Anticorrosion Behavior of Zeolite Coatings Obtained by In Situ Crystallization: A Critical Review
Abstract
:1. Introduction
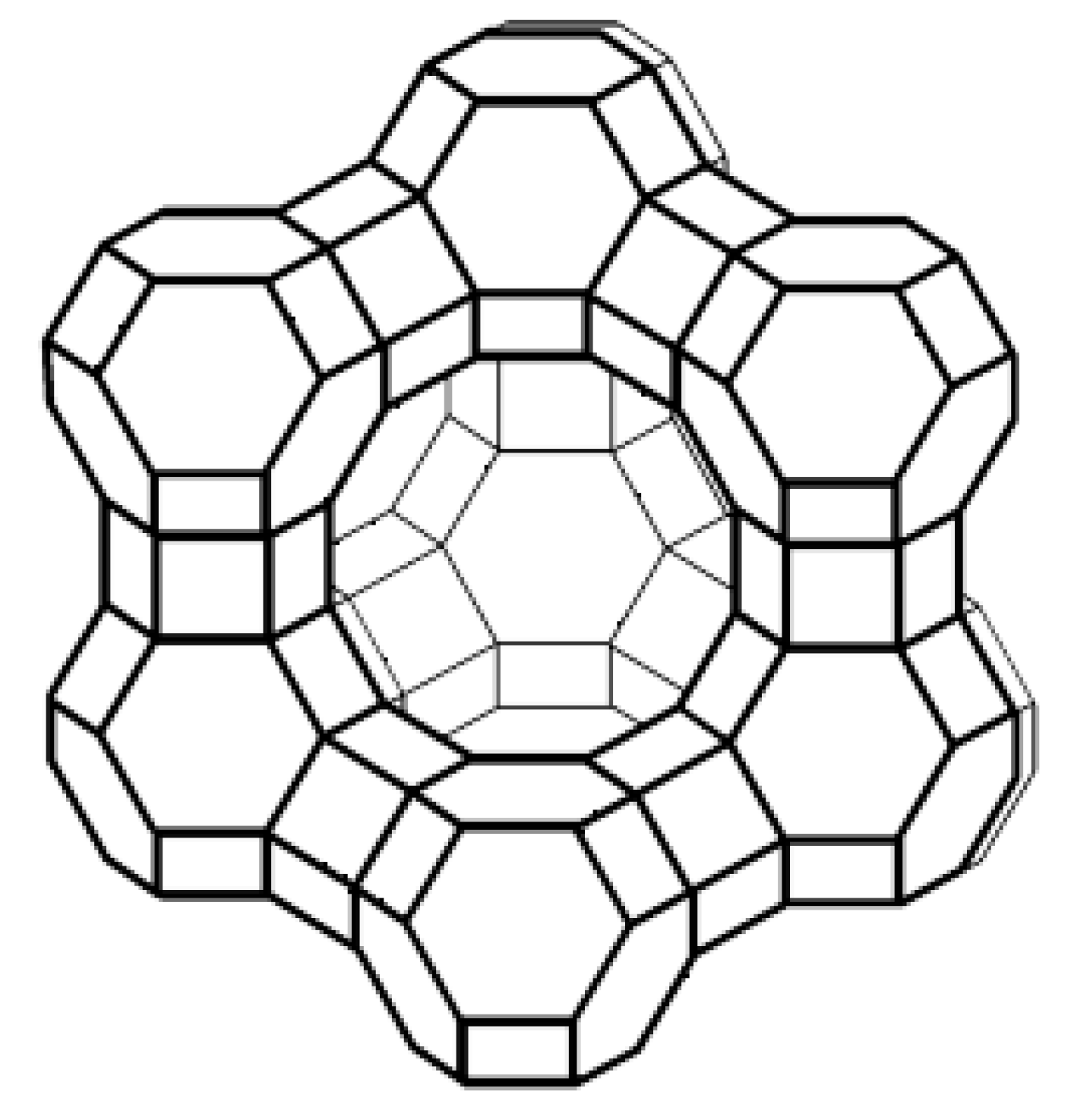
2. Microstructure of Zeolites
3. Anti-Corrosion Zeolite Coatings
3.1. In Situ Crystallization of Zeolite Coatings
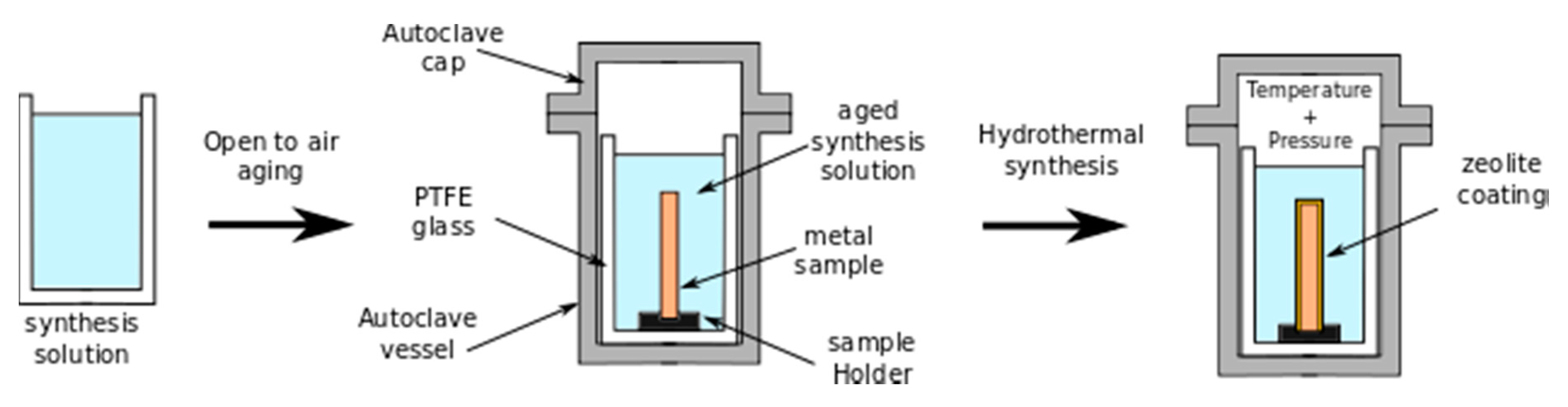
3.2. Hydrothermal In Situ Crystallization (H-InC) Method
3.3. Ionothermal In Situ Crystallization (I-InC) Method
3.4. Dry-Gel Conversion (DGC) Method
- First, a precursor sol, obtained by hydrolysis and aging of a diluted alcoholic silico/aluminate solution, is prepared.
- Then, the so prepared precursor sol is deposited on the metal substrate (e.g., by spray or dip coating)
- The coated metal substrate is dried and placed in an autoclave containing a low amount of distilled water to produce steam. The steaming phase activates the crystallization of a zeolite layer on the metal substrate.
