Design, Electron Transfer Process, and Opto-Electronic Property of Solar Cell Using Triphenylamine-Based D-π-A Architectures
Abstract
:1. Introduction
2. Computational Details
2.1. Theoretical Background
2.2. Computational Detail
3. Results and Discussion
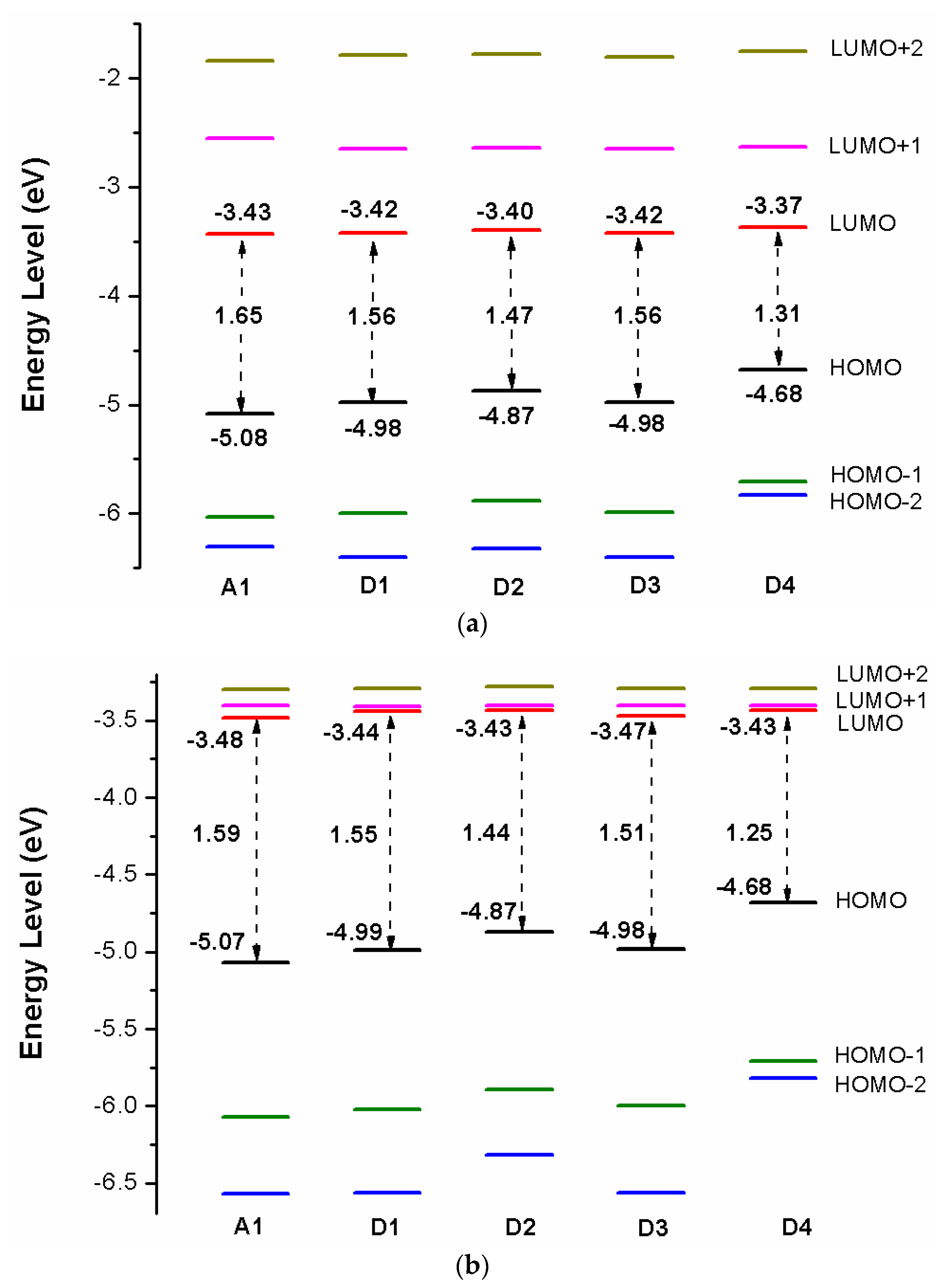
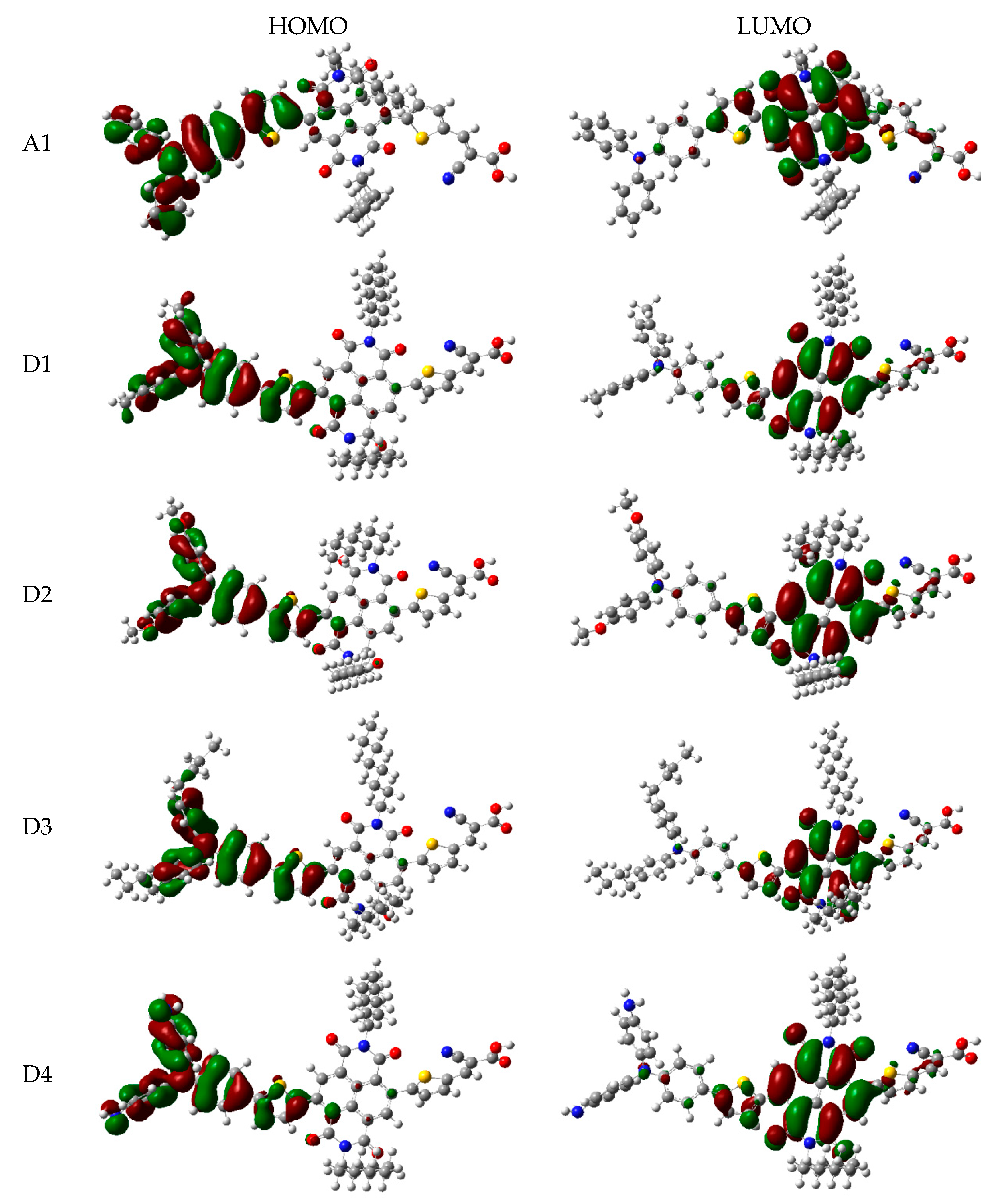
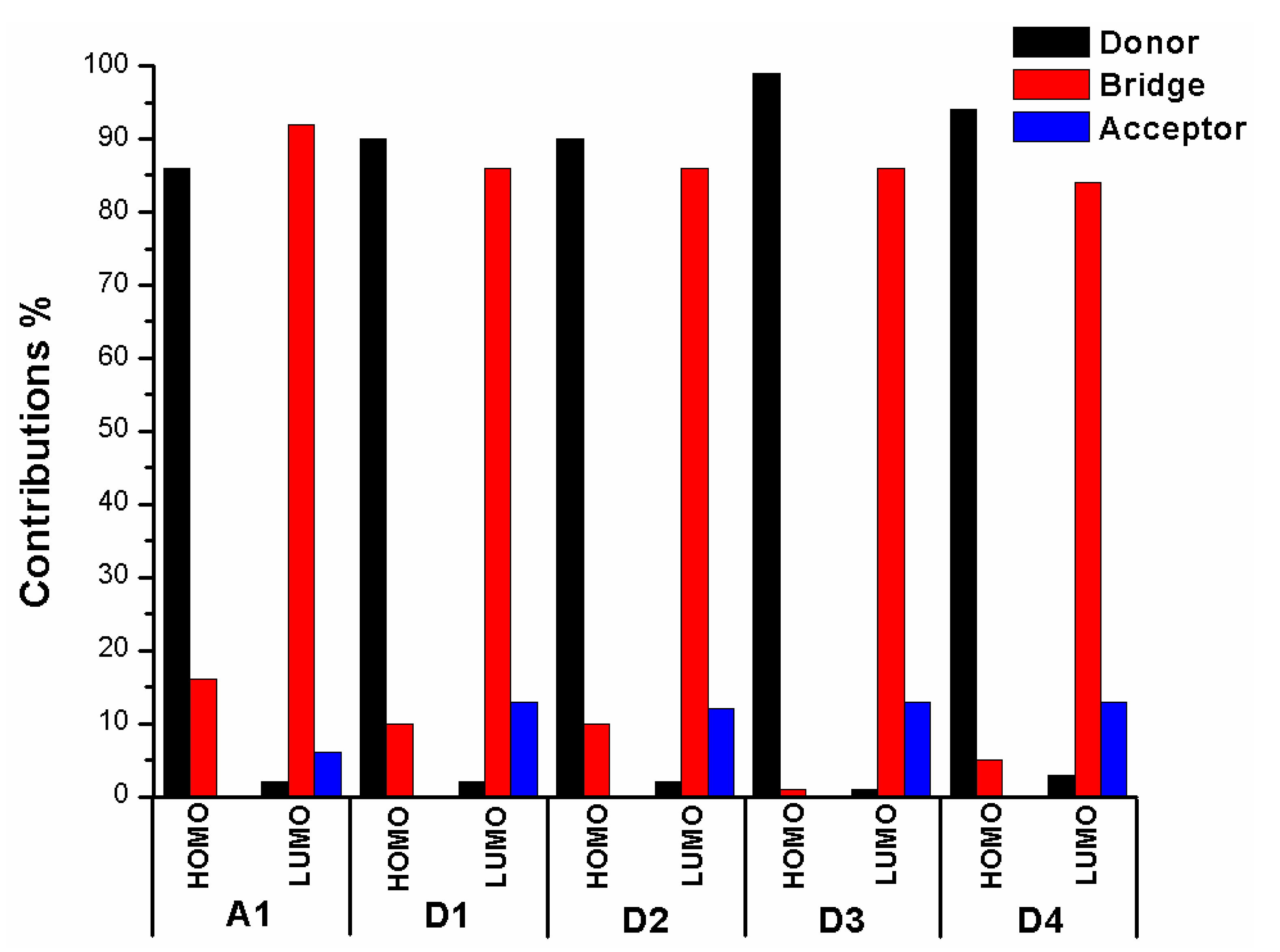
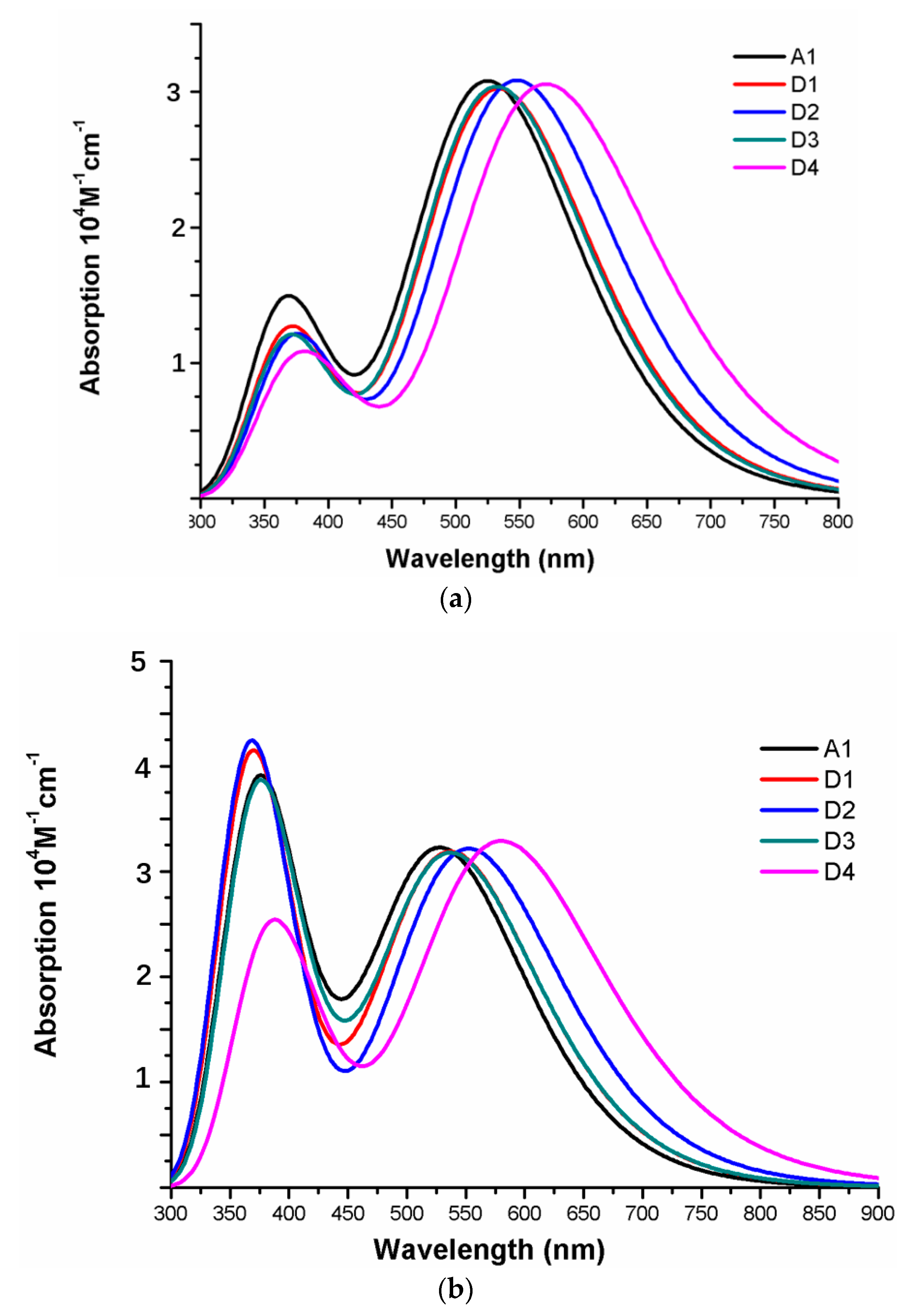
3.1. Screening of the π-Spacer
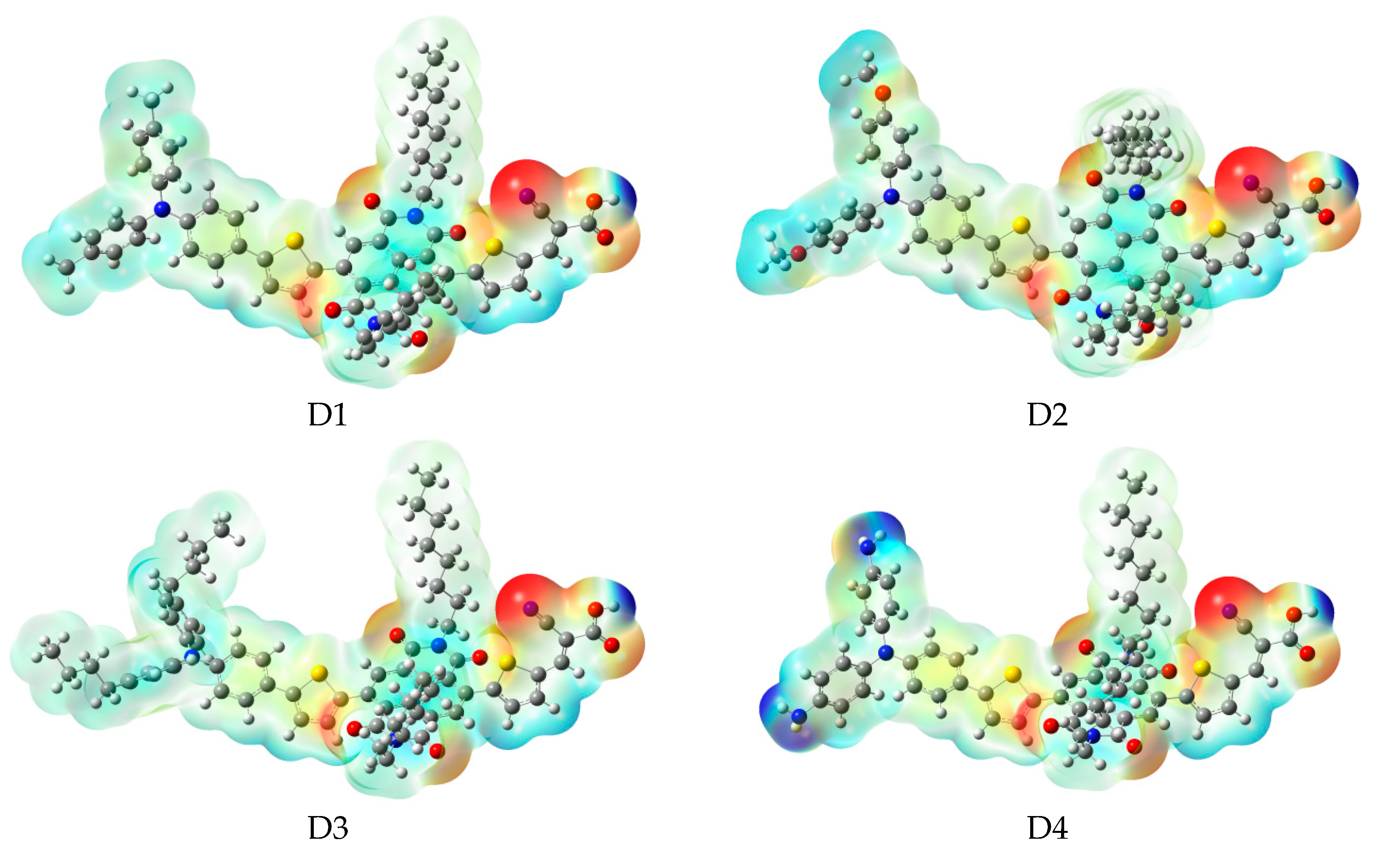
3.2. Screening of the Donors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A Low-Cost High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Duncan, W.R.; Prezhdo, O.V. Theoretical studies of photoinduced electron transfer in dye-sensitized TiO(2). Annu. Rev. Phys. Chem. 2007, 58, 143–184. [Google Scholar] [CrossRef]
- Gratzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.W.; Sumathy, K.; Qian, Q.Q.; Zhou, Z.P. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Gendron, D.; Leclerc, M. New conjugated polymers for plastic solar cells. Energy Environ. Sci. 2011, 4, 1225–1237. [Google Scholar] [CrossRef]
- Perera, I.R.; Daeneke, T.; Makuta, S.; Yu, Z.; Tachibana, Y.; Mishra, A.; Bäuerle, P.; Ohlin, C.A.; Bach, U.; Spiccia, L. Application of the Tris(acetylacetonato)iron(III)/(II) Redox Couple in p-Type Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2015, 54, 3758–3762. [Google Scholar] [CrossRef]
- Long, R.; Prezhdo, O.V. Ab Initio Nonadiabatic Molecular Dynamics of the Ultrafast Electron Injection from a PbSe Quantum Dot into the TiO2 Surface. J. Am. Chem. Soc. 2011, 133, 19240–19249. [Google Scholar] [CrossRef]
- Wang, J.G.; Li, W.H.; Xu, X.F.; Ma, F.C.; Sun, M.T. Plasmon-Exciton Coupling Interaction for Surface Catalytic Reactions. Chem. Rec. 2018, 18, 481–490. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Péchy, P.; Renouard, T.; Zakeeruddin, S.M.; Humphry-Baker, R.; Comte, P.; Liska, P.; Cevey, L.; Costa, E.; Shklover, V.; et al. Engineering of Efficient Panchromatic Sensitizers for Nanocrystalline TiO2-Based Solar Cells. J. Am. Chem. Soc. 2001, 123, 1613–1624. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; CurchodBasile, F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; NazeeruddinMd, K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Kim, H.W.; Sakong, C.; Namgoong, J.; Park, S.W.; Ko, M.J.; Lee, C.H.; Lee, W.I.; Kim, J.P. Effect of five-membered heteroaromatic linkers to the performance of phenothiazine-based dye-sensitized solar cells. Org. Lett. 2011, 13, 5784–5787. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-Y.; Taufany, F.; Nachimuthu, S.; Jiang, J.-C.; Liaw, D.-J. Design strategies of metal free-organic sensitizers for dye sensitized solar cells: Role of donor and acceptor monomers. Org. Electron. 2014, 15, 1205–1214. [Google Scholar] [CrossRef]
