USA, 2013.
- Araújo, M.V. Numerical Study of the Water and Oil Biphasic Flow in T-Connections in the Presence of Leak. Master’s Thesis, Federal University of Campina Grande, Campina Grande, Brazil, 2014; 87p. (In Portuguese). [Google Scholar]
- Çengel, Y.A.; E Ghajar, A.J. Heat and Mass Transfer: A Practical Approach; AMGH: Porto Alegre, Brazil, 2012. (In Portuguese) [Google Scholar]


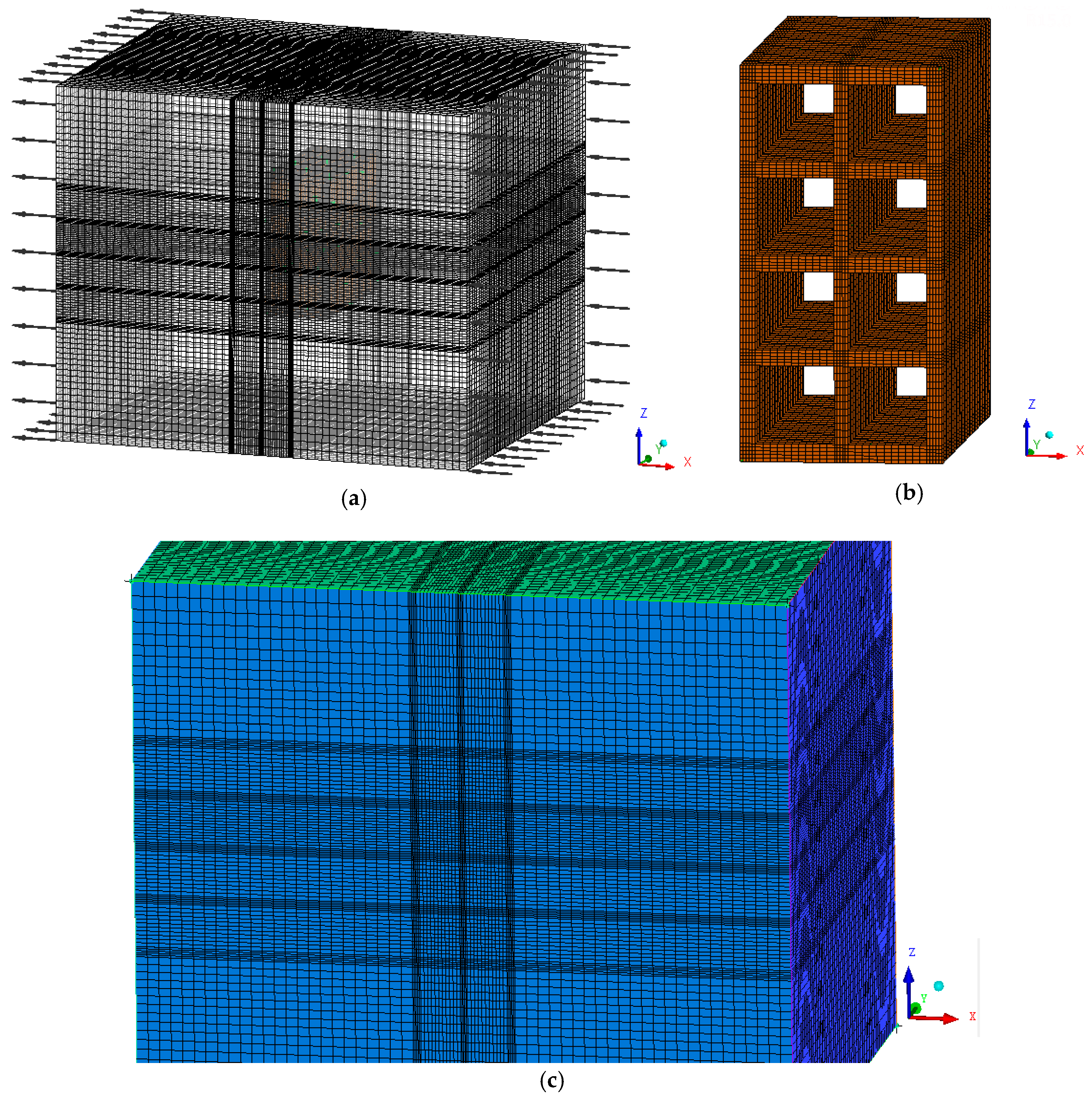

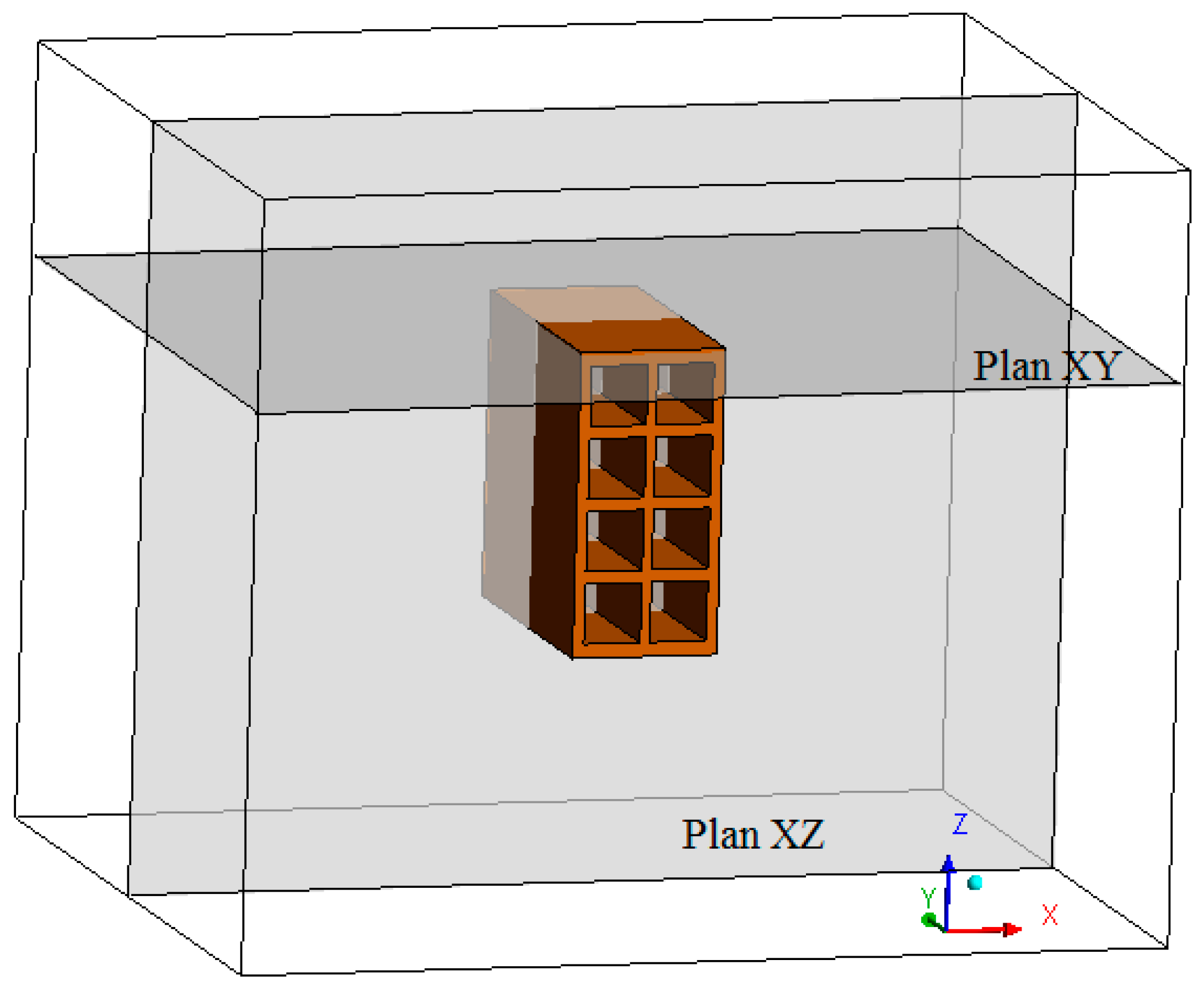





| Property | Material | |
|---|---|---|
| Solid Domain | Fluid Domain | |
| Clay Brick | Air | |
| Specific mass | 1985.8 kg/m³ [14] | 1.029 kg/m³ |
| Specific heat capacity | 1673 J/(kg·K) [14] | 1004.4 J/(kg·K) |
| Thermal conductivity | 1.675 W/(m·K) [29] | 0.0261 W/(m·K) |
| Dynamic viscosity | - | 1.831 × 10−5 kg/(m·s) |
| Mass diffusivity | - | 3.0662 × 10−5 m2/s |
| Solid Domain | Fluid Domain | ||
|---|---|---|---|
| Tbrick,0 | 26 °C | Toven,0 | 70 °C |