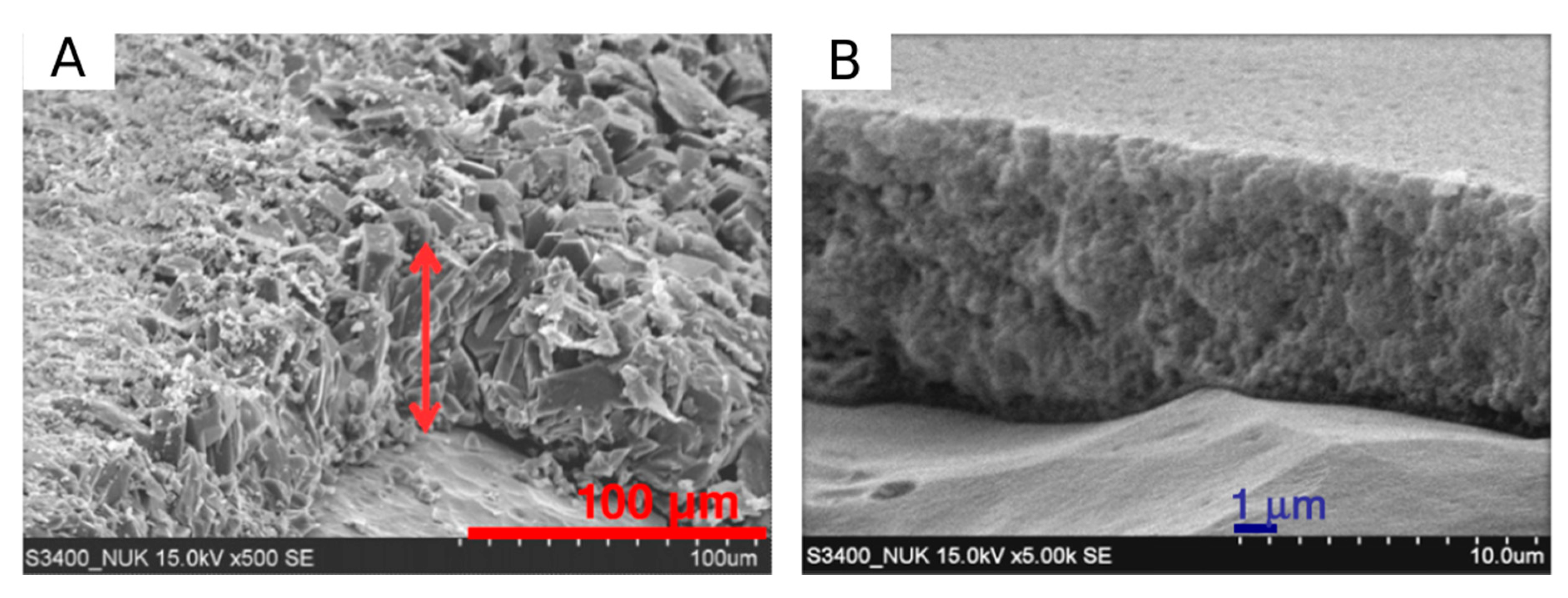
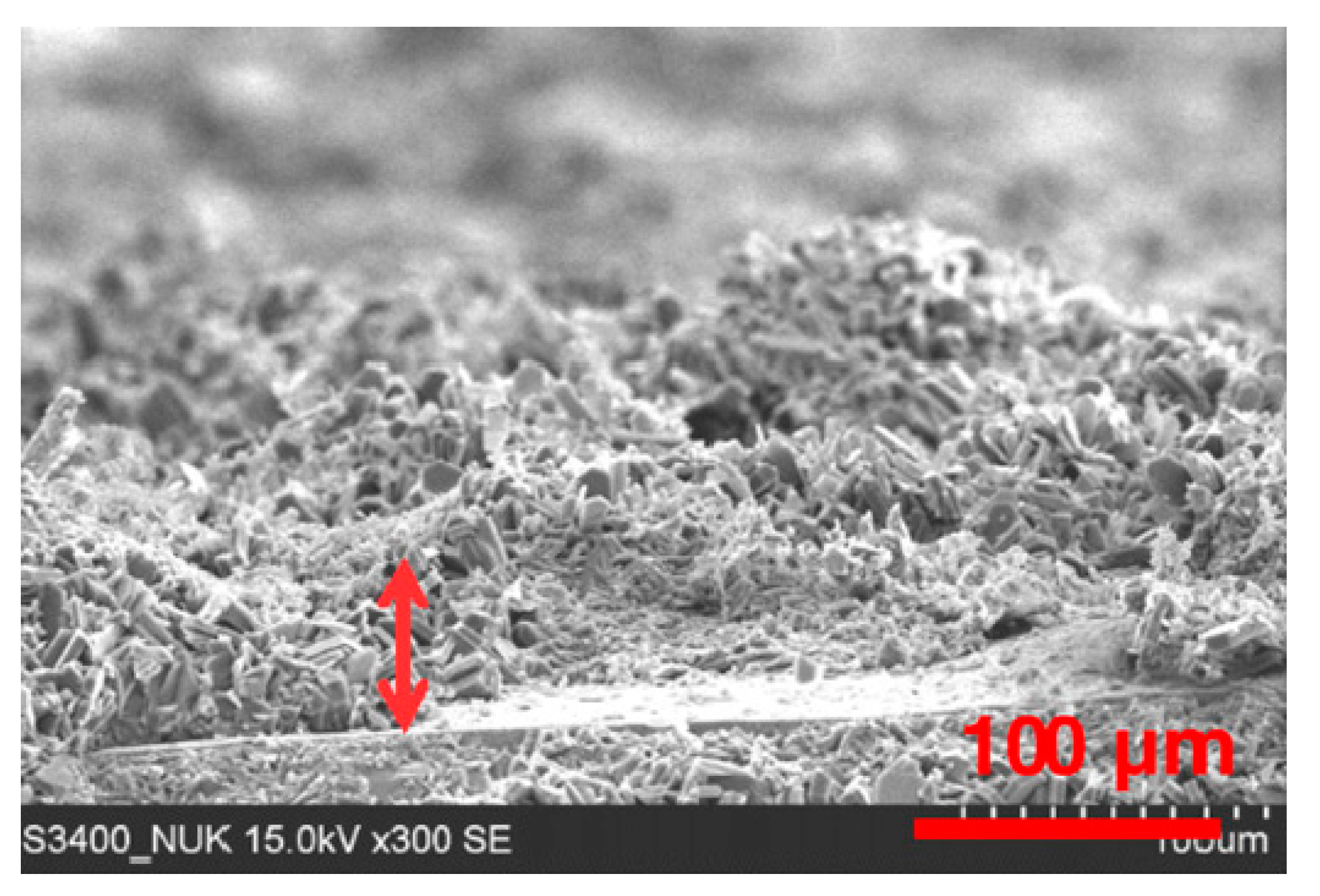
3.5. Morphological Aspects of Corrosion Resistance of the Coatings
4. Anti-Corrosion Aspects of Zeolite Coatings
4.1. Electrochemical Properties of the Zeolites
- (a)
- In the extra-zeolite electron transfer mechanism, redox species diffuse to a conductive electrode surface (e.g., a metal surface), where charge transfer occurs, after being initially ion-exchanged by the electrolyte cations.
- (b)
- In the intra-zeolite electron transfer mechanism, charge transfer occurs via electron hopping between adjacent redox species located in the zeolite structure.
- (c)
- The last mechanism involves two steps: in the first step, the electro-active species situated at the outer surface of the zeolite particles undergo electron transfer and then, in the second step, they act as mediators for the redox transformation of those located in the bulk of the solid (each step requires charge compensation by the electrolyte cations).