- Vollbrecht , J.; Bock, H.; Wiebeler, C.; Schumacher, S.; Kitzerow, H. Polycyclic Aromatic Hydrocarbons Obtained by Lateral Core Extension of Mesogenic Perylenes: Absorption and Optoelectronic Properties. Chem. A Eur. J. 2014, 20, 12026–12031. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Jiao, Y.; Ma, W.; Nazeeruddin, M.K.; Grätzel, M.; Meng, S. First principles design of dye molecules with ullazine donor for dye sensitized solar cells. J. Phys. Chem. C 2013, 117, 3772–3778. [Google Scholar] [CrossRef]
- Santhanamoorthi, N.; Lo, C.-M.; Jiang, J.-C. Molecular design of porphyrins for dye-sensitized solar cells: a DFT/TDDFT study. J. Phys. Chem. Lett. 2013, 4, 524–530. [Google Scholar] [CrossRef]
- Bellier, Q.; Pégaz, S.; Aronica, C.; Guennic, B.L.; Andraud, C.; Maury, O. Near-infrared nitrofluorene substitued aza-boron-dipyrromethenes dyes. Org. Lett. 2011, 13, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Miao, P.; Zhang, Y.; Han, J.; Zhang, X.; Gong, Y.; Gu, L.; Xu, C.; Yao, T.; Xu, P.; et al. Significantly Increased Raman Enhancement on MoX2 (X = S, Se) Monolayers upon Phase Transition. Adv. Funct. Mater. 2017, 27, 1606694. [Google Scholar] [CrossRef]
- Xu, B.; Li, Y.; Song, P.; Ma, F.; Sun, M. Photoactive layer based on T-shaped benzimidazole dyes used for solar cell: from photoelectric properties to molecular design. Sci. Rep. 2017, 7, 45688. [Google Scholar] [CrossRef] [Green Version]
- Katono, M.; Wielopolski, M.; Marszalek, M.; Bessho, T.; Moser, J.-E.; Humphry-Baker, R.; Zakeeruddin, S.M.; Grätzel, M.J. Effect of Extended π-Conjugation of the Donor Structure of Organic D–A− π–A Dyes on the Photovoltaic Performance of Dye-Sensitized Solar Cells. J. Phys. Chem. C 2014, 118, 16486–16493. [Google Scholar] [CrossRef]
- Paramasivam, M.; Gupta, A.; Raynor, A.M.; Bhosale, S.V.; Bhanuprakash, K.; Rao, V.J. Small band gap D-π-A-π-D benzothiadiazole derivatives with low-lying HOMO levels as potential donors for applications in organic photovoltaics: a combined experimental and theoretical investigation. RSC Adv. 2014, 4, 35318–35331. [Google Scholar] [CrossRef]
- Namuangruk, S.; Fukuda, R.; Ehara, M.; Meeprasert, J.; Khanasa, T.; Morada, S.; Kaewin, T.; Jungsuttiwong, S.; Sudyoadsuk, T.; Promarak, V.J. D–D−π–A-Type Organic Dyes for Dye-Sensitized Solar Cells with a Potential for Direct Electron Injection and a High Extinction Coefficient: Synthesis, Characterization, and Theoretical Investigation. J. Phys. Chem. C 2012, 116, 25653–25663. [Google Scholar] [CrossRef]
- Xie, M.; Wang, J.; Bai, F.-Q.; Hao, L.; Zhang, H.-X. Discovering the intermediate of dye regeneration in dye-sensitized solar cells: Theoretical investigations on the interaction between organic dye with different donors and X−/X3− (X = I, Br). Dyes Pigment. 2015, 120, 74–84. [Google Scholar] [CrossRef]
- Li, Y.; Xu, B.; Song, P.; Ma, F.; Sun, M.J. D–A− π–A system: Light harvesting, charge transfer, and molecular designing. J. Phys. Chem. C. 2017, 121, 12546–12561. [Google Scholar] [CrossRef]