| mwater,0 | 408 g | mvapor,0 | 2.30198 g |
| mwater_eq | 1 g | 18.00225 g/s | |
| mdry_brick | 2647 g | 0.27112 g/s |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, M.d.V.; Pereira, A.S.; Oliveira, J.L.d.; Brandão, V.A.A.; Brasileiro Filho, F.d.A.; Silva, R.M.d.; Lima, A.G.B.d. Industrial Ceramic Brick Drying in Oven by CFD. Materials 2019, 12, 1612. https://doi.org/10.3390/ma12101612
Araújo MdV, Pereira AS, Oliveira JLd, Brandão VAA, Brasileiro Filho FdA, Silva RMd, Lima AGBd. Industrial Ceramic Brick Drying in Oven by CFD. Materials. 2019; 12(10):1612. https://doi.org/10.3390/ma12101612
Chicago/Turabian StyleAraújo, Morgana de Vasconcellos, Antonildo Santos Pereira, Jéssica Lacerda de Oliveira, Vanderson Alves Agra Brandão, Francisco de Assis Brasileiro Filho, Rodrigo Moura da Silva, and Antonio Gilson Barbosa de Lima. 2019. "Industrial Ceramic Brick Drying in Oven by CFD" Materials 12, no. 10: 1612. https://doi.org/10.3390/ma12101612