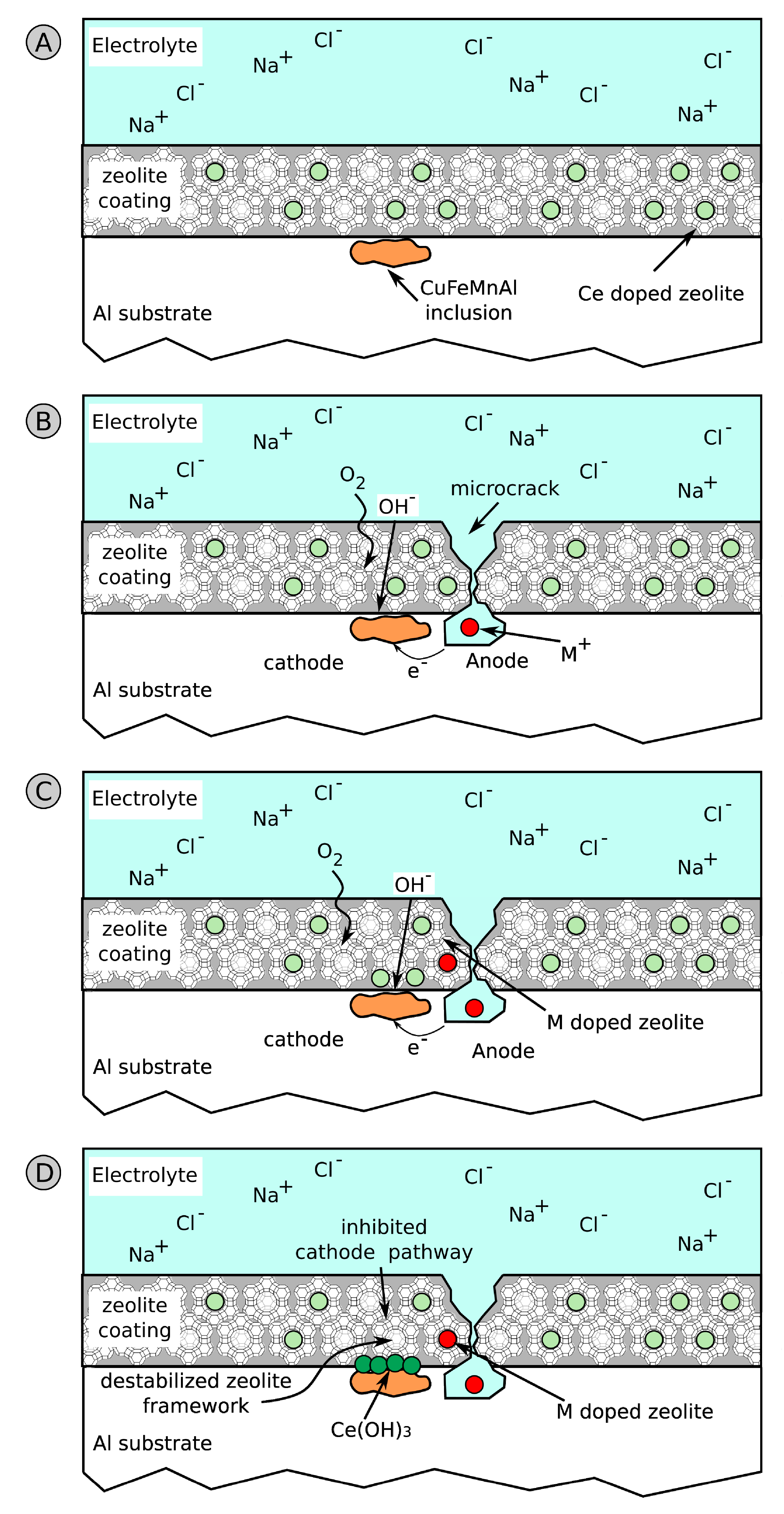
4.2. Barrier Mechanisms of Zeolite Coatings
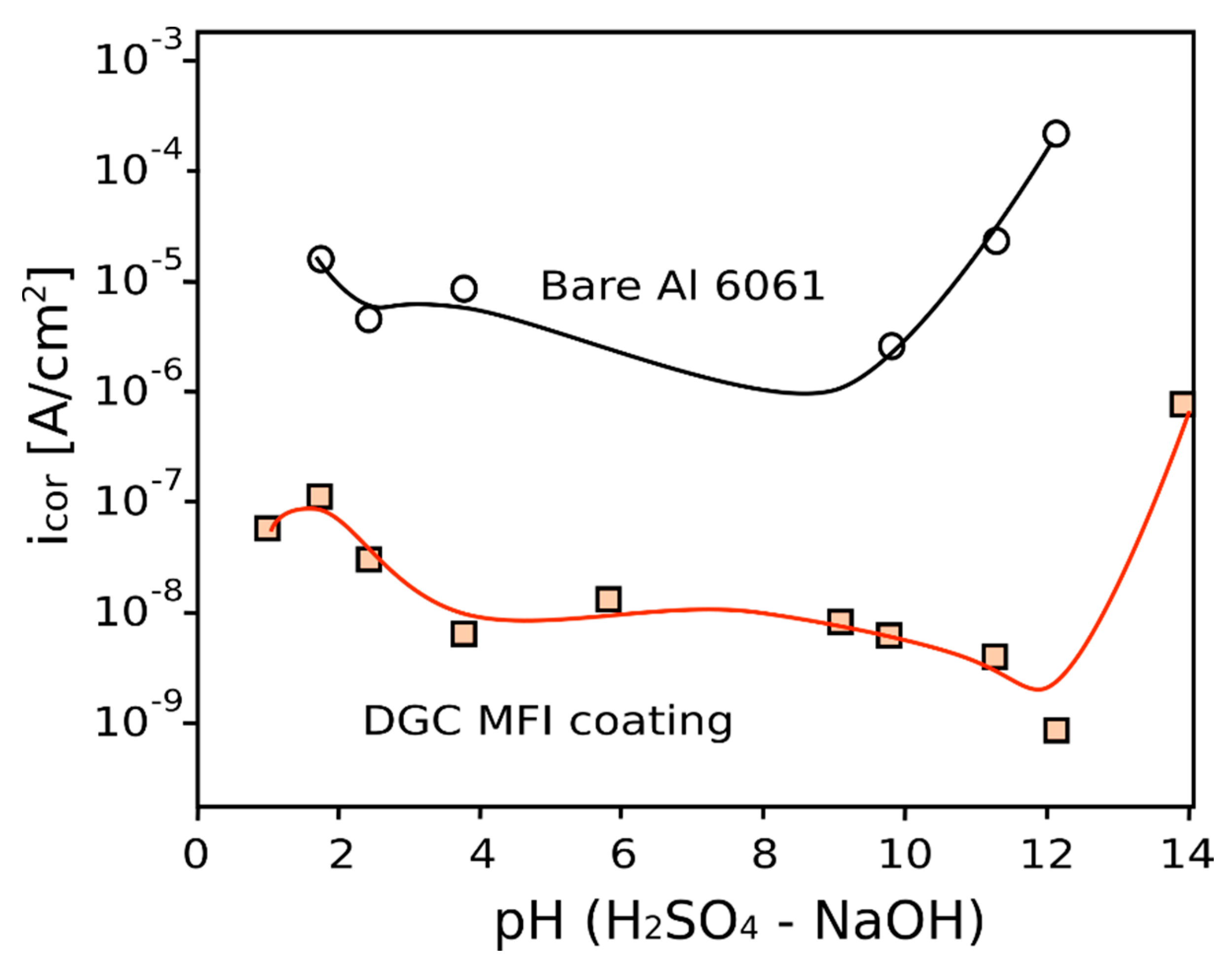
5. Anti-Corrosion Efficiency of In Situ Crystallization Zeolite Coatings
6. Functional Anti-Corrosion Coatings
7. Conclusions
8. Future Trends
Conflicts of Interest
References
- Rebrov, E.V. Sol-gel synthesis of zeolite coatings and their application in catalytic microstructured reactors. Catal. Ind. 2009, 1, 322–347. [Google Scholar] [CrossRef]
- Fawaz, E.; Salam, D.; Nouali, H.; Deroche, I.; Rigolet, S.; Lebeau, B.; Daou, T.; Fawaz, E.G.; Salam, D.A.; Nouali, H.; et al. Synthesis of Binderless ZK-4 Zeolite Microspheres at High Temperature. Molecules 2018, 23, 2647. [Google Scholar] [CrossRef] [PubMed]
- Jamali, S.H.; Vlugt, T.J.H.; Lin, L.C. Atomistic Understanding of Zeolite Nanosheets for Water Desalination. J. Phys. Chem. C 2017, 121, 11273–11280. [Google Scholar] [CrossRef]
- Sapienza, A.; Gullì, G.; Calabrese, L.; Palomba, V.; Frazzica, A.; Brancato, V.; La Rosa, D.; Vasta, S.; Freni, A.; Bonaccorsi, L.; et al. An innovative adsorptive chiller prototype based on 3 hybrid coated/granular adsorbers. Appl. Energy 2016, 179, 929–938. [Google Scholar] [CrossRef]
- Putu Hadi Setyarini, P.; Sulistyaningsih, D. Zeolite-based biomaterials for biomedical application: A review. In AIP Conference Proceedings; AIP Publishing LLC: Surakarta, Indonesia, 2018; Volume 1977, p. 030013. [Google Scholar]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Ismail, A.F.; Aziz, M.; Karamian, E.; Iqbal, N. Bioactivity, in-vitro corrosion behavior, and antibacterial activity of silver–zeolites doped hydroxyapatite coating on magnesium alloy. Trans. Nonferrous Met. Soc. China 2018, 28, 1553–1562. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, X.; Cheng, K.; Xie, G.; Liu, X.; Zhang, D.; Li, Y. Research development of anti-corrosion films and coatings of zeolites for metallic materials. Corros. Sci. Prot. Technol. 2017, 29, 457–461. [Google Scholar] [CrossRef]
- Al-Subaie, M.S.M.; Al-Turkustani, A.M.A.; Selvin, R.; Al-Mhayawi, S.R. Anticorrosion Nanocrystalline Beta Zeolite Thin Film for Advanced Applications. J. Chem. 2015, 693730. [Google Scholar] [CrossRef]
- Salim, M.M.; Ahmad, N.; Malek, N.N. Review of modified Zeolites by surfactant and Silver as antibacterial agents. J. Adv. Res. Mater. Sci. 2017, 36, 1–20. [Google Scholar]
- García, M.; Casariego, A.; Díaz, R.; Roblejo, L. Effect of edible chitosan/zeolite coating on tomatoes quality during refrigerated storage. Emirates J. Food Agric. 2014, 26, 238–246. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M.; Kölsch, P.; Schäfer, R. Zeolite membranes—state of their development and perspective. Microporous Mesoporous Mater. 2000, 38, 3–24. [Google Scholar] [CrossRef]
- Jeon, M.Y.; Kim, D.; Kumar, P.; Lee, P.S.; Rangnekar, N.; Bai, P.; Shete, M.; Elyassi, B.; Lee, H.S.; Narasimharao, K.; et al. Ultra-selective high-flux membranes from directly synthesized zeolite nanosheets. Nature 2017, 543, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, L.; Calabrese, L.; Freni, A.; Proverbio, E. Hydrothermal and microwave synthesis of SAPO (CHA) zeolites on aluminium foams for heat pumping applications. Microporous Mesoporous Mater. 2013, 167, 30–37. [Google Scholar] [CrossRef]
- Tatlier, M. Performances of MOF vs. zeolite coatings in adsorption cooling applications. Appl. Therm. Eng. 2017, 113, 290–297. [Google Scholar] [CrossRef]
- Snelders, D.J.M.; Valega Mackenzie, F.O.; Boersma, A.; Peeters, R.H.M. Zeolites as coating materials for Fiber Bragg Grating chemical sensors for extreme conditions. Sens. Actuators B Chem. 2016, 235, 698–706. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, J.; Sun, Y.; Li, X.; Wang, X.; Zhao, Z. Gas-sensing properties of composites of Y-zeolite and SnO2. J. Mater. Sci. 2018, 53, 6729–6740. [Google Scholar] [CrossRef]
- Bein, T. Synthesis and Applications of Molecular Sieve Layers and Membranes. Chem. Mater. 1996, 8, 1636–1653. [Google Scholar] [CrossRef]
- Zaarour, M.; Dong, B.; Naydenova, I.; Retoux, R.; Mintova, S. Progress in zeolite synthesis promotes advanced applications. Microporous Mesoporous Mater. 2014, 189, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J.; Herrmann, R.; Mittelbach, W.; Schwieger, W. Zeolite/aluminum composite adsorbents for application in adsorption refrigeration. Int. J. Energy Res. 2009, 33, 1233–1249. [Google Scholar] [CrossRef]