- Li, Y.; Qi, D.; Song, P.; Ma, F. Fullerene-based photoactive layers for heterojunction solar cells: Structure, absorption spectra and charge transfer process. Materials 2015, 8, 42–56. [Google Scholar] [CrossRef]
- Haid, S.; Marszalek, M.; Mishra, A.; Wielopolski, M.; Teuscher, J.; Moser, J.-E.; Humphry-Baker, R.; Zakeeruddin, S.M.; Grätzel, M.; Bäuerle, P. Significant Improvement of Dye-Sensitized Solar Cell Performance by Small Structural Modification in π-Conjugated Donor–Acceptor Dyes. Adv. Funct. Mater. 2012, 22, 1291–1302. [Google Scholar] [CrossRef]
- Mai, C.-L.; Huang, W.-K.; Lu, H.-P.; Lee, C.-W.; Chiu, C.-L.; Liang, Y.-R.; Diau, E.W.-G.; Yeh, C.-Y. Synthesis and characterization of diporphyrin sensitizers for dye-sensitized solar cells. Chem. Commun. 2010, 46, 809–811. [Google Scholar] [CrossRef]
- Imahori, H.; Matsubara, Y.; Iijima, H.; Umeyama, T.; Matano, Y.; Ito, S.; Niemi, M.; Tkachenko, N.V.; Lemmetyinen, H. Effects of meso-Diarylamino Group of Porphyrins as Sensitizers in Dye-Sensitized Solar Cells on Optical, Electrochemical, and Photovoltaic Properties. J. Phys. Chem. C 2010, 114, 10656–10665. [Google Scholar] [CrossRef]
- Han, L.; Wu, H.; Cui, Y.; Zu, X.; Ye, Q.; Gao, J. Synthesis and density functional theory study of novel coumarin-type dyes for dye sensitized solar cells. J. Photochem. Photobiol. A Chem. 2014, 290, 54–62. [Google Scholar] [CrossRef]
- Seo, K.D.; Song, H.M.; Lee, M.J.; Pastore, M.; Anselmi, C.; de Angelis, F.; Nazeeruddin, M.K.; Gräetzel, M.; Kim, H.K. Coumarin dyes-containing low-band-gap chromophores for dye-sensitised solar cells. Dyes Pigment. 2011, 90, 304–310. [Google Scholar] [CrossRef]
- Tarsang, R.; Promarak, V.; Sudyoadsuk, T.; Namuangruk, S.; Jungsuttiwong, S. Tuning the electron donating ability in the triphenylamine-based D-π-A architecture for highly efficient dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2014, 273, 8–16. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Lee, L.T.L.; Wang, M.; Chen, T.; Zhu, L. Novel D-π-A organic sensitizers containing diarylmethylene-bridged triphenylamine and different spacers for solar cell application. Tetrahedron Lett. 2015, 56, 1233–1238. [Google Scholar] [CrossRef]
- Patil, D.; Jadhav, M.; Avhad, K.; Chowdhury, T.H.; Islam, A.; Bedjac, I.; Sekar, N. A new class of triphenylamine based novel sensitizers for DSSC: A comparative study of three different anchoring groups. New J. Chem. 2018, 42, 11555–11564. [Google Scholar] [CrossRef]
- Rudolph, M.; Yoshida, T.; Miura, H.; Schlettwein, D. Improvement of light harvesting by addition of a long-wavelength absorber in dye-sensitized solar cells based on ZnO and indoline dyes. J. Phys. Chem. C 2015, 119, 1298–1311. [Google Scholar] [CrossRef]
- Cai, N.; Moon, S.-J.; Cevey-Ha, L.; Moehl, T.; Humphry-Baker, R.; Wang, P.; Zakeeruddin, S.M.; Grätzel, M. An organic D-π-A dye for record efficiency solid-state sensitized heterojunction solar cells. Nano Lett. 2011, 11, 1452–1456. [Google Scholar] [CrossRef]
- Xu, M.; Li, R.; Pootrakulchote, N.; Shi, D.; Guo, J.; Yi, Z.; Zakeeruddin, S.M.; Grätzel, M.; Wang, P. Energy-level and molecular engineering of organic D-π-A sensitizers in dye-sensitized solar cells. J. Phys. Chem. C 2008, 112, 19770–19776. [Google Scholar] [CrossRef]
- Liang, M.; Chen, J. Arylamine organic dyes for dye-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 3453–3488. [Google Scholar] [CrossRef]
- Urbani, M.; Grätzel, M.; Nazeeruddin, M.K.; Torres, T. Meso-substituted porphyrins for dye-sensitized solar cells. Chem. Rev. 2014, 114, 12330–12396. [Google Scholar] [CrossRef]
- Mishra, A.; Fischer, M.K.R.; Bäuerle, P. Metal-free organic dyes for dye-sensitized solar cells: From structure: Property relationships to design rules. Angew. Chem. Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef]
- Song, P.; Zhou, Q.; Li, Y.Z.; Ma, F.C.; Sun, M.T. Vibronic quantized tunneling controlled photoinduced electron transfer in an organic solar cell subjected to an external electric field. Phys. Chem. Chem. Phys. 2017, 19, 16105–16112. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.K.; Kang, S.O.; Ko, J.; Yum, J.H.; Fantacci, S.; de Angelis, F.; di Censo, D.; Nazeeruddin, M.K.; Grätzel, M. Molecular engineering of organic sensitizers for solar cell applications. J. Am. Chem. Soc. 2006, 128, 16701–16707. [Google Scholar] [CrossRef]
- Chen, C.; Yang, X.; Cheng, M.; Zhang, F.; Zhao, J.; Sun, L. A draft sequence for the genome of the domesticated silkworm (Bombyx mori). ACS Appl. Mater. Interfaces 2013, 5, 10960–10965. [Google Scholar] [CrossRef]
- Kim, K.-M.; Hong, J.-I. Design of high-performance dye-sensitized solar cells by variation of the dihedral angles of dyes. Tetrahedron 2016, 72, 8387–8392. [Google Scholar] [CrossRef]
- Panneerselvam, M.; Kathiravan, A.; Solomon, R.V.; Jaccob, M. The role of π-linkers in tuning the optoelectronic properties of triphenylamine derivatives for solar cell applications–A DFT/TDDFT study. Phys. Chem. Chem. Phys. 2017, 19, 6153–6163. [Google Scholar] [CrossRef]
- Biswas, A.K.; Das, A.; Ganguly, B. Can fused-pyrrole rings act as better π-spacer units than fused-thiophene in dye-sensitized solar cells? A computational study. New J. Chem. 2016, 40, 9304–9312. [Google Scholar] [CrossRef]
- Bobe, S.R.; Gupta, A.; Rananaware, A.; Bilic, A.; Xiang, W.; Li, J.; Bhosale, S.V.; Bhosale, S.V.; Evans, R.A. Insertion of a naphthalenediimide unit in a metal-free donor–acceptor organic sensitizer for efficiency enhancement of a dye-sensitized solar cell. Dyes Pigment. 2016, 134, 83–90. [Google Scholar] [CrossRef]
- Narayan, M.R. Dye sensitized solar cells based on natural photosensitizers. Renew. Sustain. Energy Rev. 2012, 16, 208–215. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.-B.; Sun, S.-L.; Geng, Y.; Wu, Y.; Su, Z.-M. Density functional theory characterization and design of high-performance diarylamine-fluorene dyes with different π spacers for dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 568–576. [Google Scholar] [CrossRef]