- Wolf, M.; Jaeschke, S. Layer Composite and Production. Thereof. Patent WO2007017015A3, 10 August 2005. [Google Scholar]
- Xu, R.; Pang, W.; Yu, J.; Huo, Q.; Chen, J. Chemistry of Zeolites and Related Porous Materials: Synthesis and Structure; Wiley & Sons: Singapore, 2007; ISBN 9780470822333. [Google Scholar]
- Mandal, S.; Planells, A.D.; Hunt, H.K. Impact of deposition and laser densification of Silicalite-1 films on their optical characteristics. Microporous Mesoporous Mater. 2016, 223, 68–78. [Google Scholar] [CrossRef]
- Lew, C.M.; Cai, R.; Yan, Y. Zeolite thin films: From computer chips to space stations. Acc. Chem. Res. 2010, 43, 210–219. [Google Scholar] [CrossRef]
- Katariya, M.N.; Jana, A.K.; Parikh, P.A. Corrosion inhibition effectiveness of zeolite ZSM-5 coating on mild steel against various organic acids and its antimicrobial activity. J. Ind. Eng. Chem. 2013, 19, 286–291. [Google Scholar] [CrossRef]
- Hamciuc, C.; Hamciuc, E.; Popovici, D.; Danaila, A.I.; Butnaru, M.; Rimbu, C.; Carp-Carare, C.; Kalvachev, Y. Biocompatible poly(ether-ether-ketone)/Ag-zeolite L composite films with antimicrobial properties. Mater. Lett. 2018, 212, 339–342. [Google Scholar] [CrossRef]
- Fernández, A.; Soriano, E.; Hernández-Muñoz, P.; Gavara, R. Migration of antimicrobial silver from composites of polylactide with silver zeolites. J. Food Sci. 2010, 75, E186–E193. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, L.; Bonaccorsi, L.; Freni, A.; Proverbio, E. Synthesis of SAPO-34 zeolite filled macrocellular foams for adsorption heat pump applications: A preliminary study. Appl. Therm. Eng. 2017, 124. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Bruzzaniti, P.; Calabrese, L.; Freni, A.; Proverbio, E.; Restuccia, G. Synthesis of SAPO-34 on graphite foams for adsorber heat exchangers. Appl. Therm. Eng. 2013, 61, 848–852. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, Z.; Yan, Y. Corrosion-Resistant Zeolite Coatings by In Situ Crystallization. Electrochem. Solid-State Lett. 2001, 4, B23. [Google Scholar] [CrossRef]
- Cai, R.; Yan, Y. Corrosion-resistant zeolite coatings. Corrosion 2008, 64, 271–278. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Tedim, J.; Ferreira, M.G.S. “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Calabrese, L.; Proverbio, E. Low temperature single-step synthesis of zeolite Y coatings on aluminium substrates. Microporous Mesoporous Mater. 2011, 144, 40–45. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Proverbio, E. Corrosion protection of aluminum 6061 in NaCl solution by silane–zeolite composite coatings. J. Coatings Technol. Res. 2012, 9, 597–607. [Google Scholar] [CrossRef]
- Dias, S.A.S.; Marques, A.; Lamaka, S.V.; Simões, A.; Diamantino, T.C.; Ferreira, M.G.S. The role of Ce(III)-enriched zeolites on the corrosion protection of AA2024-T3. Electrochim. Acta 2013, 112, 549–556. [Google Scholar] [CrossRef] [Green Version]
- Changjean, W.C.; Chiang, A.S.T.; Tsai, T.C. Anti-corrosion zeolite film by the dry-gel-conversion process. Thin Solid Films 2013, 529, 327–332. [Google Scholar] [CrossRef]
- Tsai, S.-T.; ChangJean, W.-C.; Chao, P.-H.; Fu, S.-Y.; Tsai, T.-C. Whole pH range anti-corrosion property of aluminium alloy coated with MFI zeolite film. Corros. Eng. Sci. Technol. 2018, 53, 34–38. [Google Scholar] [CrossRef]
- Shabani-nooshabadi, M.; Allahyary, E.; Jafari, Y. Enhanced Anti-corrosive Properties of Electrosynthesized Polyaniline/zeolite Nanocomposite Coatings on Steel. J. Nanostructure 2018, 8, 131–143. [Google Scholar] [CrossRef]
- Roselli, S.; Deyá, C.; Revuelta, M.; Di Sarli, A.R.; Romagnoli, R. Zeolites as reservoirs for Ce(III) as passivating ions in anticorrosion paints. Corros. Rev. 2018, 36, 305–322. [Google Scholar] [CrossRef]
- Rassouli, L.; Naderi, R.; Mahdavian, M.; Arabi, A.M. Synthesis and Characterization of Zeolites for Anti-corrosion Application: The Effect of Precursor and Hydrothermal Treatment. J. Mater. Eng. Perform. 2018, 27, 4625–4634. [Google Scholar] [CrossRef]
- Wang, Z.; Hedlund, J.; Sterte, J. Synthesis of thin silicalite-1 films on steel supports using a seeding method. Microporous Mesoporous Mater. 2002, 52, 191–197. [Google Scholar] [CrossRef]