- Preat, J.; Jacquemin, D.; Michaux, C.; Perpète, E.A. Improvement of the efficiency of thiophene-bridged compounds for dye-sensitized solar cells. Chem. Phys. 2010, 376, 56–68. [Google Scholar] [CrossRef]
- Zhang, J.; Kan, Y.-H.; Li, H.-B.; Geng, Y.; Wu, Y.; Su, Z.-M. How to design proper π-spacer order of the D-π-A dyes for DSSCs? A density functional response. Dyes Pigment. 2012, 95, 313–321. [Google Scholar] [CrossRef]
- Marinado, T.; Nonomura, K.; Nissfolk, J.; Karlsson, M.K.; Hagberg, D.P.; Sun, L.; Mori, S.; Hagfeldt, A. How the nature of triphenylamine-polyene dyes in dye-sensitized solar cells affects the open-circuit voltage and electron lifetimes. Langmuir 2010, 26, 2592–2598. [Google Scholar] [CrossRef]
- Rühle, S.; Greenshtein, M.; Chen, S.G.; Merson, A.; Pizem, H.; Sukenik, C.S.; Cahen, D.; Zaban, A. Molecular adjustment of the electronic properties of nanoporous electrodes in dye-sensitized solar cells. J. Phys. Chem. B 2005, 109, 18907–18913. [Google Scholar] [CrossRef] [PubMed]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Stratmann, R.E.; Scuseria, G.E.; Frisch, M.J. An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys. 1998, 109, 8218–8224. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.-L.; Wang, D.-M.; Geng, Z.-Y.; Zhao, X.-L.; Yan, Y.-F. Molecular engineering of indoline-based D–A− π–A organic sensitizers toward high efficiency performance from first-principles calculations. J. Phys. Chem. C 2013, 117, 17382–17398. [Google Scholar] [CrossRef]
- Kathiravan, A.; Srinivasan, V.; Khamrang, T.; Velusamy, M.; Jaccob, M.; Pavithra, N.; Anandan, S.; Velappan, K. Pyrene based D–π–A architectures: synthesis, density functional theory, photophysics and electron transfer dynamics. Phys. Chem. Chem. Phys. 2017, 19, 3125–3135. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, K.; Ju, X.-H.; Heron, B.M. Theoretical study on the light harvesting efficiency of zinc porphyrin sensitizers for DSSCs. Rsc Adv. 2014, 4, 26621–26634. [Google Scholar] [CrossRef]
- Dong, C.K.; Xiang, W.C.; Huang, F.Z.; Fu, D.C.; Huang, W.C.; Bach, U.; Cheng, Y.B.; Li, X.; Spiccia, L. Controlling Interfacial Recombination in Aqueous Dye-Sensitized Solar Cells by Octadecyltrichlorosilane Surface Treatment. Angew. Chem. Int. Ed. 2014, 53, 6933–6937. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.01, Gaussian; Wallingford’s Inc.: Oakland, CA, USA, 2009. [Google Scholar]
- Jin, J.-L.; Li, H.-B.; Geng, Y.; Wu, Y.; Duan, Y.-A.; Su, Z.-M. Theoretical Insight into the Origin of Large Stokes Shift and Photophysical Properties of Anilido-Pyridine Boron Difluoride Dyes. ChemPhysChem 2012, 13, 3714–3722. [Google Scholar] [CrossRef]
- Gajalakshmi, D.; Solomon, R.V.; Tamilmani, V.; Boobalan, M.; Venuvanalingam, P. A DFT/TDDFT mission to probe push–pull vinyl coupled thiophene oligomers for optoelectronic applications. RSC Adv. 2015, 5, 50353–50364. [Google Scholar] [CrossRef]
- Jungsuttiwong, S.; Tarsang, R.; Sudyoadsuk, T.; Promarak, V.; Khongpracha, P.; Namuangruk, S. Theoretical study on novel double donor-based dyes used in high efficient dye-sensitized solar cells: the application of TDDFT study to the electron injection process. Org. Electron. 2013, 14, 711–722. [Google Scholar] [CrossRef]
- Liu, Q.; Lin, X.C.; Mi, L.; Gao, N.; Song, P.; Ma, F.C.; Li, Y.Z. Characterizations of Efficient Charge Transfer and Photoelectric Performance in the Cosensitization of Solar Cells. Appl. Sci. 2018, 8, 1122. [Google Scholar] [CrossRef]
- Daeneke, T.; Mozer, A.J.; Uemura, Y.; Makuta, S.; Fekete, M.; Tachibana, Y.; Koumura, N.; Bach, U.; Spiccia, L. Dye regeneration kinetics in dye-sensitized solar cells. J. Am. Chem. Soc. 2012, 134, 16925–16928. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Kou, L.; Diao, L.; Zhang, Q.; Li, Z.; Wu, Q.; Lu, W.; Pan, D.; Wei, Z. Theoretical study of WS-9-Based organic sensitizers for unusual vis/NIR absorption and highly efficient dye-sensitized solar cells. J. Phys. Chem. C 2015, 119, 9782–9790. [Google Scholar] [CrossRef]
- Zhang, C.-R.; Liu, Z.-J.; Chen, Y.-H.; Chen, H.-S.; Wu, Y.-Z.; Feng, W.; Wang, D.-B. DFT and TD-DFT study on structure and properties of organic dye sensitizer TA-St-CA. Curr. Appl. Phys. 2010, 10, 77–83. [Google Scholar] [CrossRef]
- Dong, C.K.; Li, X.; Jin, P.F.; Zhao, W.; Chu, J.; Qi, J.Y. Intersubunit Electron Transfer (IET) in Quantum Dots/Graphene Complex: What Features Does IET Endow the Complex with? J. Phys. Chem. C 2012, 116, 15833–15838. [Google Scholar] [CrossRef]
- Eriksson, S.K.; Josefsson, I.; Ellis, H.; Amat, A.; Pastore, M.; Oscarsson, J.; Lindblad, R.; Eriksson, A.I.K.; Johansson, E.M.J.; Boschloo, G.; et al. Geometrical and energetical structural changes in organic dyes for dye-sensitized solar cells probed using photoelectron spectroscopy and DFT. Phys. Chem. Chem. Phys. 2016, 18, 252–260. [Google Scholar] [CrossRef]


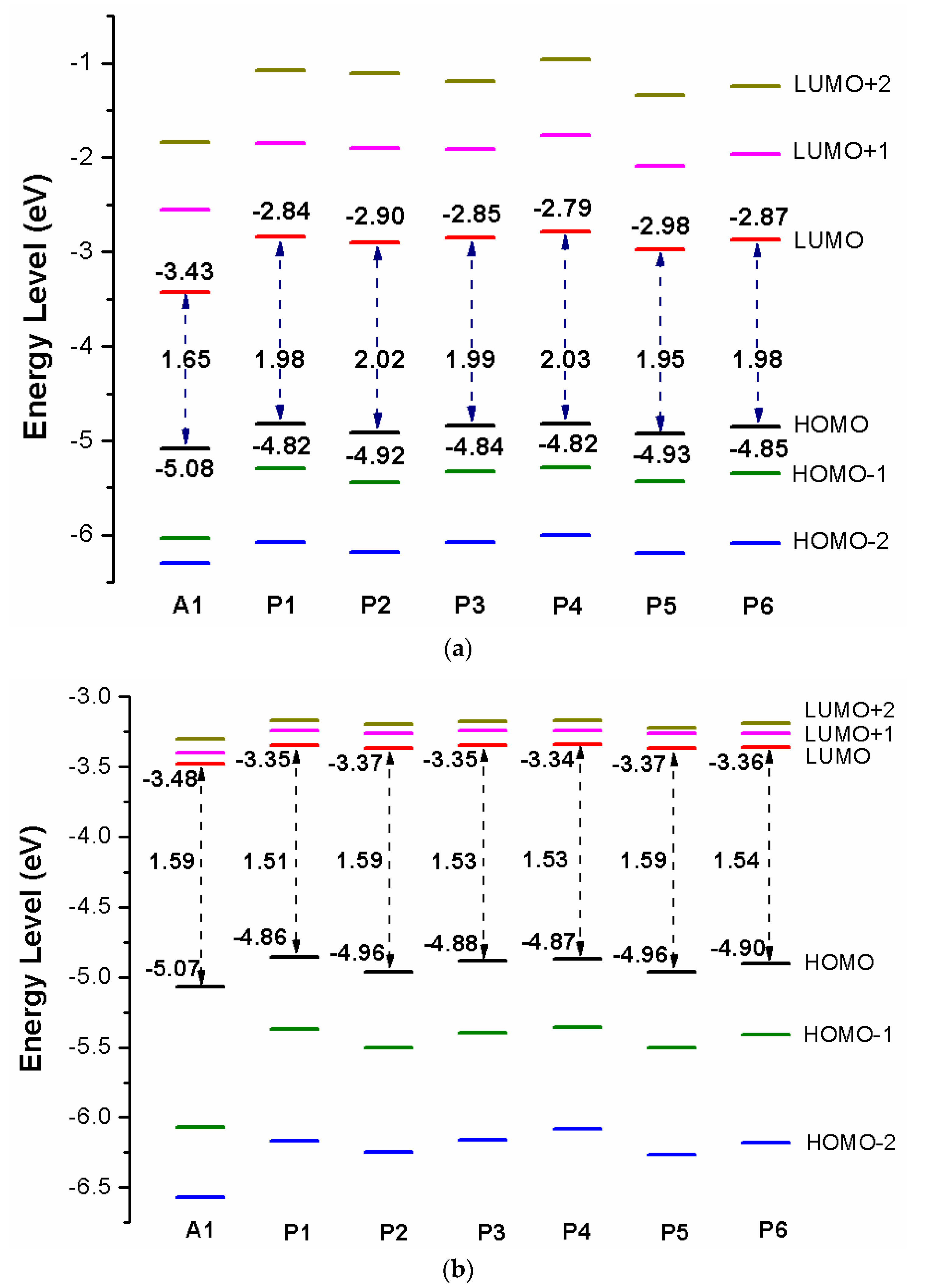
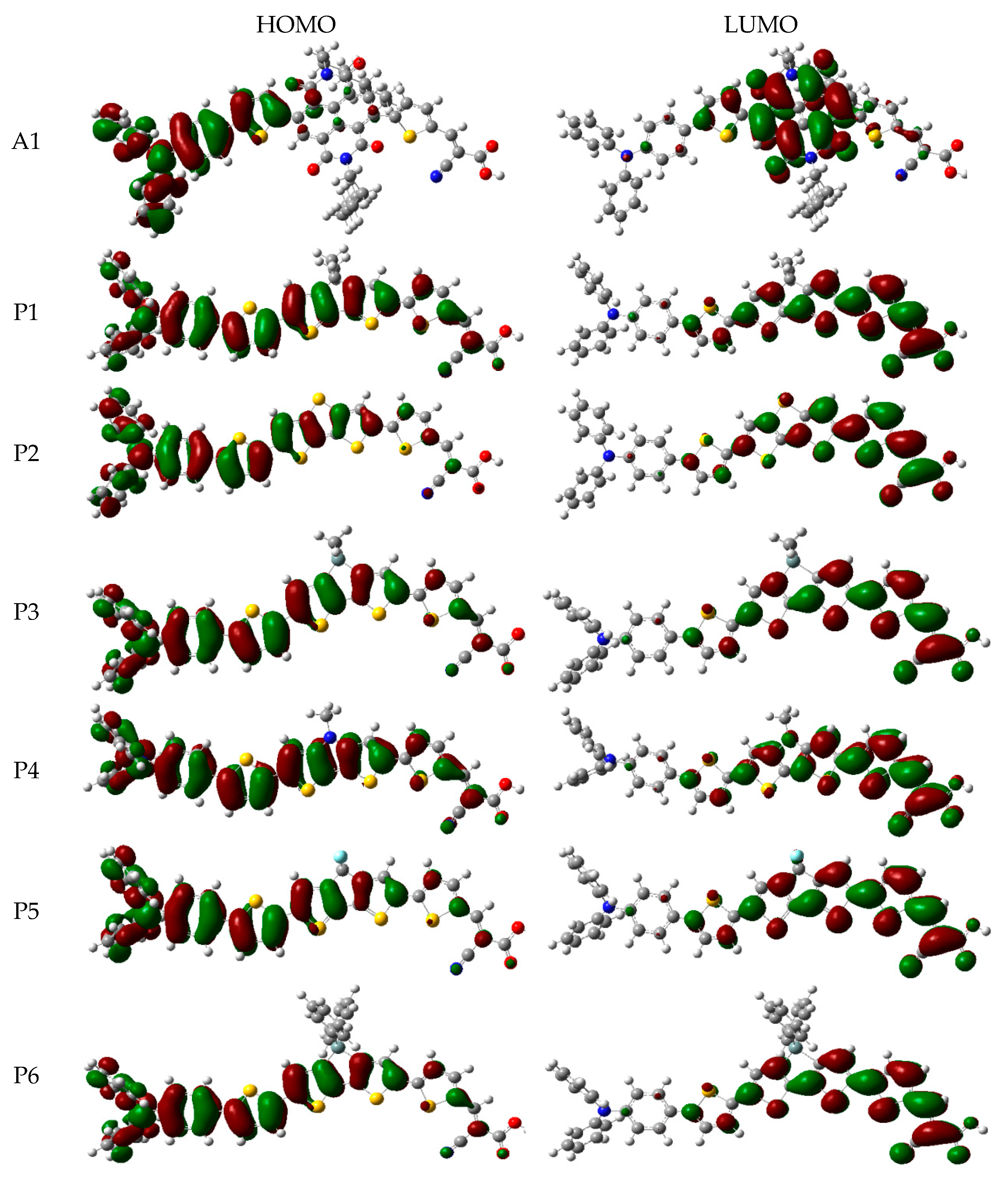
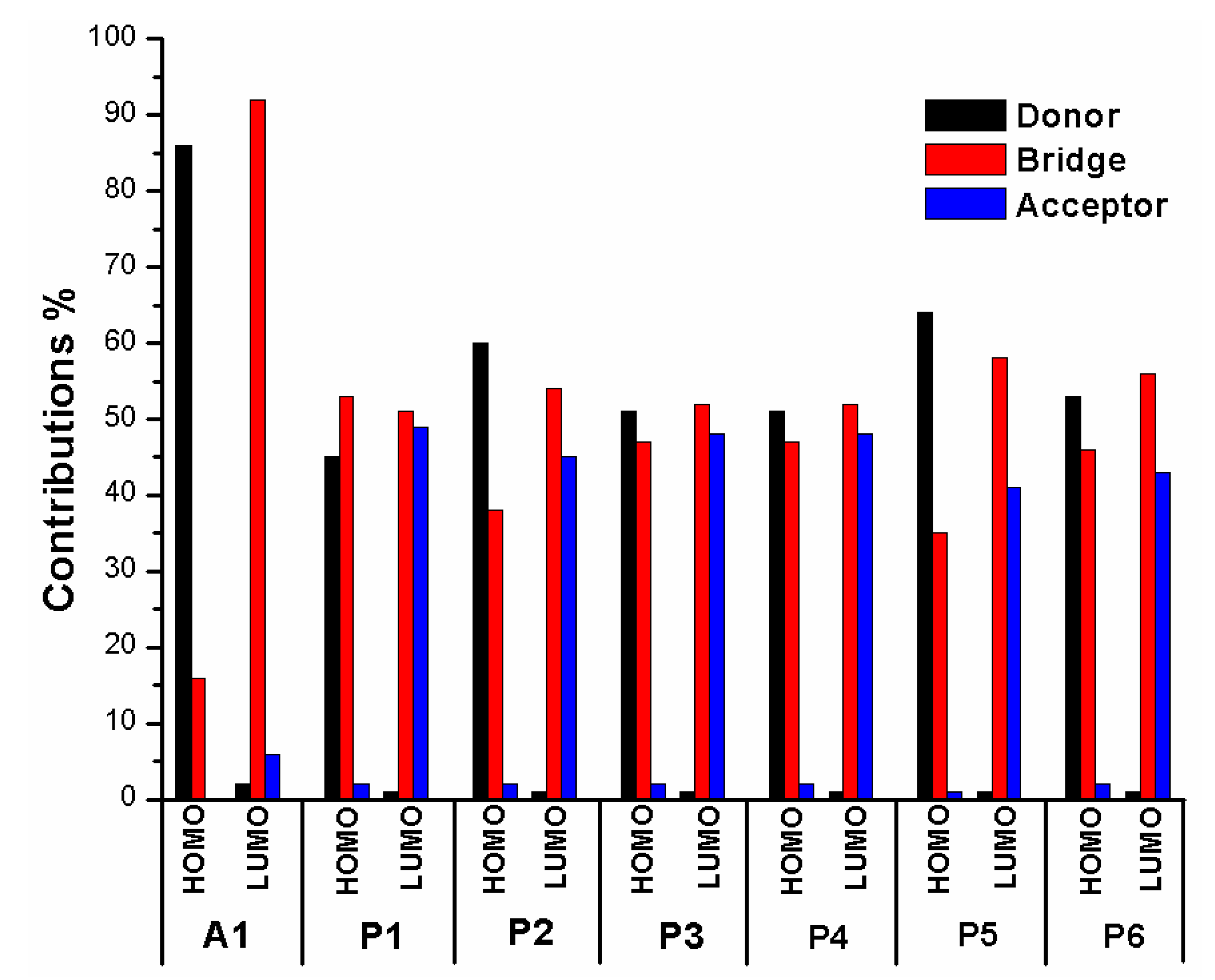

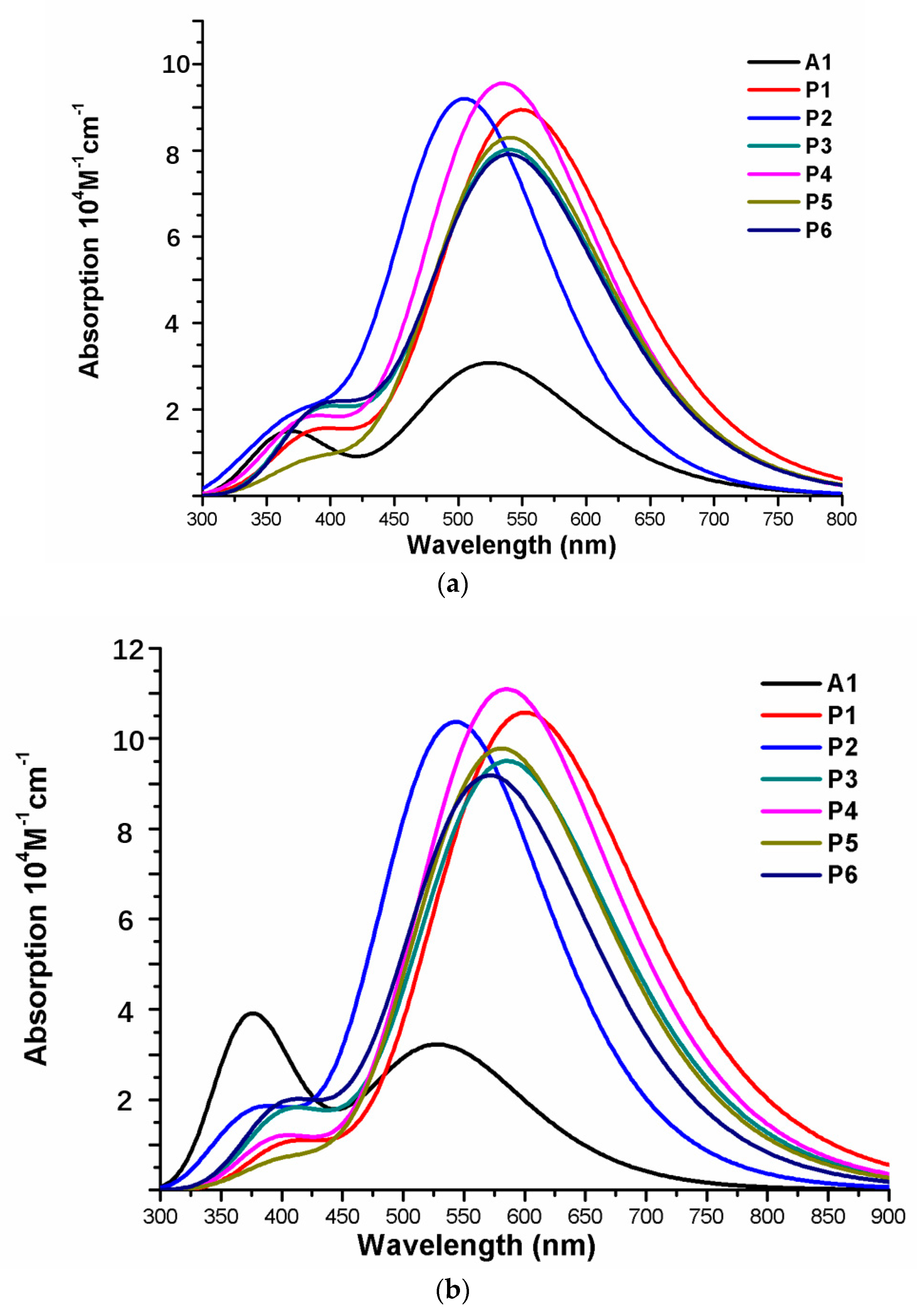


| A1 | 22.46 | −37.79 | −118.77 | −179.70 | 1.460 | 1.465 | 1.481 | 1.425 |
| P1 | −21.17 | 9.48 | 0.20 | −179.94 | 1.462 | 1.441 | 1.433 | 1.415 |
| P2 | 21.30 | −9.82 | −1.96 | −0.25 | 1.461 | 1.442 | 1.438 | 1.419 |
| P3 | −20.56 | 7.54 | 1.84 | −179.72 | 1.462 | 1.442 | 1.435 | 1.416 |
| P4 | −20.53 | 8.76 | 0.35 | 179.86 | 1.462 | 1.441 | 1.433 | 1.415 |
| P5 | −21.06 | 11.17 | 4.81 | 179.93 | 1.462 | 1.441 | 1.436 | 1.418 |
| P6 | −18.34 | 8.75 | 1.33 | −179.92 | 1.462 | 1.442 | 1.436 | 1.417 |
| D1 | 21.74 | −38.95 | −117.02 | −179.63 | 1.460 | 1.465 | 1.482 | 1.425 |
| D2 | 23.62 | −36.97 | −118.20 | −179.94 | 1.459 | 1.463 | 1.481 | 1.425 |
| D3 | 21.71 | −38.04 | −115.34 | −179.49 | 1.461 | 1.464 | 1.482 | 1.425 |