- Mitra, A.; Wang, Z.; Cao, T.; Wang, H.; Huang, L.; Yan, Y. Synthesis and Corrosion Resistance of High-Silica Zeolite MTW, BEA, and MFI Coatings on Steel and Aluminum. J. Electrochem. Soc. 2002, 149, B472. [Google Scholar] [CrossRef]
- Cai, R.; Sun, M.; Chen, Z.; Munoz, R.; O’Neill, C.; Beving, D.E.; Yan, Y. Ionothermal synthesis of oriented zeolite AEL films and their application as corrosion-resistant coatings. Angew. Chem. Int. Ed. 2008, 47, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Liu, Y.; Chu, W.; Cai, R.; Yang, W. One-step ionothermal synthesis of oriented molecular sieve corrosion-resistant coatings. Microporous Mesoporous Mater. 2018, 265, 70–76. [Google Scholar] [CrossRef]
- Matsukata, M.; Ogura, M.; Osaki, T.; Raja, P.; Rao, H.P.; Nomura, M.; Kikuchi, E. Conversion of dry gel to microporous crystals in gas phase. Top. Catal. 1999, 9, 77–92. [Google Scholar] [CrossRef]
- Hirota, Y.; Murata, K.; Tanaka, S.; Nishiyama, N.; Egashira, Y.; Ueyama, K. Dry gel conversion synthesis of SAPO-34 nanocrystals. Mater. Chem. Phys. 2010, 123, 507–509. [Google Scholar] [CrossRef]
- Bedi, R.S.; Beving, D.E.; Zanello, L.P.; Yan, Y. Biocompatibility of corrosion-resistant zeolite coatings for titanium alloy biomedical implants. Acta Biomater. 2009, 5, 3265–3271. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, L.; Bonaccorsi, L.; Di Pietro, D.; Proverbio, E. Effect of process parameters on behaviour of zeolite coatings obtained by hydrothermal direct synthesis on aluminium support. Ceram. Int. 2014, 40, 12837–12845. [Google Scholar] [CrossRef]
- Dong, Y.; Peng, Y.; Wang, G.; Wang, Z.; Yan, Y. Corrosion-resistant zeolite silicalite-1 coatings synthesized by seeded growth. Ind. Eng. Chem. Res. 2012, 51, 3646–3652. [Google Scholar] [CrossRef]
- Banerjee, P.; Woo, R.; Grayson, S.; Majumder, A.; Raman, R. Influence of Zeolite Coating on the Corrosion Resistance of AZ91D Magnesium Alloy. Materials 2014, 7, 6092–6104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.Y.; Hao, Y.C.; ChangJean, W.; Wang, M.J.; Chiang, A.S.T.; Tsai, T.C. Growth of MFI zeolite film as corrosion protection layer of aluminum alloy. Microporous Mesoporous Mater. 2015, 217, 71–80. [Google Scholar] [CrossRef]
- Beving, D.E.; McDonnell, A.M.P.; Yang, W.; Yan, Y. Corrosion Resistant High-Silica-Zeolite MFI Coating. One General Solution Formulation for Aluminum Alloy AA-2024-T3, AA-5052-H32, AA-6061-T4, and AA-7075-T6. J. Electrochem. Soc. 2006, 153, B325. [Google Scholar] [CrossRef]
- Chan, S.Y.N.; Ji, X. Comparative study of the initial surface absorption and chloride diffusion of high performance zeolite, silica fume and PFA concretes. Cem. Concr. Compos. 1999, 21, 293–300. [Google Scholar] [CrossRef]
- Chan, S.Y.N.; Ji, X. Strength, initial surface absorption and chloride diffusivity of concrete with zeolite and silica fume. HKIE Trans. Hong Kong Inst. Eng. 1998, 5, 1–7. [Google Scholar] [CrossRef]
- Beving, D.E.; O’Neill, C.R.; Yan, Y. Hydrophilic and antimicrobial low-silica-zeolite LTA and high-silica-zeolite MFI hybrid coatings on aluminum alloys. Microporous Mesoporous Mater. 2008, 108, 77–85. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Freni, A.; Proverbio, E. Silicone composite foams for adsorption heat pump applications. Sustain. Mater. Technol. 2017, 12, 27–34. [Google Scholar] [CrossRef]
- Munoz, R.; Beving, D.; Mao, Y.; Yan, Y. Zeolite y coatings on Al-2024-T3 substrate by a three-step synthesis method. Microporous Mesoporous Mater. 2005, 86, 243–248. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Proverbio, E. Synthesis of thick zeolite 4A coatings on stainless steel. Microporous Mesoporous Mater. 2004, 74, 221–229. [Google Scholar] [CrossRef]
- Pande, H.B.; Parikh, P.A. Novel application of ZSM-5 zeolite: Corrosion-resistant coating in chemical process industry. J. Mater. Eng. Perform. 2013, 22, 190–199. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Calabrese, L.; Proverbio, E.; Frazzica, A.; Freni, A.; Restuccia, G.; Piperopoulos, E.; Milone, C. Synthesis of SAPO-34/graphite composites for low temperature heat adsorption pumps. J. Energy Chem. 2013, 22, 245–250. [Google Scholar] [CrossRef]