| D4 | 22.15 | −36.17 | −116.88 | −179.67 | 1.457 | 1.462 | 1.481 | 1.424 |
| Dye | State | f | Main Configurations | |
|---|---|---|---|---|
| A1 | 1 | 2.36/525 | 0.7597 | H → L/0.58940 |
| 2 | 3.26/380 | 0.1511 | H-2 → L/0.43402 | |
| 3 | 3.43/362 | 0.2330 | H-2 → L/0.40002 | |
| P1 | 1 | 2.26/549 | 2.2053 | H → L/0.59065 |
| 2 | 3.15/393 | 0.3574 | H → L + 1/0.51442 | |
| 3 | 3.58/347 | 0.0434 | H-1 → L/0.37490 | |
| P2 | 1 | 2.45/505 | 2.2594 | H → L/0.51866 |
| 2 | 3.21/386 | 0.4115 | H → L + 1/0.48619 | |
| 3 | 3.66/338 | 0.1640 | H → L/0.42560 | |
| P3 | 1 | 2.29/541 | 1.9759 | H → L/0.56296 |
| 2 | 3.15/394 | 0.4839 | H → L + 1/0.49594 | |
| 3 | 3.56/348 | 0.0119 | H → L/0.36012 | |
| P4 | 1 | 2.32/535 | 2.3560 | H → L/0.59699 |
| 2 | 3.20/388 | 0.4152 | H → L + 1/0.53467 | |
| 3 | 3.59/345 | 0.0676 | H-2 → L/0.57213 | |
| P5 | 1 | 2.29/541 | 2.0460 | H → L/0.52808 |
| 2 | 3.14/395 | 0.1942 | H → L + 1/0.45139 | |
| 3 | 3.50/354 | 0.0343 | H-1 → L + 1/0.37664 | |
| P6 | 1 | 2.29/540 | 1.9468 | H → L/0.55318 |
| 2 | 3.13/396 | 0.5035 | H → L + 1/0.48466 | |
| 3 | 3.54/351 | 0.0117 | H → L/0.36083 | |
| D1 | 1 | 2.32/534 | 0.7459 | H → L/0.59261 |
| 2 | 3.24/382 | 0.1267 | H-1 → L/0.46484 | |
| 3 | 3.40/365 | 0.1976 | H-2 → L/0.48305 | |
| D2 | 1 | 2.26/548 | 0.7609 | H → L/0.59379 |
| 2 | 3.18/390 | 0.1150 | H-1 → L/0.48838 | |
| 3 | 3.37/368 | 0.2005 | H-2 → L/0.52160 | |
| D3 | 1 | 2.33/532 | 0.7493 | H → L/0.58869 |
| 2 | 3.24/382 | 0.1095 | H-1 → L/0.47235 | |
| 3 | 3.39/365 | 0.1981 | H-2 → L/0.50056 | |
| D4 | 1 | 2.17/571 | 0.7540 | H → L/0.59999 |
| 2 | 3.06/405 | 0.1200 | H-1 → L/0.51377 | |
| 3 | 3.36/369 | 0.1856 | H-3 → L/0.55893 | |
| A1/TiO2 | 1 | 2.34/529 | 0.7923 | H → L/0.58409 |
| 2 | 3.23/384 | 0.5380 | H-1 → L/0.36516 | |
| 3 | 3.38/366 | 0.4640 | H → L/0.37280 | |
| P1/TiO2 | 1 | 2.06/601 | 2.6104 | H → L/0.54964 |
| 2 | 3.02/411 | 0.2565 | H → L + 16/0.35495 | |
| 3 | 3.29/376 | 0.0087 | H → L + 1/00.55205 | |
| P2/TiO2 | 1 | 2.28/543 | 2.5567 | H → L/0.48438 |
| 2 | 3.12/398 | 0.3216 | H → L + 16/0.36183 | |
| 3 | 3.46/359 | 0.2092 | H → L/0.43632 | |
| P3/TiO2 | 1 | 2.12/586 | 2.3440 | H → L/0.51644 |
| 2 | 3.02/410 | 0.4186 | H-1 → L/0.32626 | |
| 3 | 3.33/372 | 0.0355 | H → L/0.41457 | |
| P4/TiO2 | 1 | 2.12/585 | 2.7395 | H → L/0.53415 |
| 2 | 3.09/401 | 0.2884 | H-1 → L/0.32472 | |
| 3 | 3.30/376 | 0.0030 | H → L + 1/0.57187 | |
| P5/TiO2 | 1 | 2.13/581 | 2.4142 | H → L/0.50933 |
| 2 | 3.01/412 | 0.1571 | H-1 → L/0.34133 | |
| 3 | 3.32/373 | 0.0276 | H → L/0.41752 | |
| P6/TiO2 | 1 | 2.17/572 | 2.2638 | H → L/0.49491 |
| 2 | 3.04/407 | 0.4603 | H-2 → L/0.30426 | |
| 3 | 3.36/369 | 0.0316 | H → L/0.45448 | |
| D1/TiO2 | 1 | 2.31/538 | 0.7861 | H → L/0.59277 |
| 2 | 3.26/380 | 0.2399 | H-1 → L/0.48023 | |
| 3 | 3.38/367 | 0.8011 | H-2 → L + 4/0.35860 | |
| D2/TiO2 | 1 | 2.24/553 | 0.7936 | H → L/0.59502 |
| 2 | 3.19/388 | 0.1238 | H-1 → L/0.51121 | |
| 3 | 3.38/367 | 0.9464 | H-3 → L/0.41438 | |
| D3/TiO2 | 1 | 2.31/538 | 0.7819 | H → L/0.58538 |
| 2 | 3.21/387 | 0.3757 | H-1 → L/0.44284 | |
| 3 | 3.35/370 | 0.6066 | H-2 → L + 4/0.30048 | |
| D4/TiO2 | 1 | 2.13/581 | 0.8108 | H → L/0.59550 |
| 2 | 3.01/411 | 0.2016 | H-1 → L/0.50064 | |
| 3 | 3.26/380 | 0.4754 | H → L + 4/0.37904 |
| Dye | State | f | Main Configurations | |
|---|---|---|---|---|
| A1 | 1 | 1.84/676 | 0.7606 | H → L/0.64473 |
| P1 | 1 | 1.87/662 | 2.3909 | H → L/0.65705 |
| P2 | 1 | 2.03/610 | 2.4533 | H → L/0.62022 |
| P3 | 1 | 1.86/665 | 2.1311 | H → L/0.64954 |
| P4 | 1 | 2.00/620 | 2.5404 | H → L/0.64919 |
| P5 | 1 | 1.81/685 | 2.1693 | H → L/0.64219 |
| P6 | 1 | 1.86/668 | 2.0739 | H → L/0.64715 |
| D1 | 1 | 1.81/685 | 0.7513 | H → L/0.64168 |
| D2 | 1 | 1.78/697 | 0.7130 | H → L/0.63564 |
| D3 | 1 | 1.81/685 | 0.7540 | H → L/0.64135 |
| D4 | 1 | 1.72/723 | 0.7148 | H → L/0.62513 |
| Dye | LHE | |||||
|---|---|---|---|---|---|---|
| A1 | 0.826 | −1.28 | 2.72 | 5.08 | 0.48 | 0.57 |
| P1 | 0.994 | −1.44 | 2.56 | 4.82 | 0.22 | 1.16 |
| P2 | 0.994 | −1.53 | 2.47 | 4.92 | 0.32 | 1.10 |
| P3 | 0.989 | −1.45 | 2.55 | 4.84 | 0.24 | 1.15 |
| P4 | 0.996 | −1.50 | 2.50 | 4.82 | 0.22 | 1.21 |
| P5 | 0.991 | −1.36 | 2.64 | 4.93 | 0.33 | 1.02 |
| P6 | 0.989 | −1.44 | −2.56 | 4.85 | 0.25 | 1.13 |
| D1 | 0.820 | −1.34 | 2.66 | 4.98 | 0.38 | 0.58 |
| D2 | 0.827 | 1.39 | 2.61 | 4.87 | 0.27 | 0.60 |
| D3 | 0.822 | −1.35 | 2.65 | 4.98 | 0.38 | 0.58 |
| D4 | 0.824 | −1.49 | 2.51 | 4.68 | 0.08 | 0.63 |
| A1/TiO2 | 0.839 | −1.23 | −2.77 | 5.07 | 0.47 | 0.52 |
| P1/TiO2 | 0.998 | −1.20 | −2.80 | 4.86 | 0.22 | 0.65 |
| P2/TiO2 | 0.997 | −1.32 | −2.68 | 4.96 | 0.36 | 0.63 |
| P3/TiO2 | 0.995 | −1.24 | −2.76 | 4.88 | 0.28 | 0.65 |
| P4/TiO2 | 0.998 | −1.25 | −2.75 | 4.87 | 0.27 | 0.66 |
| P5/TiO2 | 0.996 | −1.17 | −2.83 | 4.96 | 0.36 | 0.63 |
| P6/TiO2 | 0.995 | −1.27 | −2.73 | 4.90 | 0.30 | 0.64 |
| D1/TiO2 | 0.836 | −2.39 | 1.61 | 4.99 | 0.39 | 0.56 |
| D2/TiO2 | 0.839 | −2.51 | 1.49 | 4.87 | 0.27 | 0.57 |
| D3/TiO2 | 0.835 | −1.33 | 2.67 | 4.98 | 0.38 | 0.53 |
| D4/TiO2 | 0.845 | −1.45 | 2.55 | 4.68 | 0.08 | 0.57 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Mi, L.; Wang, H.; Li, Y.; Liang, J. Design, Electron Transfer Process, and Opto-Electronic Property of Solar Cell Using Triphenylamine-Based D-π-A Architectures. Materials 2019, 12, 193. https://doi.org/10.3390/ma12010193
Li Y, Mi L, Wang H, Li Y, Liang J. Design, Electron Transfer Process, and Opto-Electronic Property of Solar Cell Using Triphenylamine-Based D-π-A Architectures. Materials. 2019; 12(1):193. https://doi.org/10.3390/ma12010193
Chicago/Turabian StyleLi, Yuanchao, Lu Mi, Haibin Wang, Yuanzuo Li, and Jianping Liang. 2019. "Design, Electron Transfer Process, and Opto-Electronic Property of Solar Cell Using Triphenylamine-Based D-π-A Architectures" Materials 12, no. 1: 193. https://doi.org/10.3390/ma12010193