- Lauridant, N.; Daou, T.J.; Arnold, G.; Soulard, M.; Nouali, H.; Patarin, J.; Faye, D. Key steps influencing the formation of ZSM-5 films on aluminum substrates. Microporous Mesoporous Mater. 2012, 152, 1–8. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, Y. Controlling Crystal Orientation in Zeolite MFI Thin Films by Direct In Situ Crystallization. Chem. Mater. 2001, 13, 1101–1107. [Google Scholar] [CrossRef]
- Beving, D.; O’Neill, C.; Yan, Y.S. Corrosion resistant high-silica-zeolite MFI coatings. Stud. Surf. Sci. Catal. 2007, 170, 1629–1634. [Google Scholar] [CrossRef]
- Morris, R.E.; Wheatley, P.S. Gas storage in nanoporous materials. Angew. Chem. Int. Ed. 2008, 47, 4966–4981. [Google Scholar] [CrossRef]
- Morris, R.E. Ionothermal synthesis—Ionic liquids as functional solvents in the preparation of crystalline materials. Chem. Commun. 2009, 2990–2998. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Cai, R.; Liu, Y.; Gu, S. Ambient pressure dry-gel conversion method for zeolite MFI synthesis using ionic liquid and microwave heating. J. Am. Chem. Soc. 2010, 132, 12776–12777. [Google Scholar] [CrossRef]
- Rao, P.R.H.P.; Matsukata, M. Dry-gel conversion technique for synthesis of zeolite BEA. Chem. Commun. 1996, 1441–1442. [Google Scholar] [CrossRef]
- Yue, M.B.; Yang, N.; Jiao, W.Q.; Wang, Y.M.; He, M.Y. Dry-gel synthesis of shaped binderless zeolites composed of nanosized ZSM-5. Solid State Sci. 2013, 20, 1–7. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Chiang, A.S.T.; Selvin, R.; Thompson, R.W. Rapid synthesis of MFI zeolite nanocrystals. J. Phys. Chem. B 2005, 109, 18804–18814. [Google Scholar] [CrossRef] [PubMed]
- Hang Chau, J.L.; Tellez, C.; Yeung, K.L.; Ho, K. The role of surface chemistry in zeolite membrane formation. J. Memb. Sci. 2000, 164, 257–275. [Google Scholar] [CrossRef]
- Vroon, Z.A.E.P.; Keizer, K.; Burggraaf, A.J.; Verweij, H. Preparation and characterization of thin zeolite MFI membranes on porous supports. J. Memb. Sci. 1998, 144, 65–76. [Google Scholar] [CrossRef]
- Winkler, D. Predicting the Performance of Organic Corrosion Inhibitors. Metals 2017, 7, 553. [Google Scholar] [CrossRef]
- Lopez-Garrity, O.; Frankel, G.S. Corrosion inhibition of aa2024-t3 by sodium silicate. Electrochim. Acta 2014, 130, 9–21. [Google Scholar] [CrossRef]
- Voevodin, N.; Jeffcoate, C.; Simon, L.; Khobaib, M.; Donley, M. Characterization of pitting corrosion in bare and sol-gel coated aluminum 2024-T3 alloy. Surf. Coatings Technol. 2001, 140, 29–34. [Google Scholar] [CrossRef]
- Rolison, D.R. Zeolite-Modified Electrodes and Electrode-Modified Zeolites. Chem. Rev. 1990, 90, 867–878. [Google Scholar] [CrossRef]
- Walcarius, A. Electroanalytical applications of microporous zeolites and mesoporous (organo)silicas: Recent trends. Electroanalysis 2008, 20, 711–738. [Google Scholar] [CrossRef]
- Walcarius, A. Electrochemistry with Micro- and Mesoporous Silicates. In Ordered Porous Solids; Elsevier: Amsterdam, The Netherlands, 2009; pp. 523–557. ISBN 9780444531896. [Google Scholar]
- Bodoardo, S.; Geobaldo, F.; Penazzi, N.; Arrabito, M.; Rivetti, F.; Spanò, G.; Lamberti, C.; Zecchina, A. Voltammetric characterization of structural titanium species in zeotypes. Electrochem. Commun. 2000, 2, 349–352. [Google Scholar] [CrossRef]
- Gaoquan, S.; Gi, X.; Wenhua, H.; Jian, D.; Guoxiang, W. Electrochemical behavior of MOR zeolite in poly(ethylene oxide) oligomer. J. Electroanal. Chem. 1993, 344, 363–366. [Google Scholar] [CrossRef]
- Takatani, Y.; Yamakawa, K.; Yoshizawa, S. Corrosion behaviour of aluminum alloys in saturated calcium hydroxide solution. Zair. Soc. Mater. Sci. Jpn. 1983, 32, 1218–1222. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Enhancement of the hydrophobic and anti-corrosion properties of a composite zeolite coating on Al6061 substrate by modification of silane matrix. Corros. Eng. Sci. Technol. 2017, 52, 61–72. [Google Scholar] [CrossRef]
- Yamamoto, S.; Sugiyama, S.; Matsuoka, O.; Kohmura, K.; Honda, T.; Banno, Y.; Nozoye, H. Dissolution of zeolite in acidic and alkaline aqueous solutions as revealed by AFM imaging. J. Phys. Chem. 1996, 100, 18474–18482. [Google Scholar] [CrossRef]
- Palanivel, V.; Zhu, D.; Van Ooij, W.J. Nanoparticle-filled silane films as chromate replacements for aluminum alloys. Prog. Org. Coatings 2003, 47, 384–392. [Google Scholar] [CrossRef]
- Deyá, C.; Romagnoli, R.; Del Amo, B. A new pigment for smart anticorrosive coatings. J. Coatings Technol. Res. 2007, 4, 167–175. [Google Scholar] [CrossRef]
- Chico, B.; Simancas, J.; Vega, J.M.; Granizo, N.; Díaz, I.; de la Fuente, D.; Morcillo, M. Anticorrosive behaviour of alkyd paints formulated with ion-exchange pigments. Prog. Org. Coatings 2008, 61, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Schenderlein, M.; Huang, X.; Brownbill, N.J.; Blanc, F.; Shchukin, D. Influence of Functionalization of Nanocontainers on Self-Healing Anticorrosive Coatings. ACS Appl. Mater. Interfaces 2015, 7, 22756–22766. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K. Functional Coatings: By Polymer Microencapsulation; Wiley Verlag: Weinheim, Germany, 2006; ISBN 352731296X. [Google Scholar]
- Samadzadeh, M.; Boura, S.H.; Peikari, M.; Kasiriha, S.M.; Ashrafi, A. A review on self-healing coatings based on micro/nanocapsules. Prog. Org. Coatings 2010, 68, 159–164. [Google Scholar] [CrossRef]
- Stankiewicz, A.; Szczygieł, I.; Szczygieł, B. Self-healing coatings in anti-corrosion applications. J. Mater. Sci. 2013, 48, 8041–8051. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Garza, M.; Olguín, M.T.; García-Sosa, I.; Alcántara, D.; Rodríguez-Fuentes, G. Silver supported on natural Mexican zeolite as an antibacterial material. Microporous Mesoporous Mater. 2000, 39, 431–444. [Google Scholar] [CrossRef]
- Kwakye-Awuah, B.; Williams, C.; Kenward, M.A.; Radecka, I. Antimicrobial action and efficiency of silver-loaded zeolite X. J. Appl. Microbiol. 2008, 104, 1516–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, L.; Fonseca, A.M.; Botelho, G.; Aguiar, C.A.; Neves, I.C. Antimicrobial activity of faujasite zeolites doped with silver. Microporous Mesoporous Mater. 2012, 160, 126–132. [Google Scholar] [CrossRef]
- McDonnell, A.M.P.; Beving, D.; Wang, A.; Chen, W.; Yan, Y. Hydrophilic and Antimicrobial Zeolite Coatings for Gravity-Independent Water Separation. Adv. Funct. Mater. 2005, 15, 336–340. [Google Scholar] [CrossRef]
- Chanda, R.; Selvam, T.; Herrmann, R.; Schwieger, W. Reactive coating process for binder-free zeolite FAU films on metallic aluminum supports. Mater. Lett. 2018, 211, 103–106. [Google Scholar] [CrossRef]
- Calabrese, L.; Brancato, V.; Bonaccorsi, L.; Frazzica, A.; Caprì, A.; Freni, A.; Proverbio, E. Development and characterization of silane-zeolite adsorbent coatings for adsorption heat pump applications. Appl. Therm. Eng. 2017, 116, 364–371. [Google Scholar] [CrossRef]
- Aramaki, K. Preparation of protective films containing molybdate for self-healing of a scratched iron surface. Corrosion 2000, 56, 901–909. [Google Scholar] [CrossRef]
- Lin, B.L.; Lu, J.T.; Kong, G. Effect of molybdate post-sealing on the corrosion resistance of zinc phosphate coatings on hot-dip galvanized steel. Corros. Prot. 2007, 50, 962–967. [Google Scholar] [CrossRef]
- Dias, S.A.S.; Lamaka, S.V.; Nogueira, C.A.; Diamantino, T.C.; Ferreira, M.G.S. Sol–gel coatings modified with zeolite fillers for active corrosion protection of AA2024. Corros. Sci. 2012, 62, 153–162. [Google Scholar] [CrossRef]
- Ferrer, E.L.; Rollon, A.P.; Mendoza, H.D.; Lafont, U.; Garcia, S.J. Double-doped zeolites for corrosion protection of aluminium alloys. Microporous Mesoporous Mater. 2014, 188, 8–15. [Google Scholar] [CrossRef]
- Dias, S.A.S.; Lamaka, S.V.; Diamantino, T.C.; Ferreira, M.G.S. Synergistic Protection against Corrosion of AA2024-T3 by Sol-Gel Coating Modified with La and Mo-Enriched Zeolites. J. Electrochem. Soc. 2014, 161, C215–C222. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.; Miao, C.; Huang, X.; Man, R.L. Effect of cerium ion (III) on corrosion resistance of organic-inorganic hybrid coating on surface of aluminum-tube used in refrigeration equipment. Trans. Nonferrous Met. Soc. China 2015, 25, 3847–3854. [Google Scholar] [CrossRef]
- Schoonheydt, R.A.; Geerlings, P.; Pidko, E.A.; Van Santen, R.A. The framework basicity of zeolites. J. Mater. Chem. 2012, 22, 18705–18717. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G.S. Mechanism of corrosion inhibition of AA2024 by rare-earth compounds. J. Phys. Chem. B 2006, 110, 5515–5528. [Google Scholar] [CrossRef]
- Volarič, B.; Milošev, I. Rare earth chloride and nitrate salts as individual and mixed inhibitors for aluminium alloy 7075-T6 in chloride solution. Corros. Eng. Sci. Technol. 2017, 52, 201–211. [Google Scholar] [CrossRef]
- Caprì, A.; Calabrese, L.; Bonaccorsi, L.; Proverbio, E. Corrosion Resistance of Cerium Based Silane-Zeolite Coatings on AA6061. Solid State Phenom. 2015, 227, 163–166. [Google Scholar] [CrossRef]




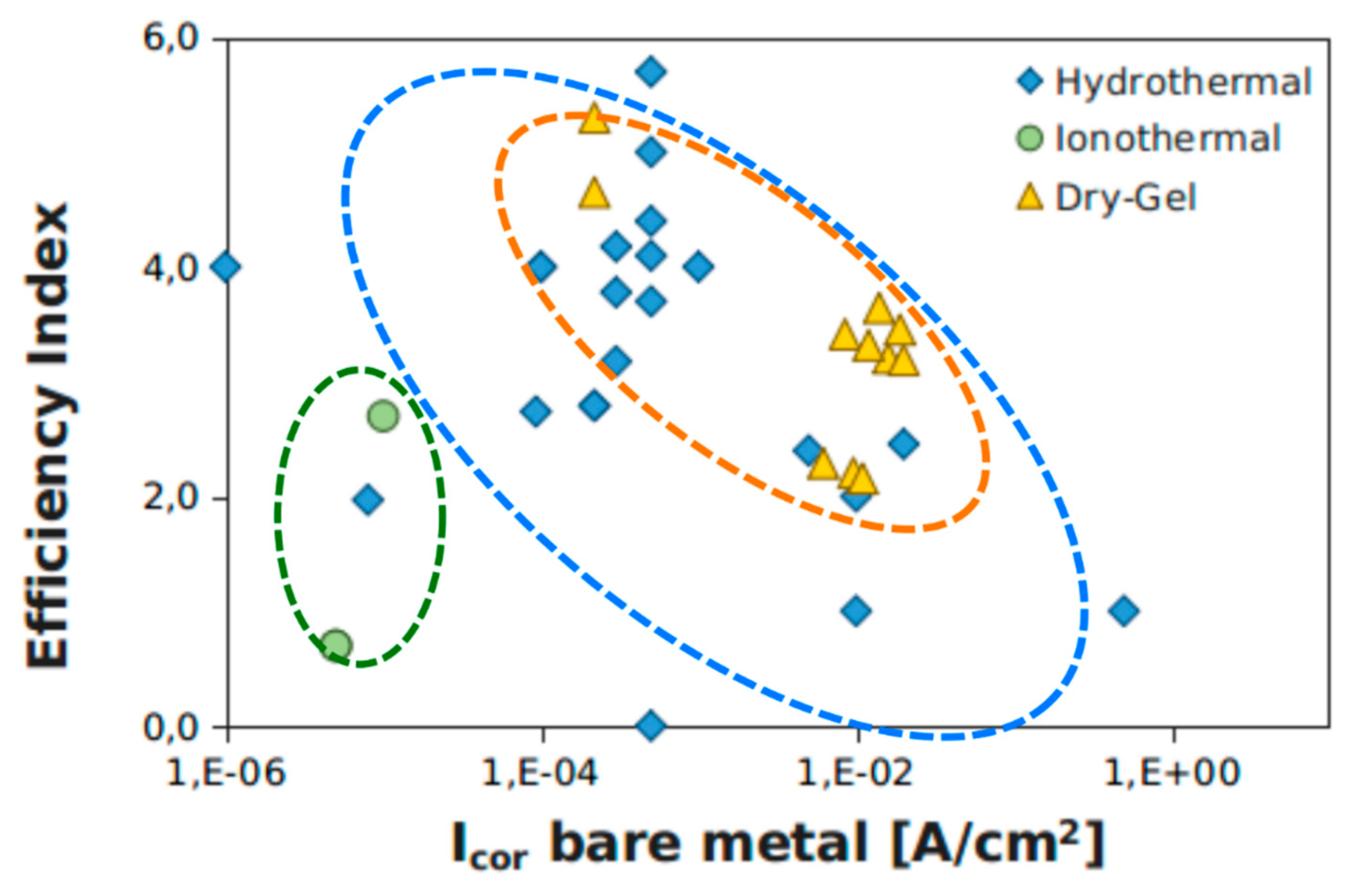

| Authors | Ref. | Year | Method | Zeolite | Substrate | Electrolyte | Icor [A/cm2] | |Z| 0.01 Hz [Ohm*cm2] | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Coating | Substrate | Coating | Substrate | |||||||
| Cheng et al. | [29] | 2001 | Hydrothermal | ZSM-5 | Al 2024-T3 | 0.5 M H2SO4 0.5 M NaCl/HCl | ~1 × 10−10 ~1 × 10−10 | ~1 × 10−6 ~1 × 10−2 | ~1 × 109 ~1 × 109 | ~1 × 105 ~1 × 102 |
| Mitra et al. | [41] | 2002 | Hydrothermal | MTW | Al 2024-T3 | 0.5 M H2SO4 | ~2 × 10−7 | ~3 × 10−4 | ||
| Mitra et al. | [41] | 2002 | Hydrothermal | MTW BEA | Al 6061-T4 | 0.5 M H2SO4 | ~2 × 10−8 ~5 × 10−8 | ~3 × 10−4 | ||
| Mitra et al. | [41] | 2002 | Hydrothermal | MTW | Al 2024-T3 | 0.1 M NaOH | ~1 × 10−7 | ~1 × 10−3 | ||
| Mitra et al. | [41] | 2002 | Hydrothermal | MTW BEA | Al 6061-T4 | 0.1 M NaOH | ~2 × 10−8 ~4 × 10−8 | ~5 × 10−4 | ||
| Mitra et al. | [41] | 2002 | Hydrothermal | MTW MFI BEA | AISI 304 | 0.5 M H2SO4 | ~1 × 10−7 ~1 × 10−9 ~5 × 10−9 | ~5 × 10−4 | ||
| Bedi et al. | [46] | 2009 | Hydrothermal | MFI | Ti6Al4V | 0.856 M NaCl 0.856 M NaCl/HCl | 8.6 × 10−8 1.7 × 10−7 | 8 × 10−6 9.3 × 10−5 | ||
| Bonaccorsi et al. | [32] | 2011 | Hydrothermal | Y | AISI 304 | 5% NaCl Ca(OH)2 sat | ~1 × 10−4 ~5 × 10−3 | ~1 × 10−2 ~2 × 10−3 | ||
| Bonaccorsi et al. | [32] | 2011 | Hydrothermal | Y | Al 6061 | 5% NaCl Ca(OH)2 sat | ~2 × 10−5 ~5 × 10−2 | ~5 × 10−3 ~5 × 10−1 | ||
| Dong et al. | [48] | 2012 | Hydrothermal | Silicalite-1 | AA 1060 | 0.5 M H2SO4 0.5 M NaCl | 1 × 10−8 1 × 10−8 | 1 × 10−4 1 × 10−4 | ||
| Banerjee et al. | [49] | 2014 | Hydrothermal | ZSM-5 | AZ91D | 0.1 M NaCl | 1 × 10−3 | 1 × 10−2 | 4.5 × 104 | 8 × 103 |
| Calabrese et al. | [47] | 2014 | Hydrothermal | Y | Al 6061 | 3.5% NaCl Ca(OH)2 sat | ~5 × 10−4 ~7 × 10−5 | ~5 × 10−4 ~2 × 10−2 | ||
| Huang et al. | [50] | 2015 | Hydrothermal | MFI | Al 6061-T6 | 3.5% NaCl | 1 × 10−6.45 | 1 × 10−3.66 | 1.7 × 103 | 2.7 × 102 |
| Tsai et al. | [36] | 2018 | Hydrothermal | MFI | Al 6061-T6 | 3.5% NaCl | 1 × 10−6.45 | 1 × 10−3.66 | 1.7 × 103 | 2.7 × 102 |
| Cai et al. | [42] | 2008 | Ionothermal | SAPO-11 ALPO-11 | Al 2024-T3 | 0.1 M NaCl | ~1 × 10−6 ~1 × 10−6 | ~5 × 10−6 | ||
| Yu et al. | [43] | 2018 | Ionothermal | ALPO-11 | Aluminum | 0.1 M NaCl | ~2 × 10−8 | ~1 × 10−5 | ||
| Authors | Ref. | Year | Method | Zeolite | Substrate | Electrolyte | Icor [A/cm2] | |Z| 0.01 Hz [Ohm∗cm2] | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Coating | Substrate | Coating | Substrate | |||||||
| Changjean et al. | [35] | 2013 | Dry gel | MFI | Al 6061-T6 | 3.5% NaCl | 1 × 10−8.96 | 1 × 10−3.66 | ||
| Pande et al. | [58] | 2013 | Dry gel | ZSM-5 | Mild Steel | 0.5M HCl 1.0M HCl 1.5M HCl | 3.3 × 10−6 1.0 × 10−5 1.3 × 10−5 | 8.5 × 10−3 1.6 × 10−2 2.0 × 10−2 | ||
| Pande et al. | [58] | 2013 | Dry gel | ZSM-5 | Mild Steel | 0.5M H2SO4 1.0M H2SO4 1.5M H2SO4 | 3.2 × 10−6 6.7 × 10−6 5.8 × 10−6 | 1.4 × 10−2 1.9 × 10−2 1.2 × 10−2 | ||
| Pande et al. | [58] | 2013 | Dry gel | ZSM-5 | Mild Steel | 0.5M H3PO4 1.0M H3PO4 1.5M H3PO4 | 3.2 × 10−5 6.0 × 10−5 7.7 × 10−5 | 6.2 × 10−3 9.6 × 10−3 1.1 × 10−2 | ||
| Al-Subaie et al. | [8] | 2015 | Dry gel | Beta | Carbon Steel | 3.0% NaCl 0.1M H2SO4 0.1M NaOH 3.5% NaCl | 1 × 10−3.54 1 × 10−1.41 1 × 10−3.83 | |||
| Huang et al. | [50] | 2015 | Dry gel | MFI | Al 6061-T6 | 3.5% NaCl | 1 × 10−8.31 | 1 × 10−3.66 | 2.5 × 104 | 2.7 × 102 |
| Tsai et al. | [36] | 2018 | Dry gel | MFI | Al 6061-T6 | 3.5% NaCl | 1 × 10−8.31 | 1 × 10−3.66 | 2.5 × 104 | 2.7 × 102 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, L. Anticorrosion Behavior of Zeolite Coatings Obtained by In Situ Crystallization: A Critical Review. Materials 2019, 12, 59. https://doi.org/10.3390/ma12010059
Calabrese L. Anticorrosion Behavior of Zeolite Coatings Obtained by In Situ Crystallization: A Critical Review. Materials. 2019; 12(1):59. https://doi.org/10.3390/ma12010059
Chicago/Turabian StyleCalabrese, Luigi. 2019. "Anticorrosion Behavior of Zeolite Coatings Obtained by In Situ Crystallization: A Critical Review" Materials 12, no. 1: 59. https://doi.org/10.3390/ma12010059





